Study of Catalytic Combustion of Dioxins on Ce-V-Ti Catalysts Modified by Graphene Oxide in Simulating Iron Ore Sintering Flue Gas
Abstract
:1. Introduction
2. Experimental and Material
2.1. Catalysts Preparation
2.2. Catalyst Characterization
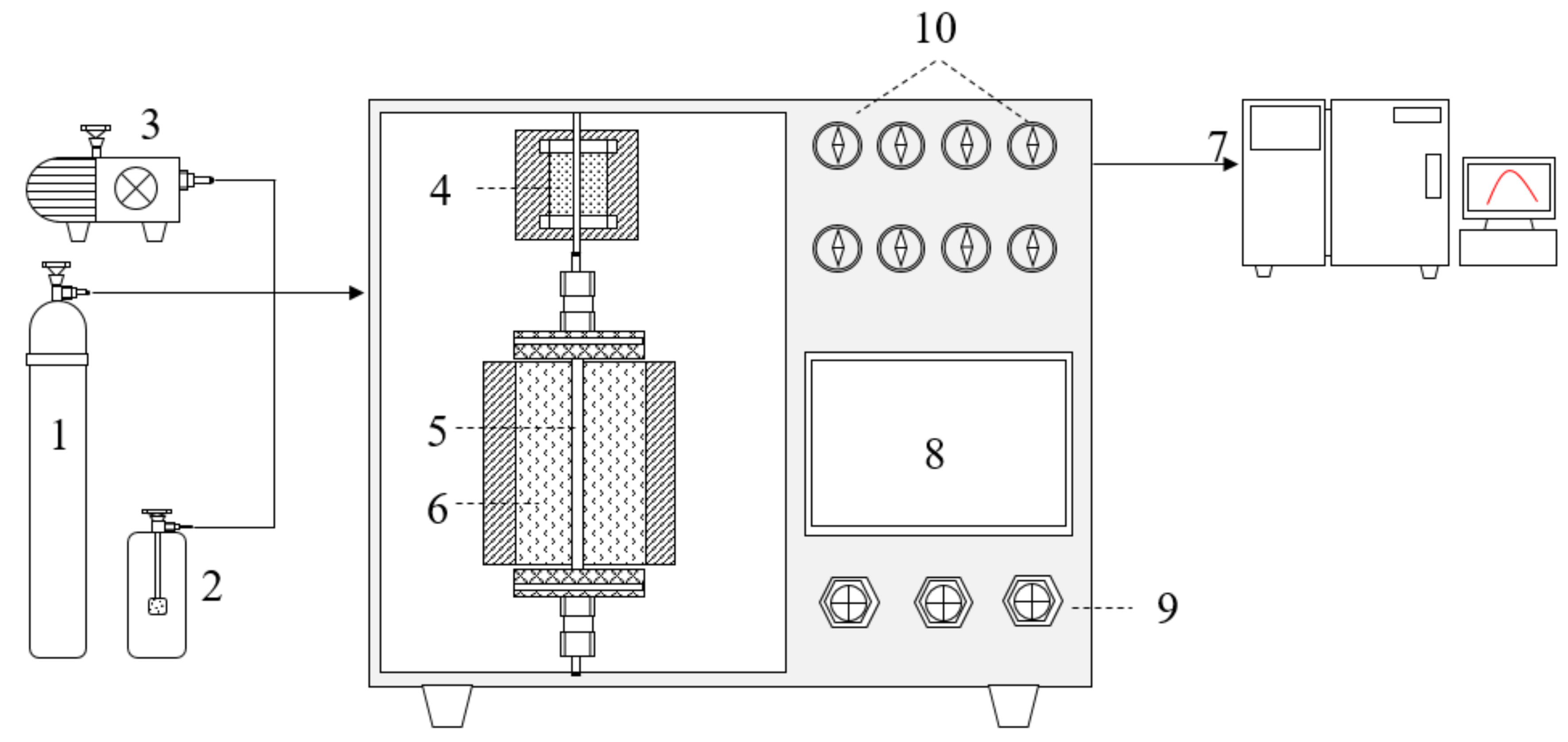
2.3. Apparatus and Methods
3. Results and Discussion
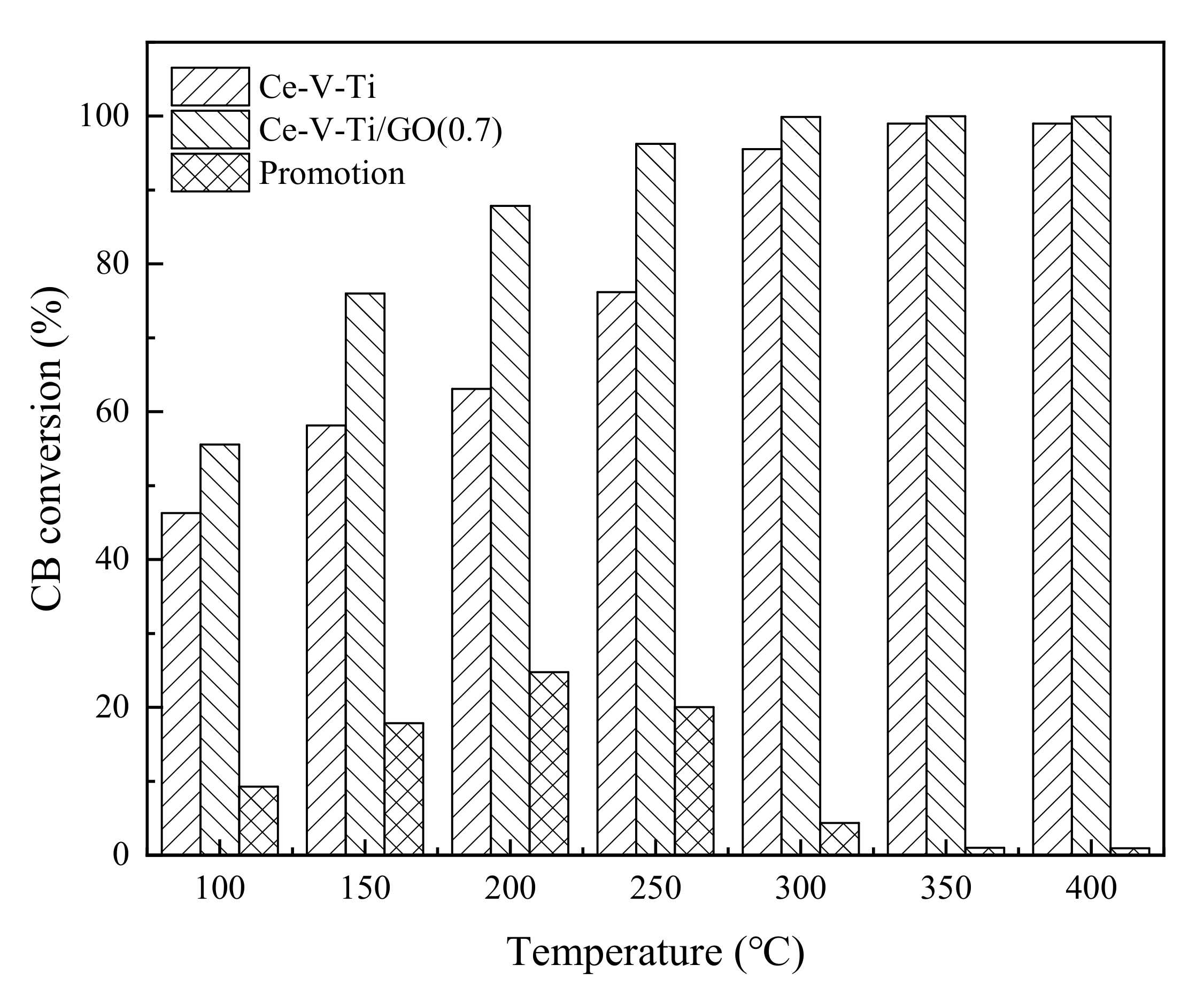
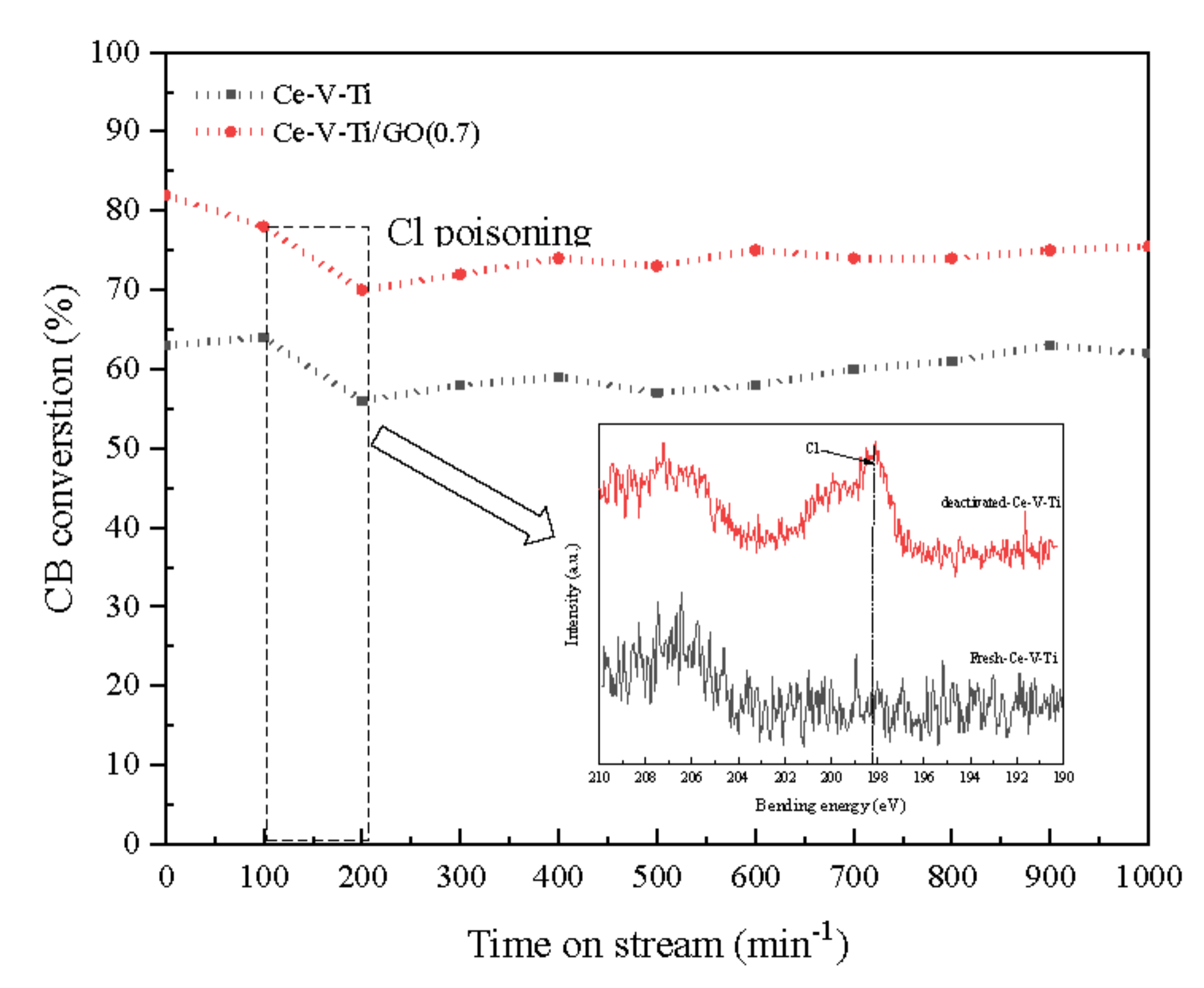
