Fabrication and Characterization of Bioresorbable Drug-coated Porous Scaffolds for Vascular Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
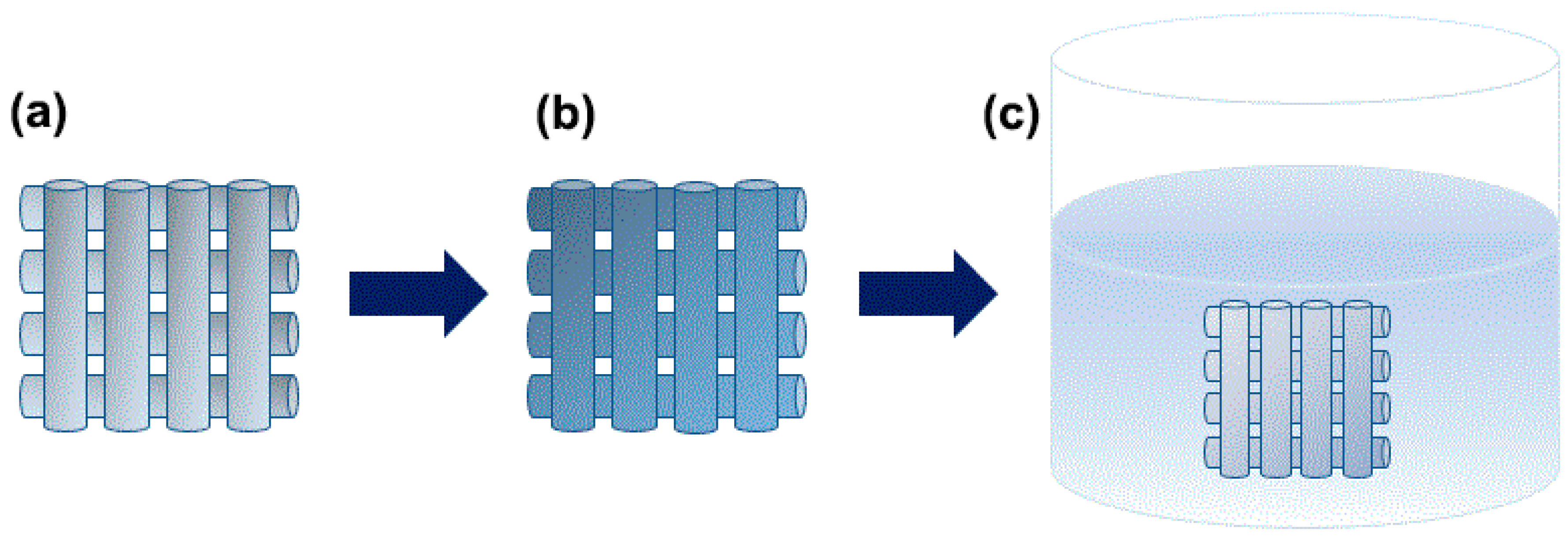
2.2. Fabrication of the Porous PCL Scaffolds by 3D printing
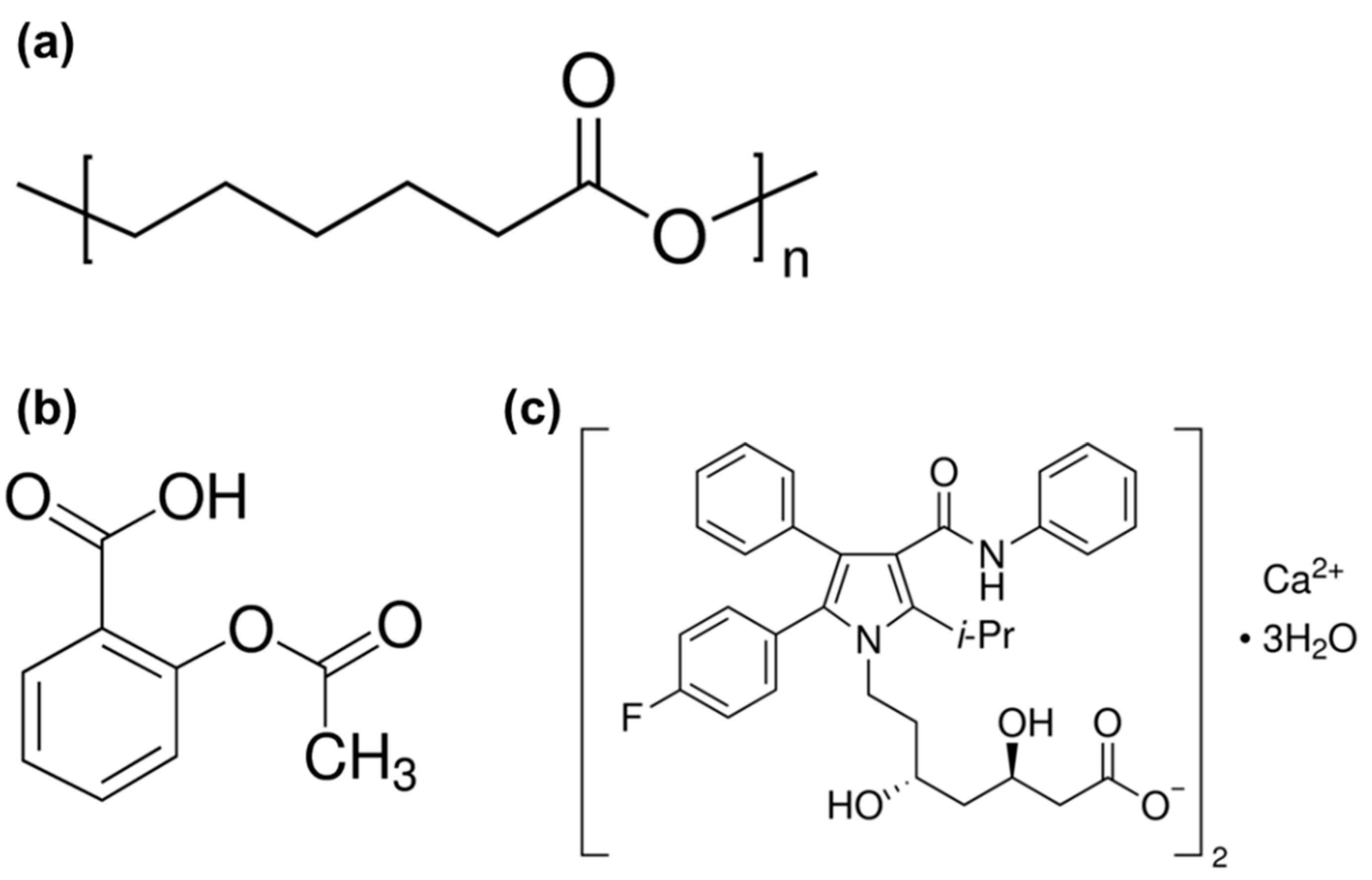
2.3. Preparation of the Atorvastatin Calcium Salt: Aspirin Stock Solutions
2.4. Drug Coating on the Porous PCL Scaffolds
2.5. Characterization
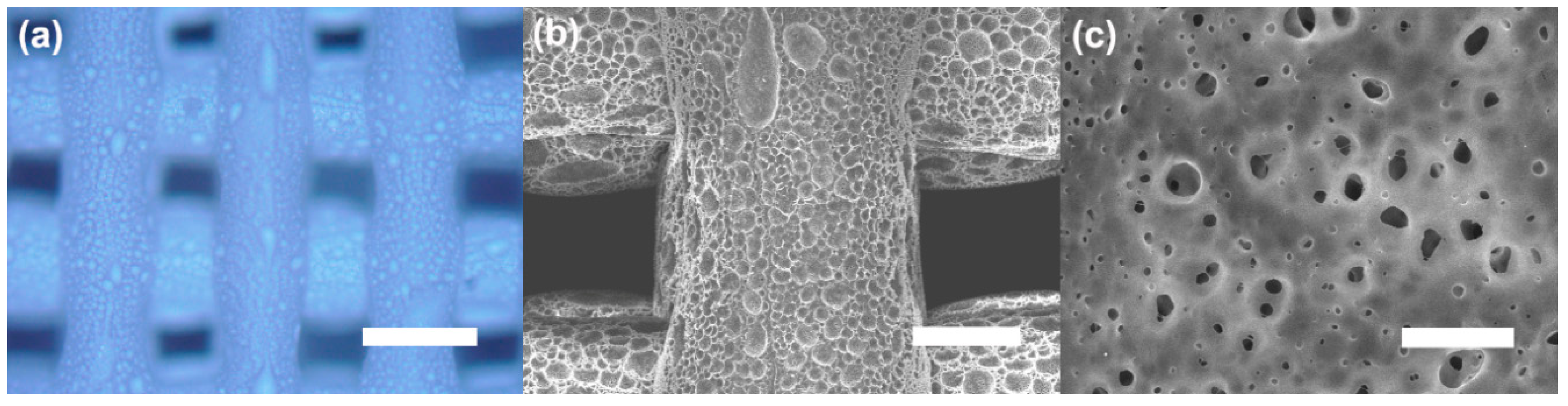
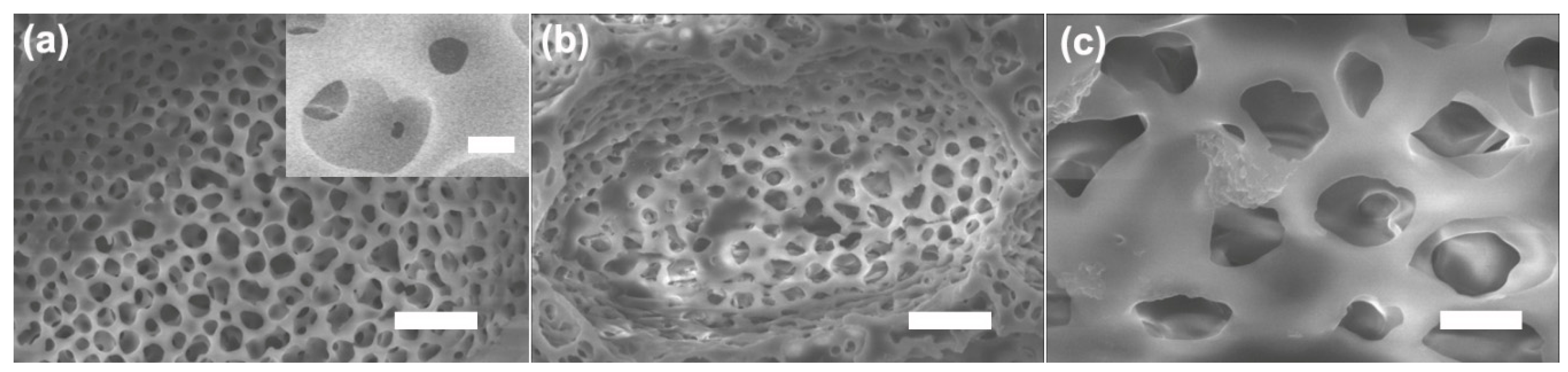
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Optical Microscopy (OM)
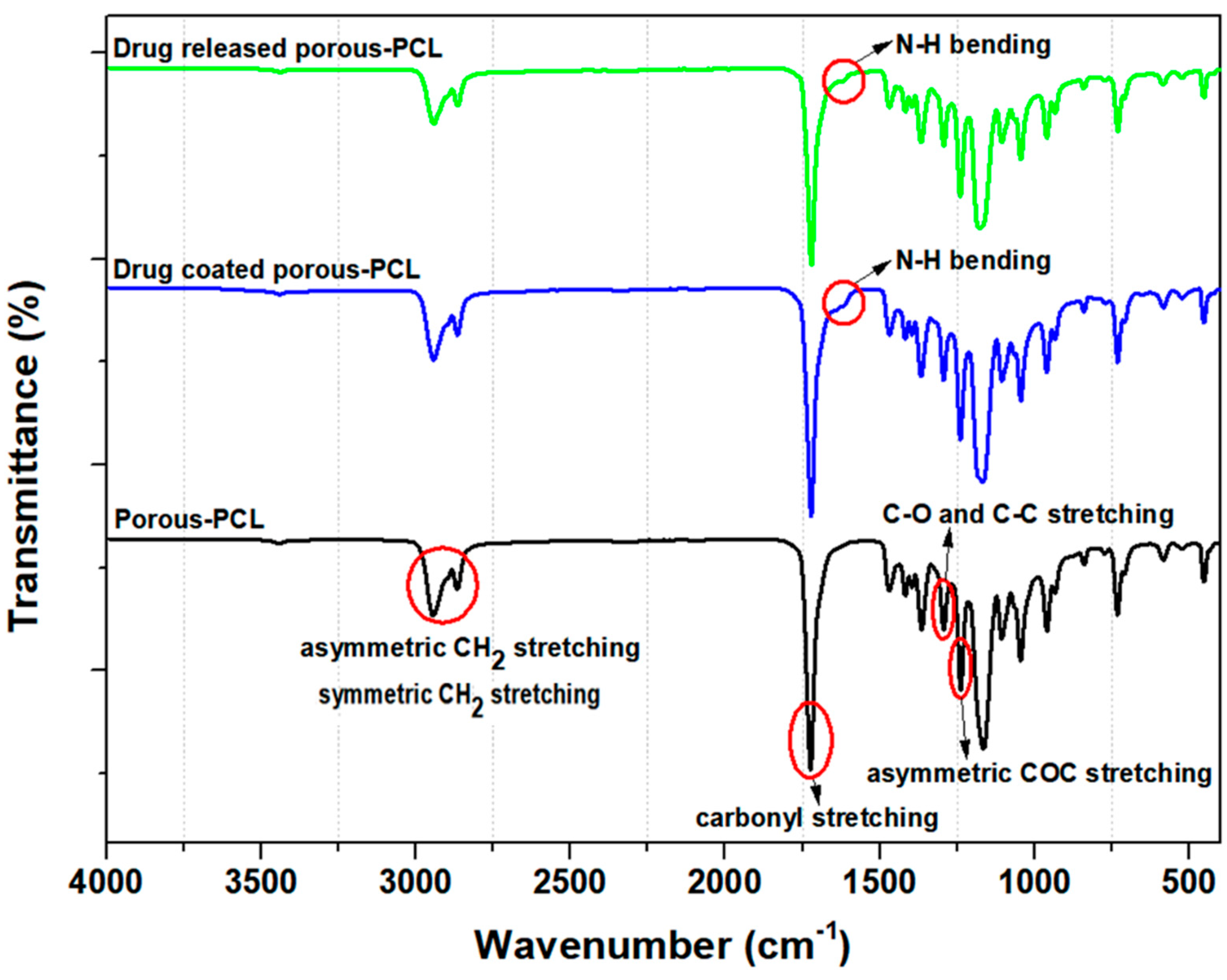
2.5.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.5.4. X-ray Photoelectron Spectroscopy (XPS)
2.5.5. Drug Content
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, S.; Hussain, C.M. Green and Sustainable Advanced Materials: Applications; John Wiley & Sons: Beverly, MA, USA, 2018. [Google Scholar]
- Kaur, G. Polymers as Bioactive Materials-I: Natural and Non-degradable Polymers. In Bioactive Glasses; Springer: Cham, Switzerland, 2017; pp. 21–51. [Google Scholar]
- Suzuki, S.; Ikada, Y. Biomaterials for Surgical Operation; Springer Science & Business Media: New York, NY, USA, 2011. [Google Scholar]
- Burg, K.; Shalaby, W.S. Bioabsorbable Polymers: Tissue Engineering. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials, 11 Volume Set; CRC Press: Boca Raton, FL, USA, 2015; pp. 429–432. [Google Scholar]
