The Preparation, Thermal Properties, and Fire Property of a Phosphorus-Containing Flame-Retardant Styrene Copolymer
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
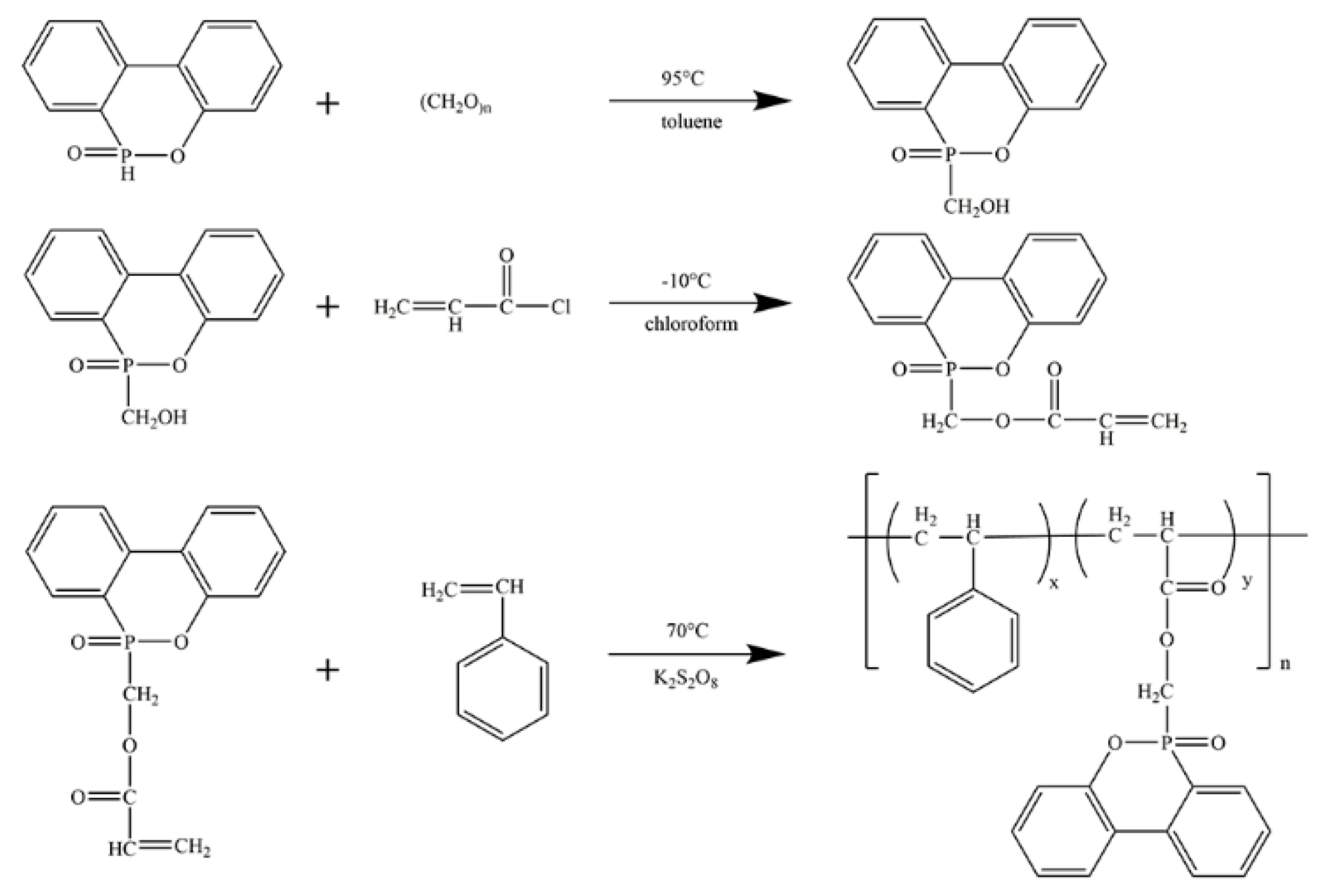
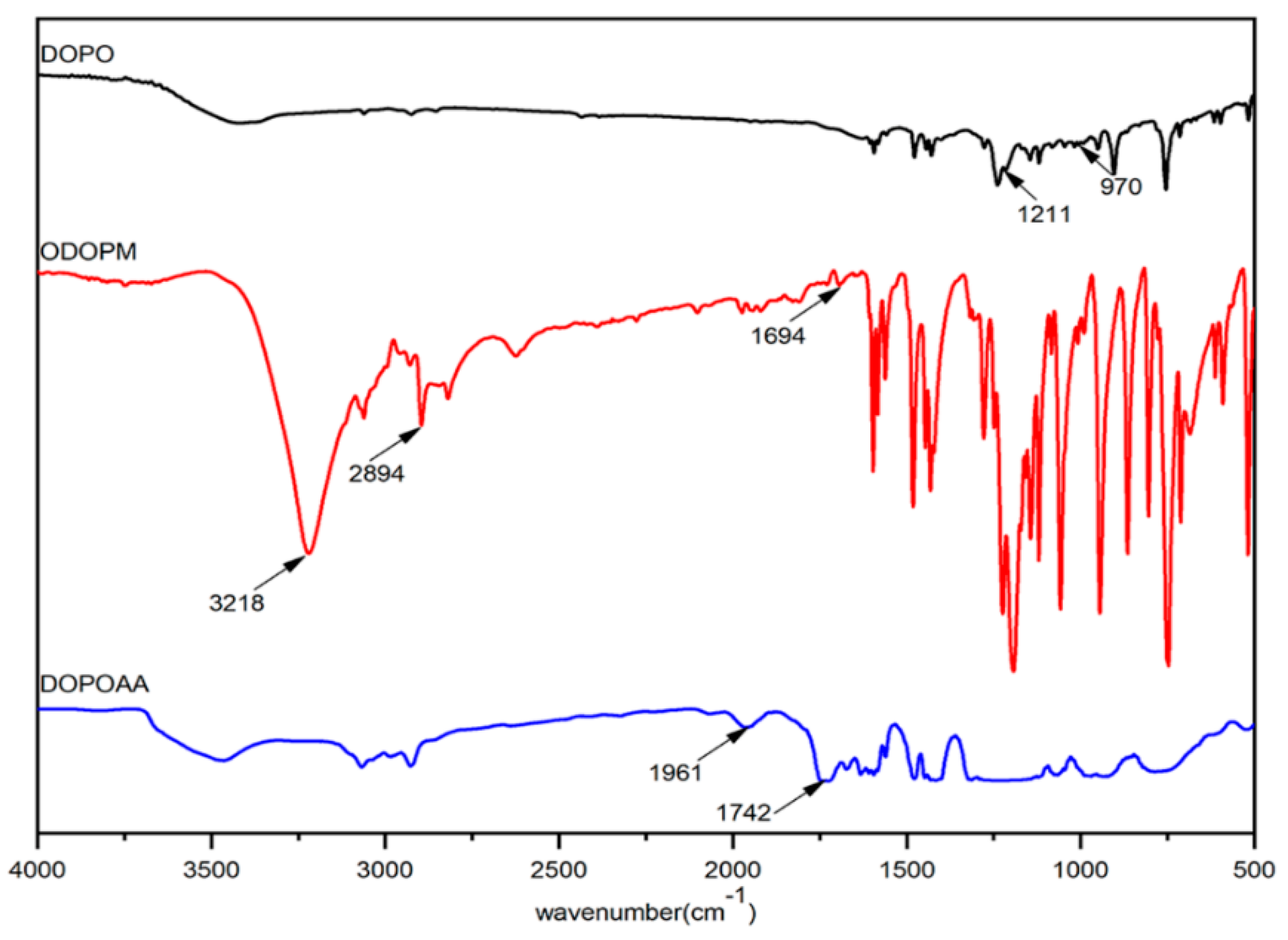