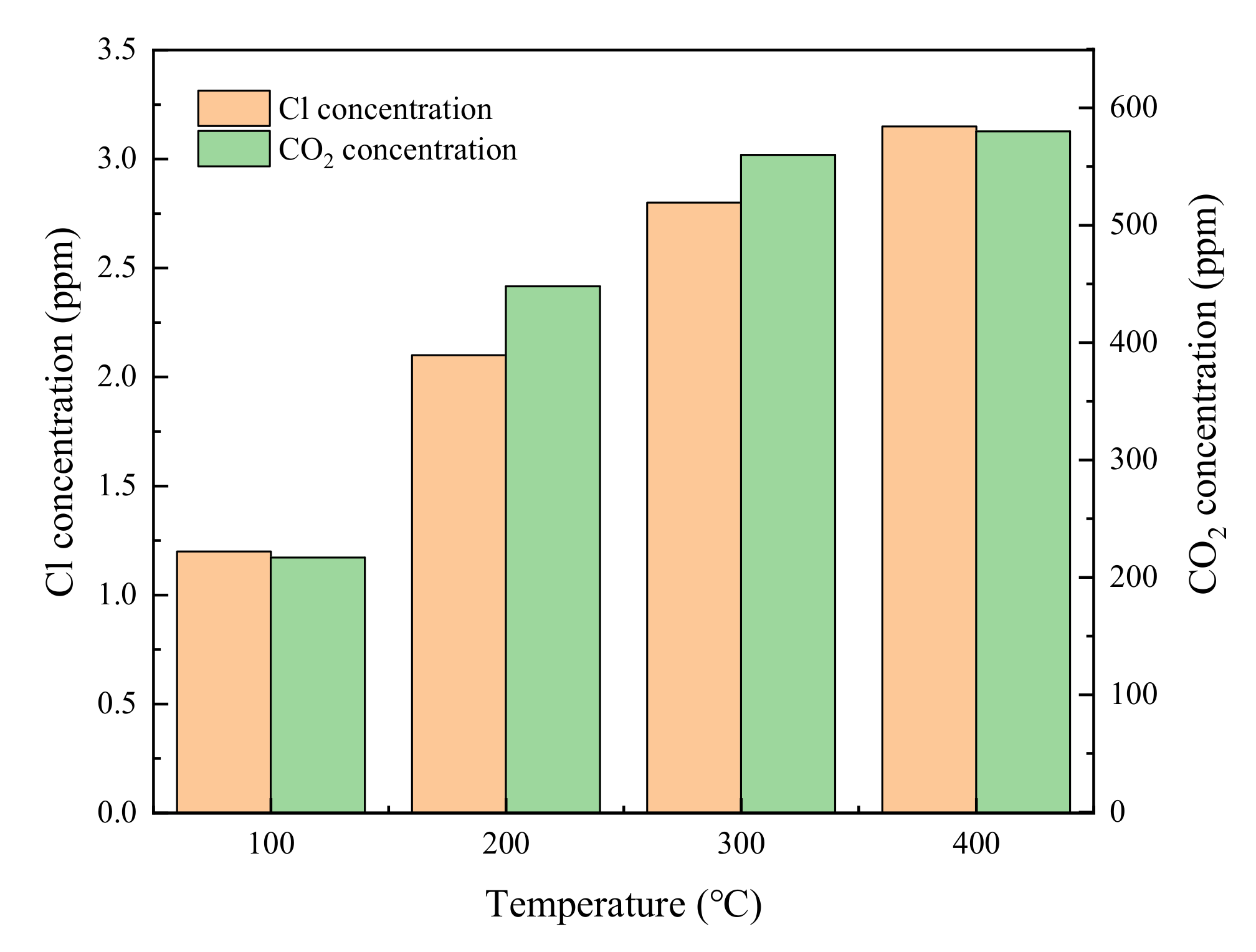
3.1. Catalytic Activity Analysis
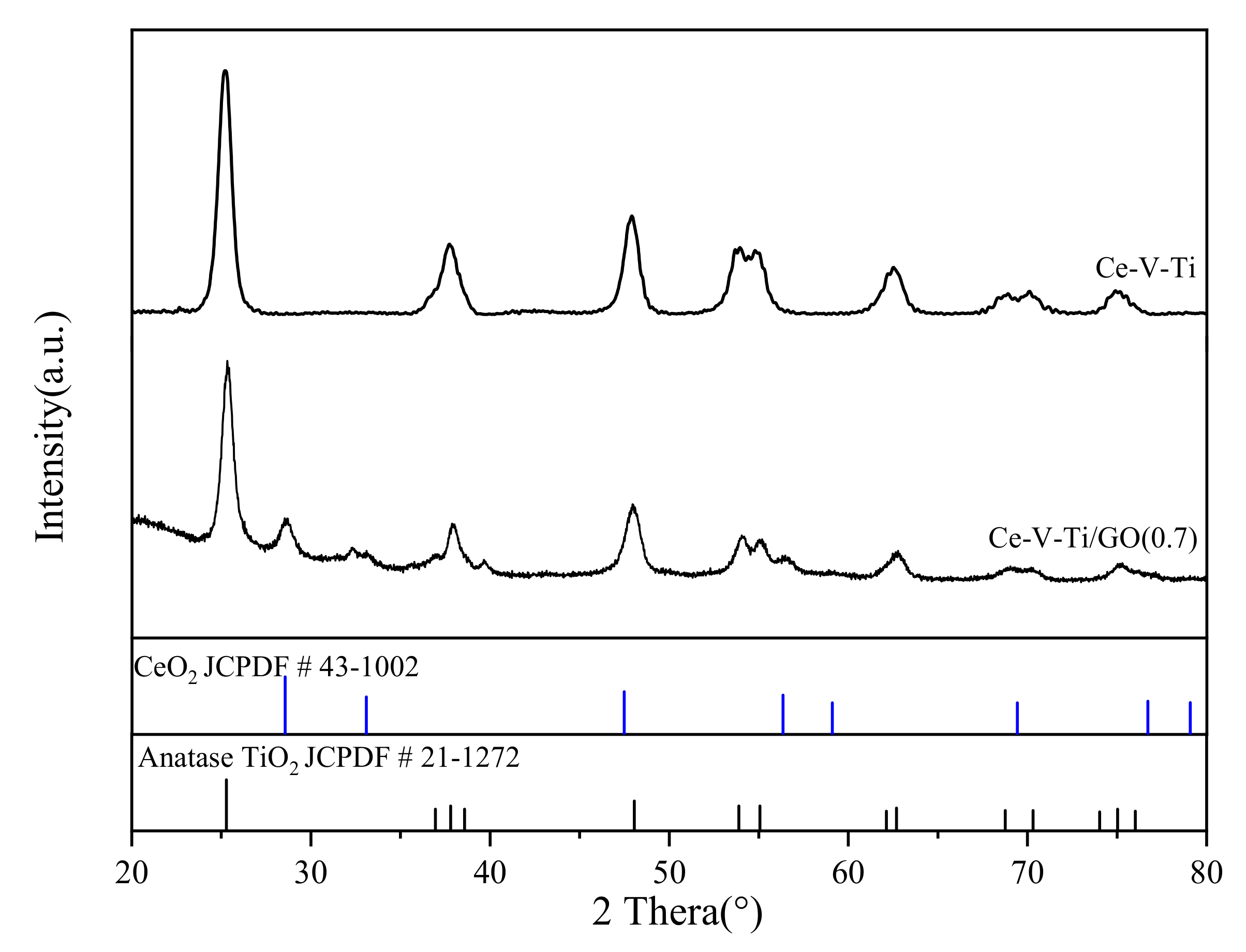
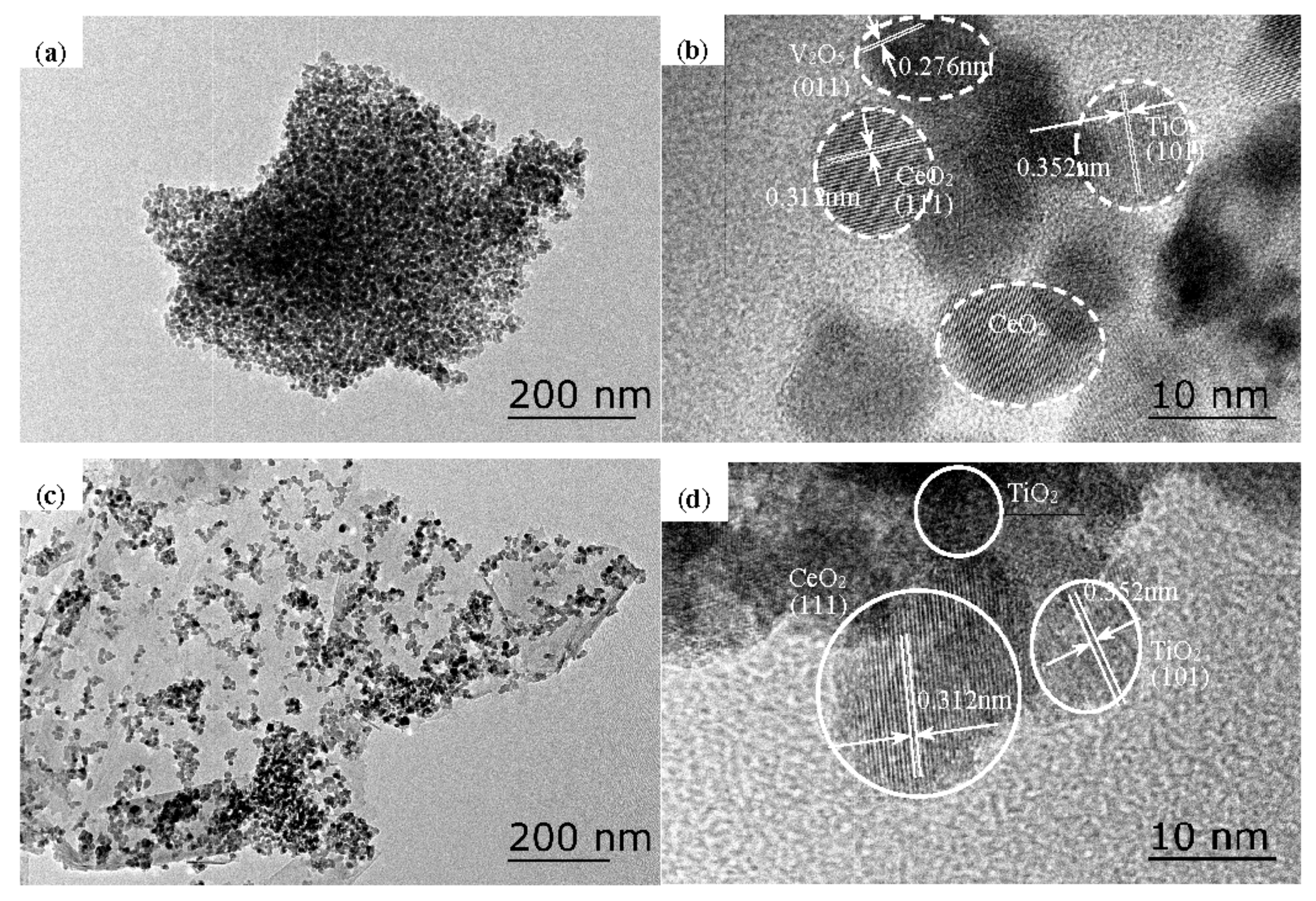
3.2. Textural Properties
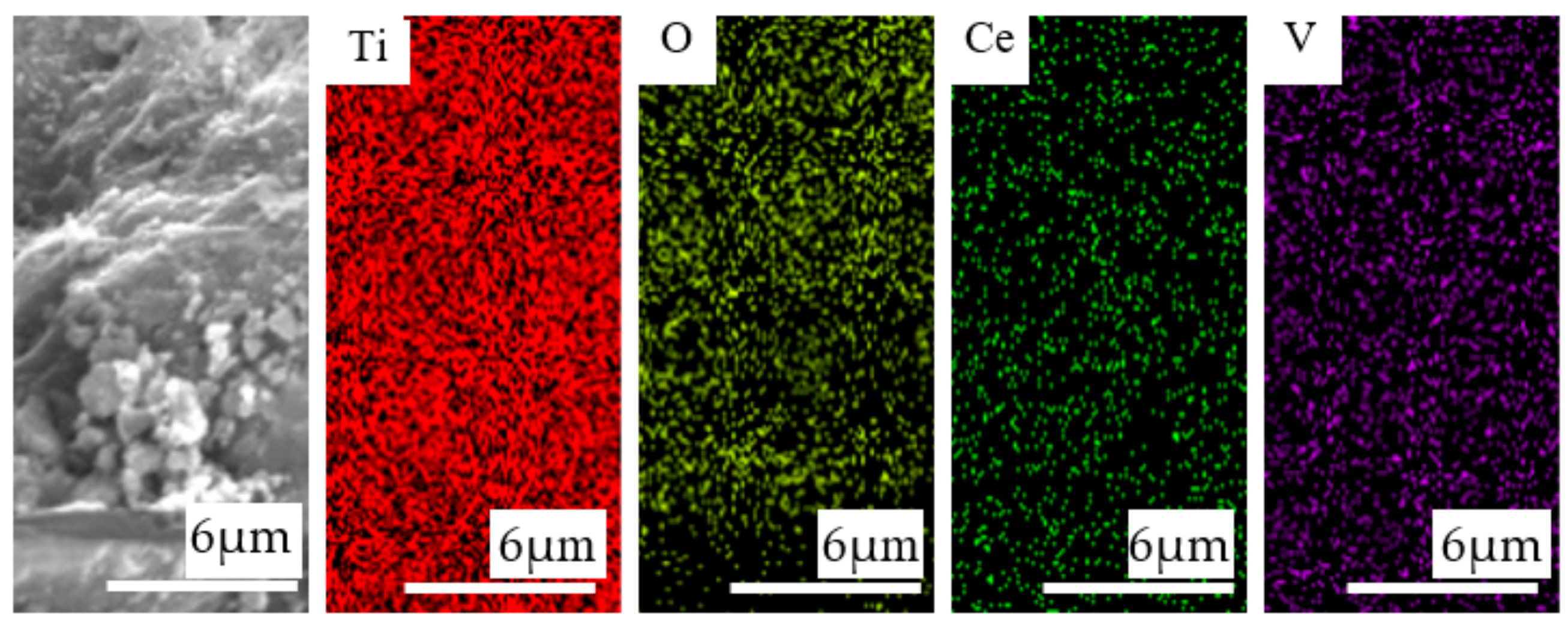