- Maitz, M.F. Applications of Synthetic Polymers in Clinical Medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Perale, G.; Hilborn, J. Bioresorbable Polymers for Biomedical Applications: From Fundamentals to Translational Medicine; Woodhead Publishing: Duxford, UK, 2016. [Google Scholar]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and Biocompatible Polymers for Tissue Engineering Application: A Review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Gu, B.K.; Choi, D.J.; Park, S.J.; Kim, M.S.; Kang, C.M.; Kim, C.-H. 3-Dimensional Bioprinting for Tissue Engineering Applications. Biomater. Res. 2016, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Hastings, G.W. Cardiovascular Biomaterials; Springer Science & Business Media: London, UK, 2012. [Google Scholar]
- Soares, J.S.; Moore, J.E. Biomechanical Challenges to Polymeric Biodegradable Stents. Ann. Biomed. Eng. 2016, 44, 560–579. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, M.; Wildemann, B.; Lendlein, A. Biodegradable Polymeric Materials. In Regenerative Medicine-from Protocol to Patient; Springer: Cham, Switzerland, 2016; pp. 65–96. [Google Scholar]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Wall, G.; Podbielska, H.; Wawrzynska, M. Functionalised Cardiovascular Stents; Woodhead Publishing: Duxford, UK, 2018. [Google Scholar]