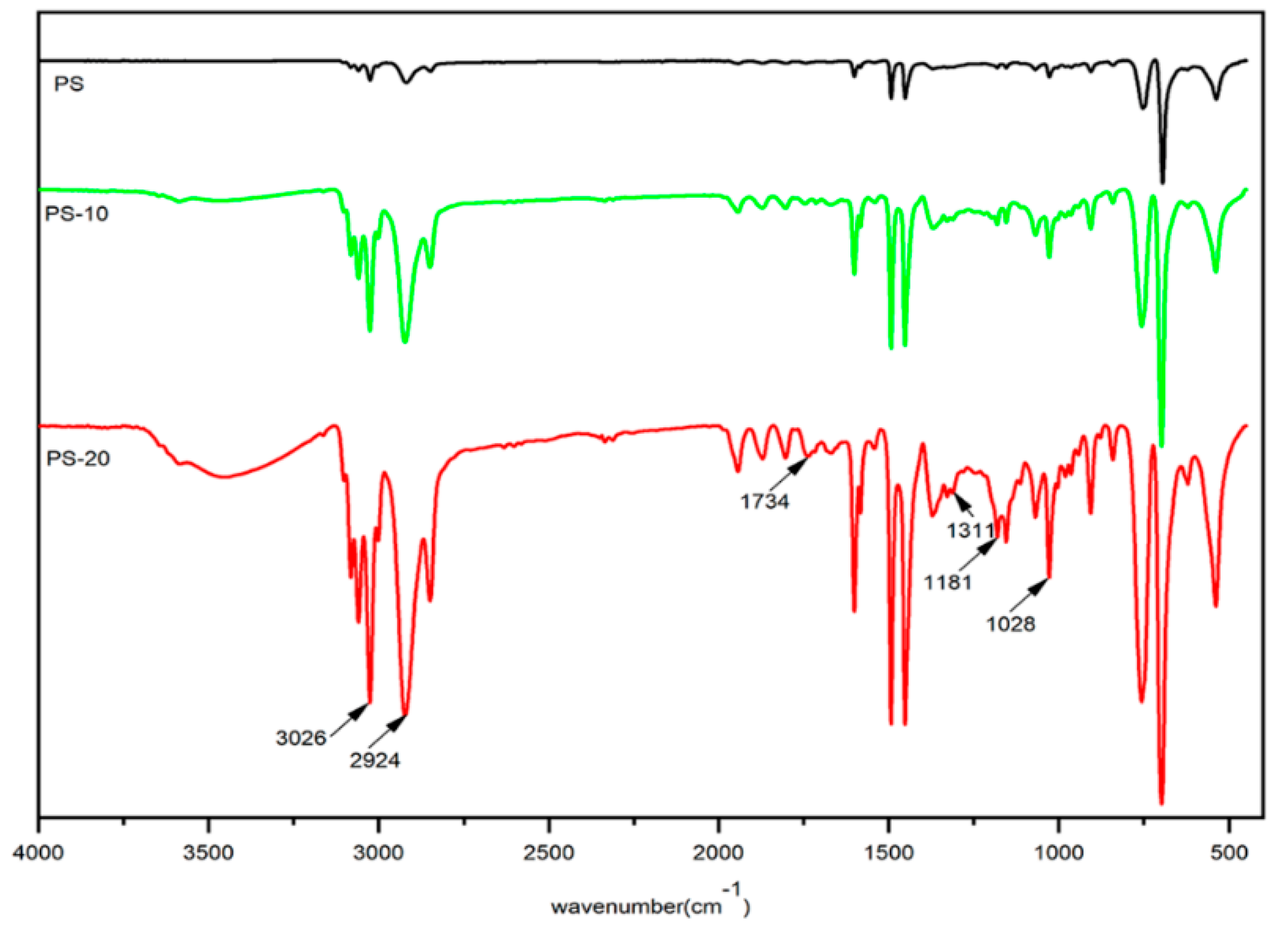
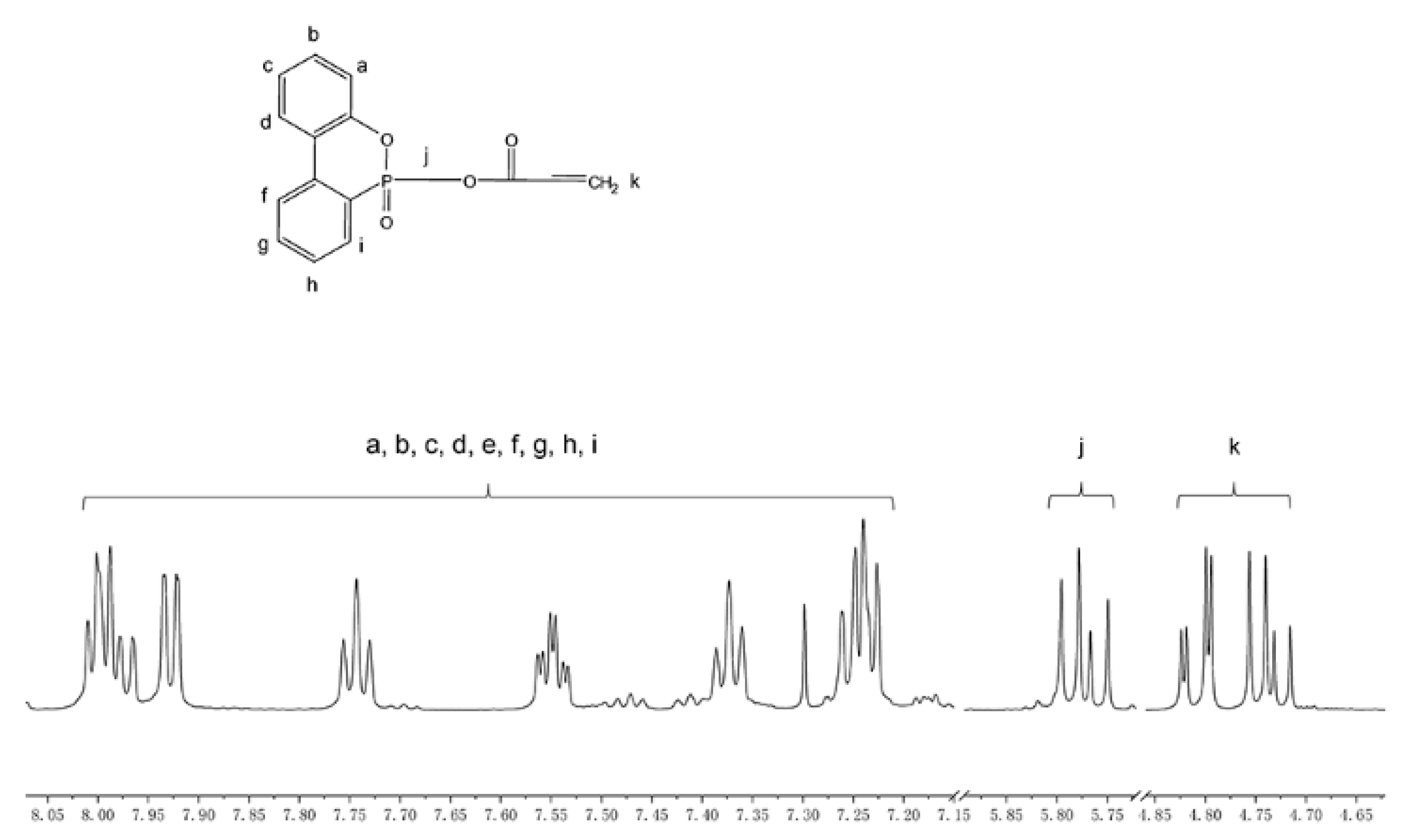
3.1. Characterization of DOPOAA and DOPOAA-Styrene Copolymer
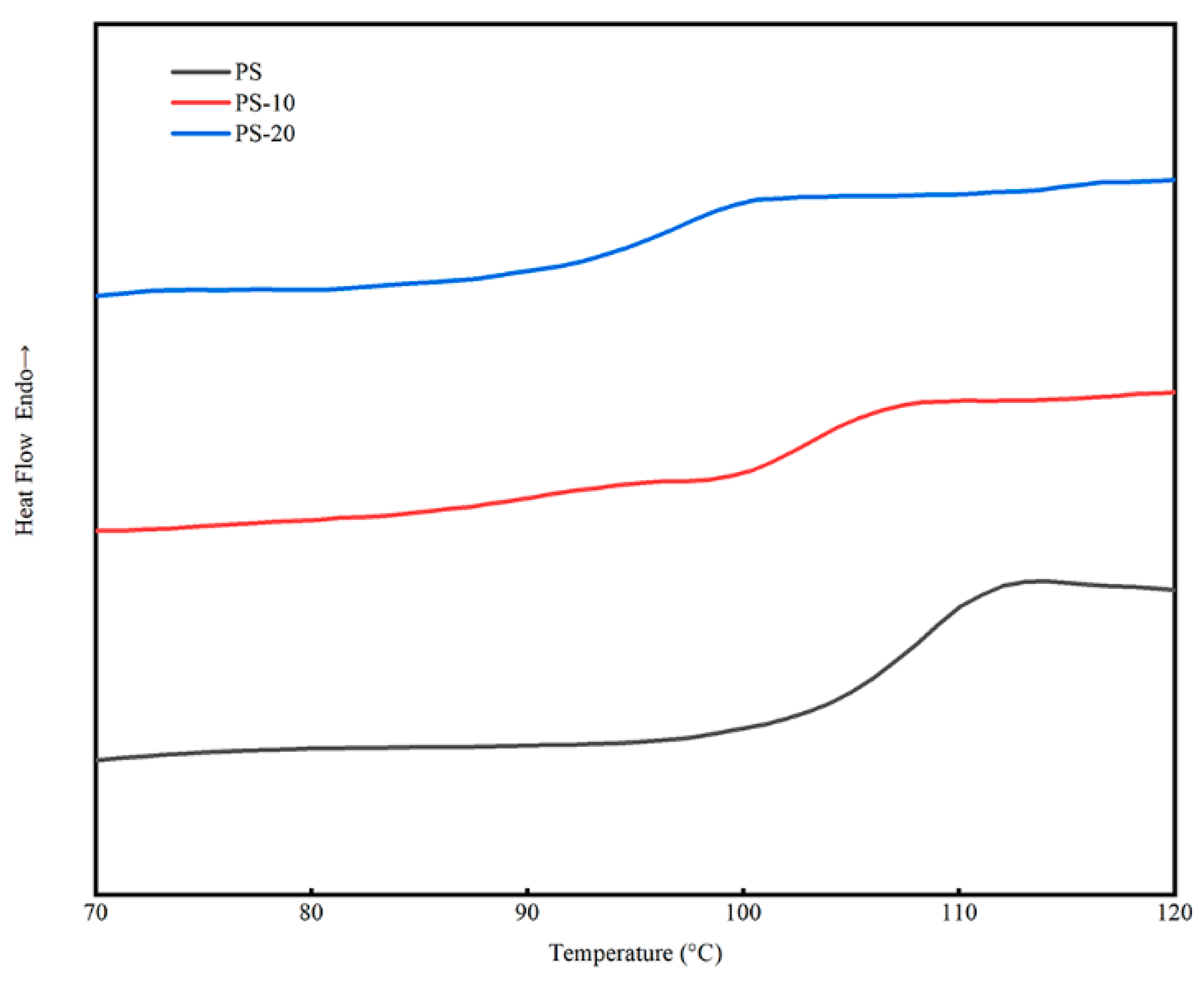
3.2. Thermal Properties
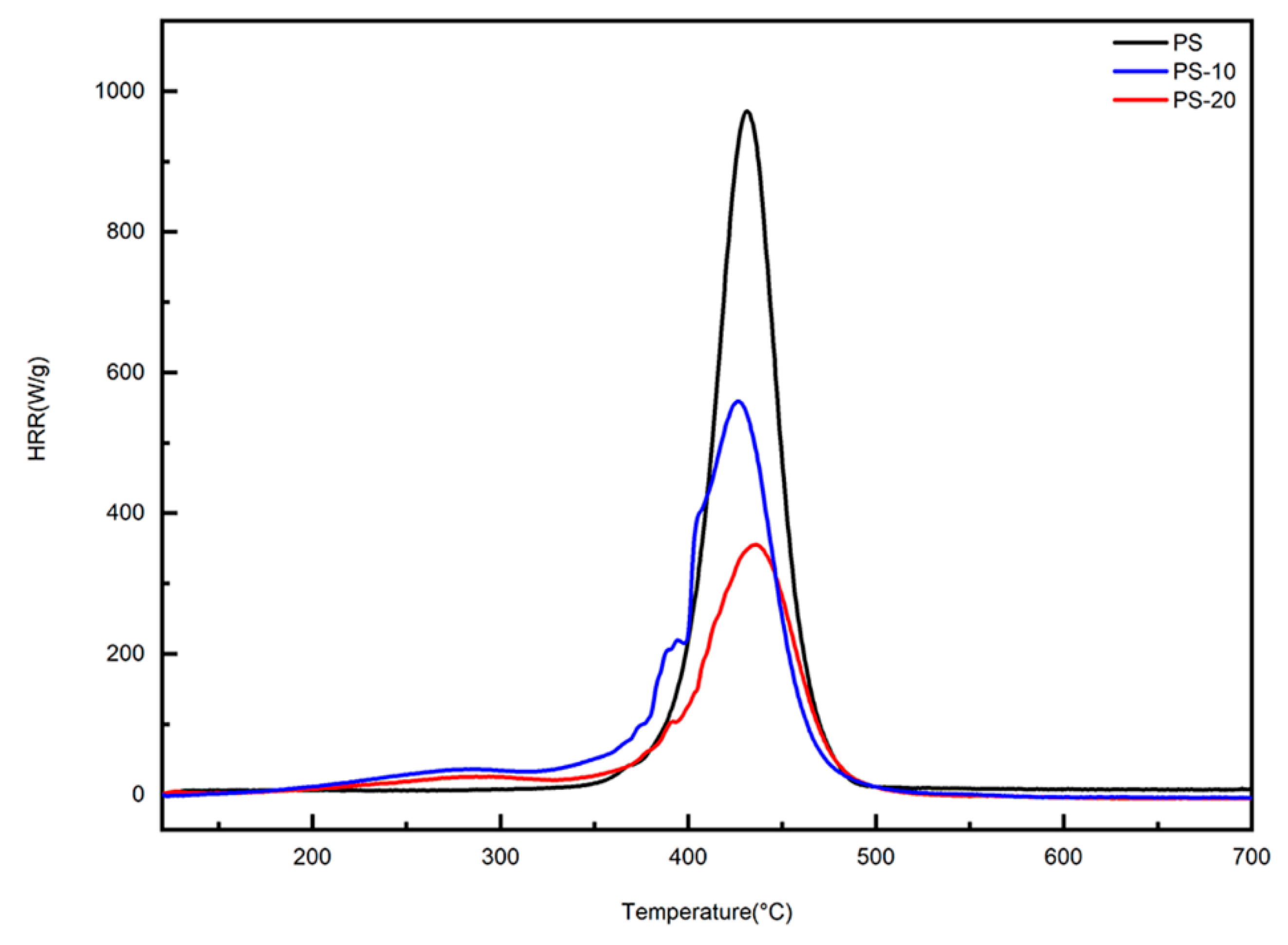
3.3. Flame-Retardant Behaviors
4. Conclusions
- A phosphorous-containing flame-retardant monomer, DOPOAA, was synthesized successfully. Then, its structure was characterized by FT-IR and 1H-NMR, and a series of the copolymers of St and DOPOAA were prepared at different ratios;
- TGA data showed that the residues at 700 °C rose with the increase of DOPOAA content, compared with the pure PS. In the MCC test, with the increase of DOPOAA, the PHRR value of copolymers was evidently reduced compared to pure PS, and the LOI value of copolymers increased. Judging, from the results of TG, MCC, and LOI tests, it is possible that the addition of DOPOAA could ameliorate the thermal stability and flame-retardant properties of PS. In conclusion, it is a tangible method to incorporate the flame retardancy monomer into the PS chain to prepare flame-retardant PS.