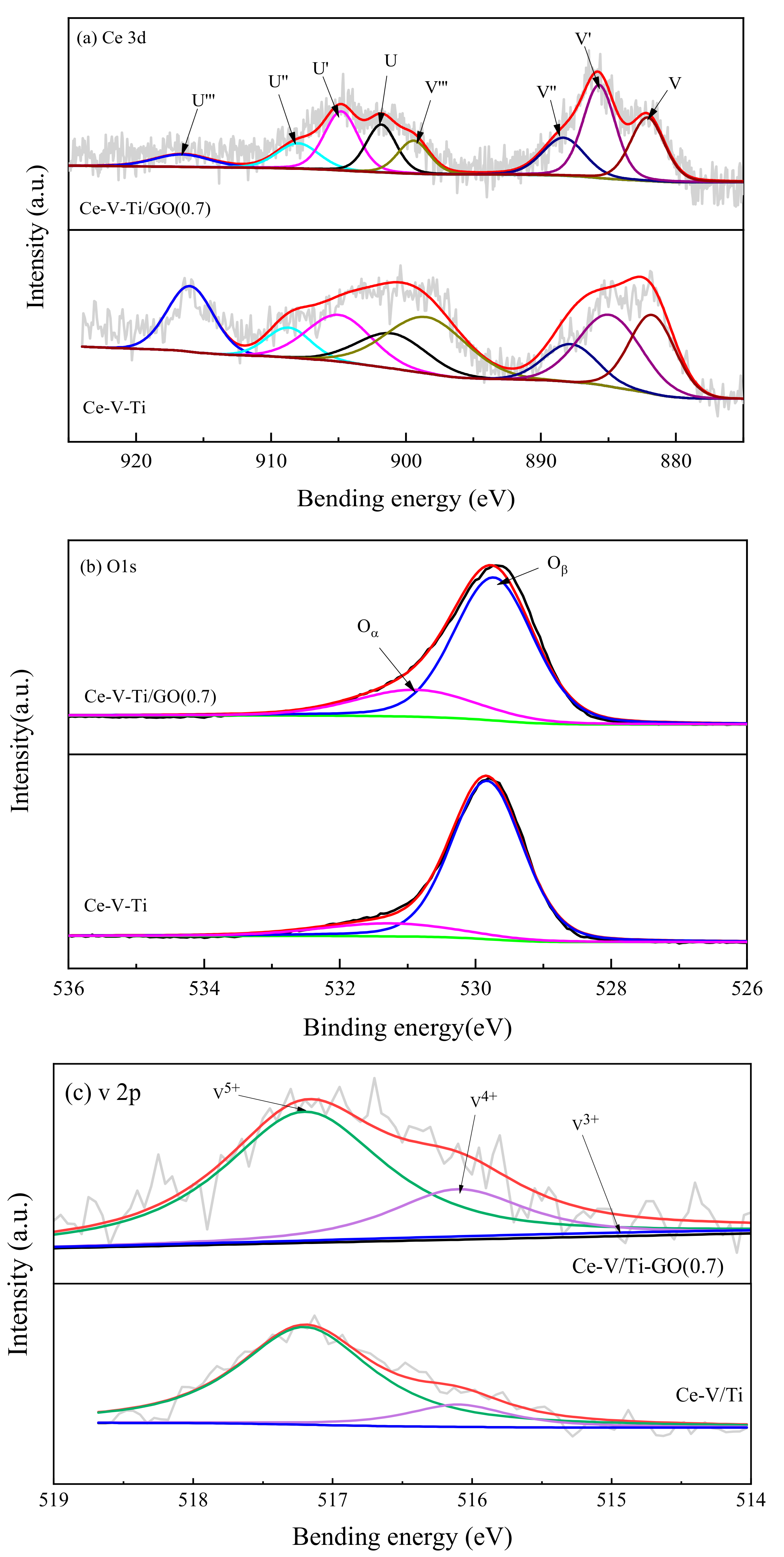
3.3. Surface Properties
3.4. FTIR Spectra and H2-TPR Analysis
3.5. Proposed Reaction Mechanism Analysis
4. Conclusions
- (1)
- The catalytic activity of Ce-V-Ti catalysts was improved significantly after modification with GO. The CB conversion over Ce-V-Ti/GO catalysts achieved 60% at 100 °C and 80% at 150 °C, which showed excellent catalytic activity at low temperature.