- Sin, L.T. Polylactic Acid: PLA Biopolymer Technology and Applications; William Andrew: Kidlington, UK, 2012. [Google Scholar]
- Mclauchlin, A.; Thomas, N. Biodegradable polymer nanocomposites. In Advances in Polymer Nanocomposites; Elsevier: Sawston, UK, 2012; pp. 398–430. [Google Scholar]
- Zhang, X.; Peng, X.; Zhang, S. Synthetic biodegradable medical polymers: Polymer blends. In Science and Principles of Biodegradable and Bioresorbable Medical Polymers; Elsevier: Duxford, UK, 2017; pp. 217–254. [Google Scholar]
- Ricklefs, M.; Korossis, S.; Haverich, A.; Schilling, T. Polymeric Scaffolds for Bioartificial Cardiovascular Prostheses. In Scaffolds in Tissue Engineering-Materials, Technologies and Clinical Applications; IntechOpen: London, UK, 2017. [Google Scholar]
- Ghorbani, F.; Moradi, L.; Shadmehr, M.B.; Bonakdar, S.; Droodinia, A.; Safshekan, F. In-vivo Characterization of a 3D Hybrid Scaffold Based on PCL/Decellularized Aorta for Tracheal Tissue Engineering. Mater. Sci. Eng. C 2017, 81, 74–83. [Google Scholar] [CrossRef]
- Chen, X.; Guan, Y.; Wang, L.; Sanbhal, N.; Zhao, F.; Zou, Q.; Zhang, Q. Antimicrobial textiles for sutures, implants, and scaffolds. In Antimicrobial Textiles; Elsevier: Duxford, UK, 2016; pp. 263–285. [Google Scholar]
- Jenkins, M.; Stamboulis, A. Durability and Reliability of Medical Polymers; Elsevier: Sawston, UK, 2012. [Google Scholar]