Author Contributions
Funding
Conflicts of Interest
References
- Carastan, D.J.; Demarquette, N.R. Polystyrene/clay nanocomposites. Metall. Rev. 2007, 52, 345–380. [Google Scholar] [CrossRef]
- Chevallier, C.; Becquart, F.; Taha, M. Polystyrene/polycarbonate blends compatibilization: Morphology, rheological and mechanical properties. Mater. Chem. Phys. 2013, 139, 616–622. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Cheng, H.K.F.; Cai, J.; Li, L.; Chan, S.H.; Zhao, J.; Yu, S. Improvement of mechanical and thermal properties of carbon nanotube composites through nanotube functionalization and processing methods. Mater. Chem. Phys. 2009, 117, 313–320. [Google Scholar] [CrossRef]
- Su, W.-Y.; Min, K.; Quirk, R.P. In situ copolymerization and compatibilization of polyester and polystyrene blends. II. Thermally and chemically induced reaction and mechanical properties. Polymer 2001, 42, 5121–5134. [Google Scholar] [CrossRef]
- Yu, J.; Yang, C.; Tian, L.; Liao, D. A study on optimum insulation thicknesses of external walls in hot summer and cold winter zone of China. Appl. Energy 2009, 86, 2520–2529. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Yuan, B.; Hu, Y.; Song, L. Functionalized graphene oxide for fire safety applications of polymers: A combination of condensed phase flame retardant strategies. J. Mater. Chem. 2012, 22, 23057. [Google Scholar] [CrossRef]
- Zhang, M.; Buekens, A.; Li, X. Brominated flame retardants and the formation of dioxins and furans in fires and combustion. J. Hazard. Mater. 2016, 304, 26–39. [Google Scholar] [CrossRef]
- Cristale, J.; Belé, T.G.A.; Lacorte, S.; Marchi, M.R.R. Occurrence of flame retardants in landfills: A case study in Brazil. Environ. Res. 2019, 168, 420–427. [Google Scholar] [CrossRef]
- Alaee, M.; Wenning, R.J. The significance of brominated flame retardants in the environment: Current understanding, issues and challenges. Chemosphere 2002, 96, 579–582. [Google Scholar] [CrossRef]
- Tai, Q.; Chen, L.; Song, L.; Nie, S.; Hu, Y.; Yuen, R.K.K. Preparation and thermal properties of a novel flame retardant copolymer. Polym. Degrad. Stab. 2010, 95, 830–836. [Google Scholar] [CrossRef]
- Yan, Y.W.; Chen, L.; Jian, R.-K.; Kong, S.; Wang, Y.-Z. Intumescence: An effect way to flame retardance and smoke suppression for polystryene. Polym. Degrad. Stab. 2012, 97, 1423–1431. [Google Scholar] [CrossRef]
- Chang, S.; Xie, T.; Yang, G. Effects of interfacial modification on the thermal, mechanical, and fire properties of high-impact polystyrene/microencapsulated red phosphorous. Appl. Polym. Sci. 2008, 110, 2139–2144. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H. Thermal degradation mechanism of flame retarded epoxy resins with a DOPO-substitued organophosphorus oligomer by TG-FTIR and DP-MS. Anal. Appl. Pyrolysis 2011, 92, 164–170. [Google Scholar] [CrossRef]
- Neisius, N.M.; Lutz, M.; Rentsch, D.; Hemberger, P.; Gaan, S. Synthesis of DOPO-based phosphonamidates and their thermal properties. Ind. Eng. Chem. Res. 2014, 53, 2889–2896. [Google Scholar] [CrossRef]
- Armitage, P.; Ebdon, J.R.; Hunt, B.J.; Jones, M.S.; Thorpe, F.G. Chemical modification of polymers to improve flame retardance—I. The influence of boron-containing groups. Polym. Degrad. Stab. 1996, 54, 387–393. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.; Lu, H.; Lv, P.; Jie, G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Chen, H.B.; Zhang, Y.; Chen, L.; Shao, Z.B.; Liu, Y.; Wang, Y.Z. Novel inherently flame-retardant poly (trimethylene terephthalate) copolyester with the phosphorus-containing linking pendent group. Ind. Eng. Chem. Res. 2010, 49, 7052–7059. [Google Scholar] [CrossRef]