- (2)
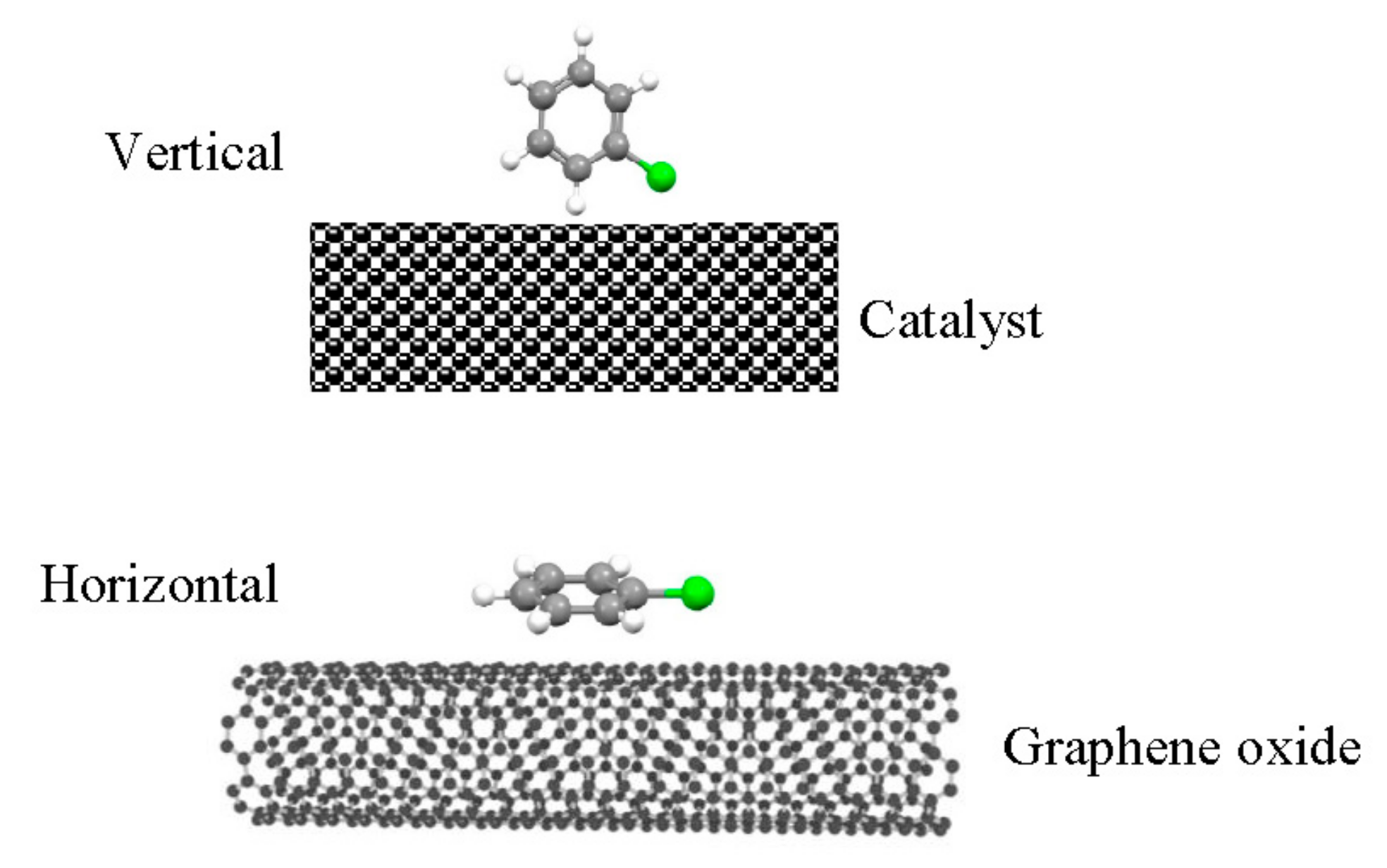
- After being modified with GO, the specific surface area and adsorb ability of CB were both enhanced. The concentration of Ce3+ and V4+ on the surface was enlarged, which corresponded with oxygen vacancies. Moreover, the adsorption mode could be changed from a vertical adsorption mode to a parallel adsorption mode by interaction of π-π bonds between CB and GO.
- (3)
- Ce played a major catalytic role and V acted as a co-catalytic composition during catalytic combustion. The chemical interaction between Ce and Ti could improve the amount of oxygen vacancies and Ce3+ ions. V3+ could help to promote the reduction reaction.
Author Contributions
Funding
Conflicts of Interest
References
- Sun, P.; Wang, W.; Dai, X.; Weng, X.; Wu, Z. Mechanism study on catalytic oxidation of chlorobenzene over MnXCe1-XO2/H-ZSM5 catalysts under dry and humid conditions. Appl. Catal. B Environ. 2016, 198, 389–397. [Google Scholar] [CrossRef]
- Qian, L.; Chun, T.; Long, H.; Li, J.; Di, Z. Emission reduction research and development of PCDD/Fs in the iron ore sintering. Process Saf. Environ. 2018, 117, 82–91. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Catalytic oxidation of vinyl chloride emissions over Co-Ce composite oxide catalysts. Chem. Eng. J. 2017, 315, 392–402. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, H.; Dong, F.; Zha, F.; Tang, Z. Highly efficient catalytic combustion of o-dichlorobenzene over three-dimensional ordered mesoporous cerium manganese bimetallic oxides: A new concept of chlorine removal mechanism. Mol. Catal. 2019, 463, 119–129. [Google Scholar] [CrossRef]
- Aristizábal, B.H.; Maya, C.; Correa, C.M.D. Ortho-dichlorobenzene oxidation over Pd/Co loaded sulfated zirconia and mordenite catalysts. Appl. Catal. A Gen. 2008, 335, 211–219. [Google Scholar] [CrossRef]
- Ma, X.; Shen, J.; Pu, W.; Sun, H.; Pang, Q.; Ma, X. Water-resistant Fe-Ca-Ox/TiO2 catalysts for low temperature 1,2-dichlorobenzene oxidation. Appl. Catal. A Gen. 2013, 466, 68–76. [Google Scholar] [CrossRef]
- Amorós-Pérez, A.; Cano-Casanova, L.; Castillo-Deltell, A.; Lillo-Ródenas, Á.M.; Román-Martínez, C.M. TiO2 Modification with Transition Metallic Species (Cr, Co, Ni, and Cu) for Photocatalytic Abatement of Acetic Acid in Liquid Phase and Propene in Gas Phase. Materials 2019, 12, 40. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Wang, Q.; Stevens, W.R.; Lee, C.W.; Gullett, B.K.; Zhao, Y. Study on the decomposition of trace benzene over V2O5-WO3/TiO2-based catalysts in simulated flue gas. Appl. Catal. B Environ. 2014, 147, 322–329. [Google Scholar] [CrossRef]
- Gutiérrez-Ortiz, J.I.; López-Fonseca, R.; Aurrekoetxea, U.; González-Velasco, J.R. Low-temperature deep oxidation of dichloromethane and trichloroethylene by H-ZSM-5-supported manganese oxide catalysts. J. Catal. 2003, 218, 148–154. [Google Scholar] [CrossRef]
- López-Fonseca, R.; de Rivas, B.; Gutiérrez-Ortiz, J.I.; González-Velasco, J.R. Enhanced activity of zeolites by chemical dealumination for chlorinated VOC abatement. Appl. Catal. B Environ. 2003, 41, 31–42. [Google Scholar] [CrossRef]
- Deng, W.; Dai, Q.; Lao, Y.; Shi, B.; Wang, X. Low temperature catalytic combustion of 1,2-dichlorobenzene over CeO2-TiO2 mixed oxide catalysts. Appl. Catal. B Environ. 2016, 181, 848–861. [Google Scholar] [CrossRef]
- He, F.; Luo, J.Q.; Liu, S. Novel metal loaded KIT-6 catalysts and their applications in the catalytic combustion of chlorobenzene. Chem. Eng. J. 2016, 294, 362–370. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, X.; Lu, G. Low-temperature catalytic combustion of trichloroethylene over cerium oxide and catalyst deactivation. Catal. Commun. 2008, 3, 192–202. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, X.; Dai, Q.; Li, D. Effect of Ce and La on the structure and activity of MnOx catalyst in catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2012, 111, 141–149. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Samia, A.; Faiza, M.; Muhammad, A.A. Facile synthesis of graphene oxide with significant enhanced properties for optoelectronic and energy devices. Ceram. Int. 2018, 44, 6823–6828. [Google Scholar]
- Chiraz, G.; Romain, D.; Pierre, E.; Damien, P.D.; Abdelhamid, G.; Eric, M.G. Sol-gel derived V2O5-TiO2 mesoporous materials as catalysts for the total oxidation of chlorobenzene. Catal. Commun. 2011, 1, 1–5. [Google Scholar]