- Lee, S.J.; Lee, D.; Yoon, T.R.; Kim, H.K.; Jo, H.H.; Park, J.S.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Surface Modification of 3D-printed Porous Scaffolds via Mussel-inspired Polydopamine and Effective Immobilization of rhBMP-2 to Promote Osteogenic Differentiation for Bone Tissue Engineering. Acta Biomater. 2016, 40, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Lanza, R.; Langer, R.; Vacanti, J.P. Principles of Tissue Engineering; Academic Press: London, UK, 2011. [Google Scholar]
- He, W.; Benson, R. Polymeric biomaterials. In Applied Plastics Engineering Handbook; Elsevier: Kindlington, UK, 2017; pp. 145–164. [Google Scholar]
- Brannigan, R.P.; Dove, A.P. Synthesis, Properties and Biomedical Applications of Hydrolytically Degradable Materials Based on Aliphatic Polyesters and Polycarbonates. Biomater. Sci. 2017, 5, 9–21. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Zhang, S. Biodegradable Medical Polymers: Fundamental Sciences. In Science and Principles of Biodegradable and Bioresorbable Medical Polymers; Elsevier: Duxforf, UK, 2017; pp. 1–33. [Google Scholar]
- Hoornaert, A.; d’Arros, C.; Heymann, M.-F.; Layrolle, P. Biocompatibility, Resorption and Biofunctionality of a New Synthetic Biodegradable Membrane for Guided Bone Regeneration. Biomed. Mater. 2016, 11, 045012. [Google Scholar] [CrossRef]