- Kundu, C.K.; Yu, B.; Gangireddy, C.S.R.; Mu, X.; Wang, B.; Wang, X.; Song, L.; Hu, Y. UV Grafting of a dopo-based phosphoramidate monomer onto polyamide 66 fabrics for flame retardant treatment. Ind. Eng. Chem. Res. 2017, 56, 1376–1384. [Google Scholar] [CrossRef]
- Wang, H.; Wang, S.; Du, X.; Wang, H.; Cheng, X.; Du, Z. Synthesis of a novel flame retardant based on DOPO derivatives and its application in waterborne polyurethane. RSC Adv. 2019, 9, 7411–7419. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Chen, X.; Wang, M.; Hou, P.; Cao, J.; Li, J.; Xu, Y.; Zeng, B.; Dai, L. A novel halogen-free co-curing agent with linear multi-aromatic rigid structure as flame-retardant modifier in epoxy resin. Polym. Adv. Technol. 2018, 29, 603–611. [Google Scholar] [CrossRef]
- Wang., P.; Yang, F.; Li., L.; Cai, Z. Flame retardancy and mechanical properties of epoxy thermosets modified with a novel DOPO-based oligomer. Polym. Degrad. Stab. 2016, 129, 156–167. [Google Scholar] [CrossRef]
- Howell, B.A.; Dumitrascu, A. Comparison of the impact of phosphorus and phosphorus/nitrogen on the flammability of styrenic oligomers. ACS Symp. Ser. 2012, 1118, 235–250. [Google Scholar]
- Wang, L.; Jiang, J.; Jiang, P.; Yu, J. Synthesis, characteristic of a novel flame retardant containing phosphorus, silicon and its application in ethylene vinyl-acetate copolymer (EVM) rubber. J. Polym. Res. 2010, 17, 891–902. [Google Scholar] [CrossRef]
- Zhong, H.; Wu, D.; Wei, P.; Jiang, P.; Hao, J. Synthesis characterization of a novel flame retardant containing silicon and its application in PC/ABS alloy. J. Mater. Sci. 2007, 42, 10106–10112. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, M.; Ding, Y.; Wang, F.; Gao, C.; Liu, P.; Wen, B.; Zhang, S.; Yang, M. Synergistic effect of DOPO immobilized silica nanoparticles in the intumescent flame retarded polypropylene composites. Polym. Adv. Technol. 2013, 24, 732–739. [Google Scholar] [CrossRef]
- Huo, S.; Wang, J.; Yang, S.; Chen, X.; Zhang, B.; Wu, Q.; Zhang, B. Flame-retardant performance and mechanism of epoxy thermosets modified with a novel reactive flame retardant containing phosphorus, nitrogen, and sulfur. Polym. Adv. Technol. 2017, 29, 497–506. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Zhang, X.-Y.; Liu, J.; Li, M.-M.; Guo, F.; Xia, X.-N.; Xu, W.-J. Synthesis of novel phosphorus-containing epoxy hardeners and thermal stability and flame-retardant properties of cured products. J. Appl. Polym. Sci. 2012, 125, 1219–1225. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Z.; Fang, Z. Effect of styrene-maleic anhydride as a reactive compatibilizer on the mechanical properties and flammability of intumescent flame retardant polystyrene. J. Appl. Polym. Sci. 2010, 118, 152–158. [Google Scholar] [CrossRef]
- Czégény, Z.; Blazsó, M. Effect of phosphorous flame retardants on the thermal decomposition of vinyl polymers and copolymers. J. Anal. Appl. Pyrolysis 2008, 81, 218–224. [Google Scholar] [CrossRef]
- Xiong, Y.; Jiang, Z.; Xie, Y.; Zhang, X.; Xu, W. Development of a DOPO-containing melamine epoxy hardeners and its thermal and flame-retardant properties of cured products. J. Appl. Polym. Sci. 2013, 127, 4352–4358. [Google Scholar] [CrossRef]
- Hu, W.; Zhan, J.; Hong, N.; Hull, T.R.; Stec, A.A.; Song, L.; Wang, J.; Hu, Y. Flame retardant polystyrene copolymers: Preparation, thermal properties, and fire toxicities. Polym. Adv. Technol. 2014, 25, 631–637. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, C.; He, M.; Ke, Z.; Liu, Y.; Tai, Q.; Xiao, X.; Hu, Y. Preparation of a novel styrene copolymer: Simultaneously improving the thermal stability and toughness. J. Appl. Polym. Sci. 2018, 135, 46120. [Google Scholar] [CrossRef]