- Huang, H.; Gu, Y.; Wang, X. Catalytic combustion of chlorobenzene over VOx/CeO2 catalysts. J. Catal. 2015, 326, 54–68. [Google Scholar] [CrossRef]
- Heo, U.S.; Kim, D.W.; Kim, K.S.; Park, D.W.; Heo, U.S. A facile synthesis of anatase TiO2-Graphene nanocomposites using plasma and heat treatment. Appl. Surf. Sci. 2019, 474, 118–126. [Google Scholar] [CrossRef]
- Zhao, K.; Han, W.; Tang, Z.; Lu, J.; Hu, X. High-Efficiency Environmental-Friendly Fe-W-Ti Catalyst for Selective Catalytic Reduction of NO with NH3: The Structure-Activity Relationship. Catal. Surv. Asia 2018, 22, 20–30. [Google Scholar] [CrossRef]
- Freidel, I.M.; Frost, A.C.; Herbert, K.J.; Meyer, F.J.; Summers, J.C. New catalyst technologies for the destruction of halogenated hydrocarbons and volatile organics. Catal. Today 1993, 17, 367–382. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Y.; Yang, Y.; Hu, R.; Yang, G.; Feng, L.; Suo, G. Microstructure evolution and controlled hydrolytic hydrogen generation strategy of Mg-rich Mg-Ni-La ternary alloys. Energy 2019, 188, 116081–116091. [Google Scholar] [CrossRef]
- Zuo, S.; Ding, M.; Tong, J.; Feng, L.; Qi, C. Study on the preparation and characterization of a titanium-pillared clay-supported CrCe catalyst and its application to the degradation of a low concentration of chlorobenzene. Appl. Clay Sci. 2015, 105, 118–123. [Google Scholar] [CrossRef]
- Kan, J.; Deng, L.; Li, B.; Huang, Q.; Zhu, S.; Shen, S.; Chen, Y. Performance of Co-doped Mn-Ce catalysts supported on cordierite for low concentration chlorobenzene oxidation. Appl. Catal. A Gen. 2017, 530, 21–29. [Google Scholar] [CrossRef]
- Dai, Q.; Bai, S.; Wang, J.; Li, M.; Wang, X.; Lu, G. The effect of TiO2 doping on catalytic performances of Ru/CeO2 catalysts during catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2013, 142, 222–233. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Y.; Hu, R.; Shi, H.; Feng, L.; Suo, G. Catalytic effect of EG and MoS2 on hydrolysis hydrogen generation behavior of high-energy ball-milled Mg-10wt.%Ni alloys in NaCl solution-A powerful strategy for superior hydrogen generation performance. Int. J. Energy Res. 2019, 43, 8426–8438. [Google Scholar]
- He, C.; Yu, Y.; Shen, Q.; Chen, J.; Qiao, N. Catalytic behavior and synergistic effect of nanostructured mesoporous CuO-MnOx-CeO2 catalysts for chlorobenzene destruction. Appl. Surf. Sci. 2014, 297, 59–69. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, X.; Li, D.; Dai, Q. Catalytic combustion of chlorobenzene over Mn-Ce-La-O mixed oxide catalysts. J. Hazard. Mater. 2011, 188, 132–139. [Google Scholar]
- Wang, X.; Kang, Q.; Li, D. Low-temperature catalytic combustion of chlorobenzene over MnOx-CeO2 mixed oxide catalysts. Catal. Commun. 2008, 9, 2158–2162. [Google Scholar] [CrossRef]
- Murillo, R.; García, T.; Aylón, E.; Callén, M.S.; Navarro, M.V.; López, J.M.; Mastral, A.M. Adsorption of phenanthrene on activated carbons: Breakthrough curve modeling. Carbon 2004, 42, 2009–2017. [Google Scholar] [CrossRef]
- Mastral, A.M.; García, T.; Callén, M.S.; Navarro, M.V. Assessement of Phenanthrene Removal from Hot Gas by Porous Carbons. Energy Fuel 2000, 15, 1–7. [Google Scholar] [CrossRef]
- Nuño, M.; Adamaki, V.; Tobaldi, D.M.; Hortigüela-Gallo, M.J.; Otero-Irurueta, G.; Bowen, C.R.; Ball, R.J. Solid-Gas Phase Photo-Catalytic Behaviour of Rutile and TiOn (1 <n <2) Sub-Oxide Phases for Self-Cleaning Applications. Materials 2019, 12, 170. [Google Scholar]
- Zhang, H.; Xu, Y.; Liu, X. Study on preparation mechanism of Ce-Cu/TiO2 hollow microspheres with uniform particle size distribution based on fourier transform infrared spectrum and X-ray diffraction. Spectrosc. Spectr. Anal. 2019, 39, 2360–2365. [Google Scholar]
- He, H.; Dai, H.X.; Au, C.T. Defective structure, oxygen mobility, oxygen storage capacity, and redox properties of RE-based (RE = Ce, Pr) solid solutions. Catal. Today 2004, 90, 245–254. [Google Scholar] [CrossRef]
- Hou, X.; Wang, L.; Yang, Y. Enhanced hydrogen generation behaviors and hydrolysis thermodynamics of as-cast Mg-Ni-Ce magnesium-rich alloys in simulate seawater. Int. J. Hydrogen Energy 2019, 44, 24086–24097. [Google Scholar] [CrossRef]