- Simmons, K.B. Selection and Application of Synthetic Polymeric Materials in Surgical Sutures. Master of Science, Master Thesis, University of Georgia, Athens, GA, USA, 2017. [Google Scholar]
- Ormiston, J.A.; Serruys, P.W. Bioabsorbable Coronary Stents. Circ.-Cardiovasc. Interv. 2009, 2, 255–260. [Google Scholar] [CrossRef]
- Ako, J.; Bonneau, H.N.; Honda, Y.; Fitzgerald, P.J. Design Criteria for the Ideal Drug-Eluting Stent. Am. J. Cardiol. 2007, 100, S3–S9. [Google Scholar] [CrossRef]
- Gentile, P.; Carmagnola, I.; Nardo, T.; Chiono, V. Layer-by-Layer Assembly for Biomedical Applications in the Last Decade. Nanotechnology 2015, 26, 422001. [Google Scholar] [CrossRef]
- Alexy, R.D.; Levi, D.S. Materials and Manufacturing Technologies Available for Production of a Pediatric Bioabsorbable Stent. BioMed Res. Int. 2013, 2013, 137985. [Google Scholar] [CrossRef]
- Livingston, M.; Tan, A. Classification of Joints Used in Steerable Instruments for Minimally Invasive Surgery—A Review of the State of the Art. J. Med. Devices 2016, 10, 010801. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, S.J.; Lim, K.S.; Bae, I.H.; Lee, J.H.; Kim, W.D.; Jeong, M.H.; Park, J.-K. In vivo Evaluation and Characterization of a Bio-absorbable Drug-coated Stent Fabricated Using a 3D-printing System. Mater. Lett. 2015, 141, 355–358. [Google Scholar] [CrossRef]