- Howell, B.A.; Daniel, Y.G. Incorporation of comonomer exo-5-(diphenylphosphato) isosorbide-2-endo-acrylate to generate flame retardant poly (styrene). Polymers 2019, 11, 2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schäfer, A.; Seibold, S.; Lohstroh, W.; Walter, O.; Döring, M. Synthesis and properties of flame-retardant epoxy resins based on DOPO and one of its analog DPPO. J. Appl. Polym. Sci. 2010, 105, 685–696. [Google Scholar] [CrossRef]
- Hoang, D.; Kim, W.; An, H.; Kim, J. Flame retardancies of novel organo-phosphorus flame retardants based on DOPO derivatives when applied to ABS. Macromol. Res. 2015, 23, 442–448. [Google Scholar] [CrossRef]
- Jiang, S.; Yang, H.; Qian, X.; Shi, Y.; Zhou, K.; Xu, H.; Shan, X.; Lo, S.; Yuan, H.; Zhou, G. A novel transparent cross-linked poly (methyl methacrylate)-based copolymer with enhanced mechanical, thermal, and flame-retardant properties. Ind. Eng. Chem. Res 2014, 53, 3880–3887. [Google Scholar] [CrossRef]
- Bai, Z.; Song, L.; Hu, Y.; Yuen, R.K.K. Preparation, flame retardancy, and thermal degradation of unsaturated polyester resin modified with a novel phosphorus containing acrylate. Ind. Eng. Chem. Res. 2013, 52, 12855–12864. [Google Scholar] [CrossRef]
- Qian, L.; Qiu, Y.; Sun, N.; Xu, M.; Xu, G.; Xin, F.; Chen, Y. Pyrolysis route of a novel flame retardant constructed by phosphaphenanthrene and triazine-trione groups and its flame-retardant effect on epoxy resin. Polym. Degrad. Stab. 2014, 107, 98–105. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Yang, S.; Cheng, J.; Ding, G.; Huo, S. Facile construction of one-component intrinsic flame-retardant epoxy resin system with fast curing ability using imidazole-blocked bismaleimide. Compos. Part B Eng. 2019, 177, 107380. [Google Scholar] [CrossRef]
- Liang, S.; Hemberger, P.; Neisius, N.M.; Bodi, A.; Hansjörg, G.; Joelle, L.G.; Gaan, S. Elucidating the thermal decomposition of dimethyl methylphosphonate by vacuum ultraviolet (VUV) photoionization: Pathways to the PO radical, a key species in flame-retardant mechanisms. Chem. Eur. J. 2015, 21, 1073–1080. [Google Scholar] [CrossRef]


| Sample | St (g) | DOPOAA (g) | DOPO Content (%) | DOPOAA in Copolymer (%) | Yield (%) | Mn (×104) | Mw (×104) |
|---|---|---|---|---|---|---|---|
| PS | 5 | 0 | 0 | 0 | 87.6 | 3.23 | 5.69 |
| PS-10 | 3.79 | 1.21 | 10 | 3.34 | 69 | 6.16 | 16.26 |
| PS-20 | 2.91 | 2.01 | 20 | 5.97 | 66.3 | 6.81 | 20.97 |
| Sample | T5% (°C) | Tmax1 (°C) | Tmax2 (°C) | Rmax1 (%/°C) | Rmax2 (%/°C) | Residue at 700 °C (%) |
|---|---|---|---|---|---|---|
| PS | 365 | 414 | - | −2.75 | - | 0.21 |
| DOPOAA | 269 | 307 | 414 | −1.37 | −0.52 | 5.62 |
| PS-10 | 138 | 412 | - | −1.87 | - | 0.89 |
| PS-20 | 162 | 413 | - | −1.49 | - | 0.91 |
| Sample | PHRR (W·g−1) | Temperature (°C) | LOI (%) |
|---|---|---|---|
| PS | 972 | 432.1 | 18.1 |
| PS-10 | 558 | 427.2 | 21.9 |
| PS-20 | 354 | 435.1 | 26.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, Y.; Liu, L.; Xiao, T. The Preparation, Thermal Properties, and Fire Property of a Phosphorus-Containing Flame-Retardant Styrene Copolymer. Materials 2020, 13, 127. https://doi.org/10.3390/ma13010127
Sun Y, Wang Y, Liu L, Xiao T. The Preparation, Thermal Properties, and Fire Property of a Phosphorus-Containing Flame-Retardant Styrene Copolymer. Materials. 2020; 13(1):127. https://doi.org/10.3390/ma13010127
Chicago/Turabian StyleSun, Yu, Yazhen Wang, Li Liu, and Tianyuan Xiao. 2020. "The Preparation, Thermal Properties, and Fire Property of a Phosphorus-Containing Flame-Retardant Styrene Copolymer" Materials 13, no. 1: 127. https://doi.org/10.3390/ma13010127