- Harish, S.; Sabarinathan, M.; Archana, J.; Navaneethan, M. Functional properties and enhanced visible light photocatalytic performance of V 3 O 4, nanostructures decorated ZnO nanorods. Appl. Surf. Sci. 2017, 418, 171–178. [Google Scholar] [CrossRef]
- Dong, G.; Bai, Y.; Zhang, Y.; Zhao, Y. Effect of the V4+(3+) V5+ ratio on the denitration activity for V2O5-WO3TiO2 catalysts. N. J. Chem. 2015, 39, 3588–3596. [Google Scholar] [CrossRef]
- Ganduglia-pirovano, M.V.; Popa, C.; Sauer, J.; Abbott, H.; Uhl, A.; Baron, M.; Stacchiola, D.; Bondarchuk, O.; Shaikhutdinov, S.; Freund, H.J. Role of Ceria in Oxidative Dehydrogenation on Supported Vanadia Catalysts. J. Am. Chem. Soc. 2010, 132, 2345–2349. [Google Scholar] [CrossRef]
- Zhang, H.; Fangm, Y. Temperature dependent photoluminescence of surfactant assisted electrochemically synthesized ZnSe nanostructures. J. Alloys Compd. 2019, 781, 201–208. [Google Scholar] [CrossRef]
- Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Characterization of activated carbon, graphitized carbon fibers and synthetic diamond powder using TPD and DRIFTS. Carbon 1998, 36, 1821–1831. [Google Scholar] [CrossRef]
- Shangguan, J.; Li, C.; Miao, M.; Yang, Z. Surface characterization and SO2 removal activity of activated semi-coke with heat treatment. N. Carbon Mater. 2008, 23, 37–43. [Google Scholar] [CrossRef]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Swiatkowski, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Poelman, H.; Sels, B.F.; Olea, M.; Eufinger, K.; Paul, J.S.; Sack, I. New supported vanadia catalysts for oxidation reactions prepared by sputter deposition. J. Catal. 2007, 245, 156–172. [Google Scholar] [CrossRef]
- Arena, F.; Trunfio, G.; Negro, J.; Spadaro, L. Optimization of the MnCeOx system for the catalytic wet oxidation of phenol with oxygen (CWAO). Appl. Catal. B Environ. 2008, 85, 40–47. [Google Scholar] [CrossRef]
- Arena, F.; Trunfio, G.; Negro, J.; Fazio, B.; Spadaro, L. Basic Evidence of the Molecular Dispersion of MnCeOx Catalysts Synthesized via a Novel “Redox-Precipitation” Route. Chem. Mater. 2007, 38, 2269–2276. [Google Scholar] [CrossRef]
- Nagano, S.; Tamon, H.; Adzumi, T.; Nakagawa, K.; Suzuki, T. Activated carbon from municipal waste. Carbon 2000, 38, 915–920. [Google Scholar] [CrossRef]
- Zhou, X.J.; Buekens, A.; Li, X.D.; Chen, K.F. Adsorption of polychlorinated dibenzo-p-dioxins/dibenzofurans on activated carbon from hexane. Chemosphere 2016, 144, 1264–1269. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef]


| Samples | C150 °C | T90 | Reaction Condition | Ref. |
|---|---|---|---|---|
| Ce0.5Ti0.5 | 0% | 375 °C | 1000 ppm CB; 10% O2/N2; GHSV = 30,000 h−1 | [22] |
| Cr0.75Ce0.25/Ti | 20% | 225 °C | 500 ppm CB; GHSV = 20,000 h−1 | [23] |
| Mn6Co2Ce2 | 20% | 325 °C | 500 ppm CB; GHSV = 15,000 h−1 | [24] |
| Ru (1%)/TiO2-CeO2 | 5% | 225 °C | 550 ppm CB; GHSV = 15,000 h−1 | [25] |
| VOx (2.1%)/CeO2 | 5% | 225 °C | 1000 ppm CB; GHSV = 30,000 h−1 | [26] |
| Cu0.15Mn0.15Ce0.7Ox | 2% | 255 °C | 600 ppm CB; 21%O/N2; GHSV = 30,000 h−1 | [27] |
| Mn (0.86)-CeLa | 20% | 250 °C | 1000 ppm CB; 10%O/N2; GHSV = 15,000 h−1 | [28] |
| MnOX (0.86)-CeO2 | 20% | 236 °C | 1000 ppm CB; 10%O/N2; GHSV = 15,000 h−1 | [29] |
| Sample | Surface Area (m2/g) a | Total Pore (cm3/g) b | Average Pore (nm) c |
|---|---|---|---|
| Ce-V-Ti/GO(0.7) | 123.8 | 0.15 | 6.4 |
| Ce-V-Ti | 95.7 | 0.29 | 6.5 |
| Sample | Atomic (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ce | V | O | C | Ce | O | |||
| Ce3+ | Ce4+ | Oα | Oβ | |||||
| Ce-V-Ti | 5.56 | 0.91 | 63.44 | 20 | 80 | 88 | 12 | |
| Ce-V-Ti/GO(0.7) | 6.96 | 1.01 | 65.82 | 1.66 | 30 | 70 | 53 | 47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Q.; Ding, L.; Long, H.-M.; Chun, T.-J. Study of Catalytic Combustion of Dioxins on Ce-V-Ti Catalysts Modified by Graphene Oxide in Simulating Iron Ore Sintering Flue Gas. Materials 2020, 13, 125. https://doi.org/10.3390/ma13010125
Shi Q, Ding L, Long H-M, Chun T-J. Study of Catalytic Combustion of Dioxins on Ce-V-Ti Catalysts Modified by Graphene Oxide in Simulating Iron Ore Sintering Flue Gas. Materials. 2020; 13(1):125. https://doi.org/10.3390/ma13010125
Chicago/Turabian StyleShi, Qi, Long Ding, Hong-Ming Long, and Tie-Jun Chun. 2020. "Study of Catalytic Combustion of Dioxins on Ce-V-Ti Catalysts Modified by Graphene Oxide in Simulating Iron Ore Sintering Flue Gas" Materials 13, no. 1: 125. https://doi.org/10.3390/ma13010125