- Im, E.; Cho, Y.-H.; Suh, Y.; Cho, D.-K.; Her, A.-Y.; Kim, Y.H.; Lee, K.; Kang, W.C.; Yun, K.H.; Yoo, S.-Y.; et al. High-intensity Statin Treatments in Clinically Stable Patients on Aspirin Monotherapy 12 Months After Drug-eluting Stent Implantation: A Randomized Study. Revista Española de Cardiología (Engl. Ed.) 2018, 71, 423–431. [Google Scholar] [CrossRef]
- Joshy, K.; Snigdha, S.; Thomas, S. Plasma Modified Polymeric Materials for Scaffolding of Bone Tissue Engineering. In Non-Thermal Plasma Technology for Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 439–458. [Google Scholar]
- Bakare, R.A. Synthesis and Characterization of Protein-Conjugated Silver Nanoparticles/Silver Salt Loaded Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV) Film for Prevention of Bacterial Infections and Potential Use in Bone Tissue Engineering Applications. Department of Chemistry. Ph.D. Thesis, Howard University, Washington, DC, USA, 2015. [Google Scholar]
- Ghosal, K.; Manakhov, A.; Zajíčková, L.; Thomas, S. Structural and Surface Compatibility Study of Modified Electrospun Poly(ε-caprolactone) (PCL) Composites for Skin Tissue Engineering. AAPS PharmSciTech 2017, 18, 72–81. [Google Scholar] [CrossRef]
- Shkarina, S.; Shkarin, R.; Weinhardt, V.; Melnik, E.; Vacun, G.; Kluger, P.; Loza, K.; Epple, M.; Ivlev, S.I.; Baumbach, T.; et al. 3D Biodegradable Scaffolds of Polycaprolactone with Silicate-containing hydroxyapatite Microparticles for Bone Tissue Engineering: High-resolution Tomography and in vivo Study. Sci. Rep. 2018, 8, 8907. [Google Scholar] [CrossRef]
- Gümüşderelioğlu, M.; Dalkıranoğlu, S.; Aydın, R.S.T.; Çakmak, S. A Novel Dermal Substitute Based on Biofunctionalized Electrospun PCL Nanofibrous Matrix. J. Biomed. Mater. Res. A 2011, 98, 461–472. [Google Scholar] [CrossRef]
- Pan, X.; Sun, B.; Mo, X. Electrospun Polypyrrole-coated Polycaprolactone Nanoyarn Nerve Guidance Conduits for Nerve Tissue Engineering. Front. Mater. Sci. 2018, 12, 438–446. [Google Scholar] [CrossRef]
- Chen, S.-H.; Chen, C.-H.; Shalumon, K.; Chen, J.-P. Preparation and Characterization of Antiadhesion Barrier Film from Hyaluronic Acid-grafted Electrospun Poly(caprolactone) Nanofibrous Membranes for Prevention of Flexor Tendon Postoperative Peritendinous Adhesion. Int. J. Nanomed. 2014, 9, 4079–4092. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Wang, Z.; Feng, S.; Xu, H.; Zhao, Q.; Wang, S.; Fang, J.; Qiao, M.; Kong, D. Immobilization of Anti-CD31 Antibody on Electrospun Poly(e-caprolactone) Scaffolds Through Hydrophobins for Specific Adhesion of Endotherial Cells. Colloids Surf. B 2011, 85, 32–39. [Google Scholar] [CrossRef]
- Pappa, A.M.; Karagkiozaki, V.; Krol, S.; Kassavetis, S.; Konstantinou, D.; Pitsalidis, C.; Tzounis, L.; Pliatsikas, N.; Logothetidis, S. Oxygen-plasma-modified Biomimetic nanofibrous Scaffolds for Enhanced Compatibility of Cardiovascular Implants. Beilstein J. Nanotechnol. 2015, 6, 254–262. [Google Scholar] [CrossRef]
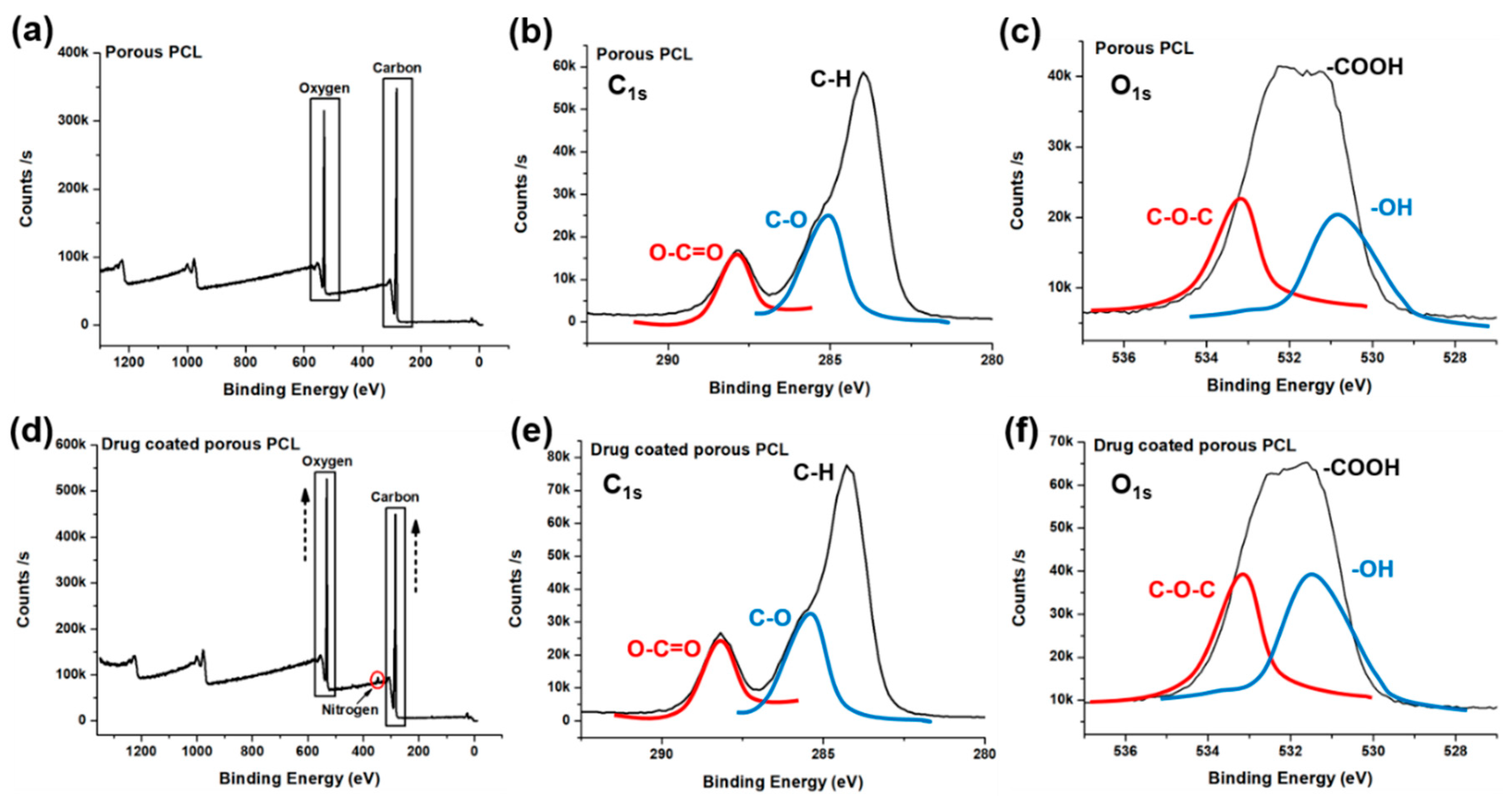
| Experimental Groups | Carbon | Oxygen | Nitrogen | Calcium |
|---|---|---|---|---|
| Porous PCL | 76.55 | 23.45 | 0 | 0 |
| Drug coated porous PCL | 73.99 | 25.55 | 0.25 | 0.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Park, S.A.; Kim, J.; Lee, J. Fabrication and Characterization of Bioresorbable Drug-coated Porous Scaffolds for Vascular Tissue Engineering. Materials 2019, 12, 1438. https://doi.org/10.3390/ma12091438
Kim J, Park SA, Kim J, Lee J. Fabrication and Characterization of Bioresorbable Drug-coated Porous Scaffolds for Vascular Tissue Engineering. Materials. 2019; 12(9):1438. https://doi.org/10.3390/ma12091438
Chicago/Turabian StyleKim, Jueun, Su A. Park, Jei Kim, and Jaejong Lee. 2019. "Fabrication and Characterization of Bioresorbable Drug-coated Porous Scaffolds for Vascular Tissue Engineering" Materials 12, no. 9: 1438. https://doi.org/10.3390/ma12091438
APA StyleKim, J., Park, S. A., Kim, J., & Lee, J. (2019). Fabrication and Characterization of Bioresorbable Drug-coated Porous Scaffolds for Vascular Tissue Engineering. Materials, 12(9), 1438. https://doi.org/10.3390/ma12091438




