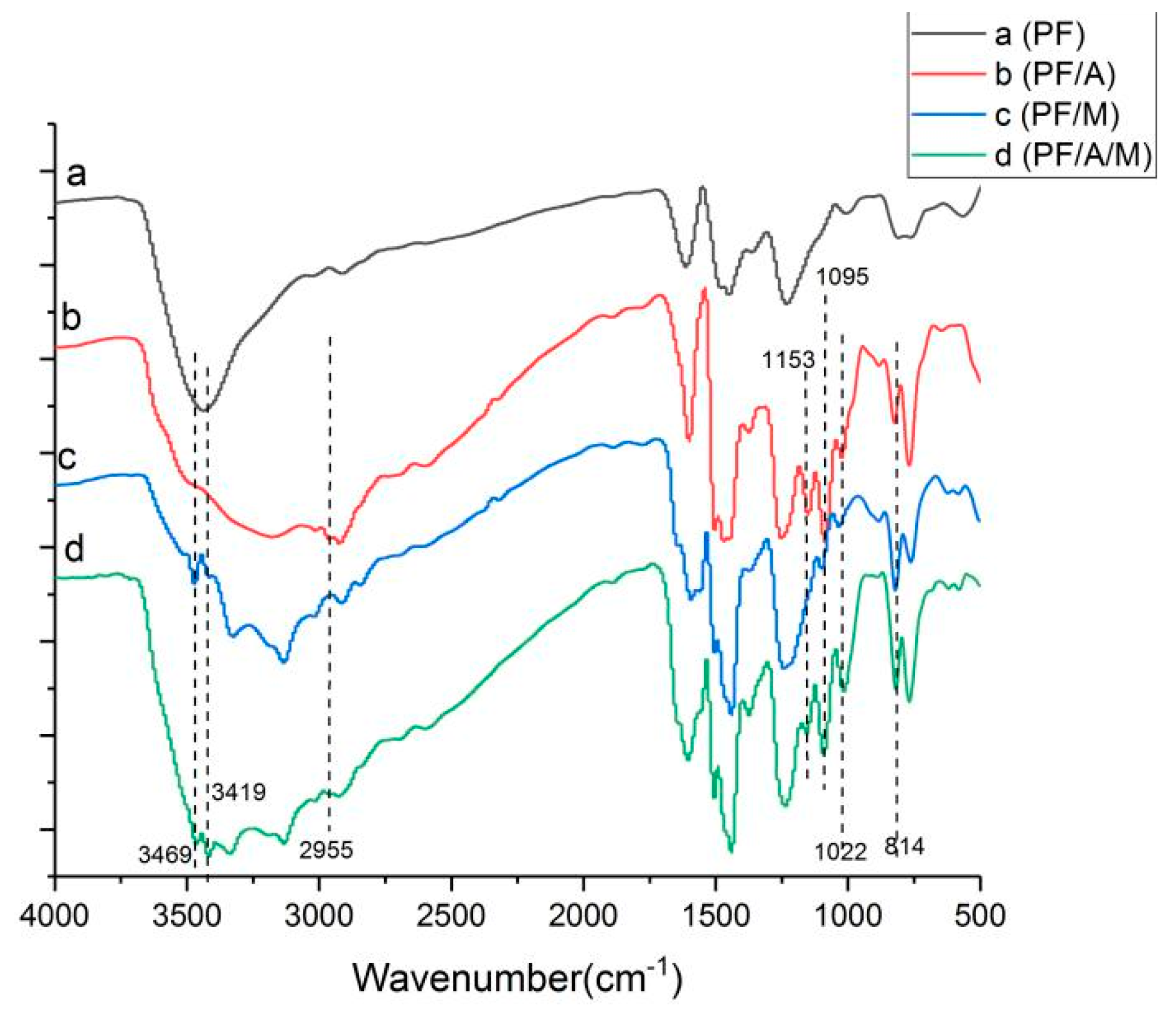
3.1. Fourier Transform Infrared Spectroscopy (FTIR) Test Results
Phenolic resin and modified phenolic resin were characterized by Fourier transform infrared spectroscopy, as shown in
Figure 1. The Fourier spectra of the melamine-modified phenolic resin are shown in
Figure 1, and the absorption peaks at 3469 cm
−1 and 3419 cm
−1 are the performance of the anti-symmetrical vibration of NH
2. The 1022 cm
−1 is formed by NH twisting vibration, and the characteristic absorption peak at 814 cm
−1 is the characteristic absorption of deformation vibration of a triazine ring [
18]. The absorption peaks of these bands are also found in
Figure 1 of the phenolic resin system in which aluminum diethylphosphinate and melamine are simultaneously added. In
Figure 1 of the aluminum diethylphosphinate modified phenolic resin system, the stretching vibration of P=C double bond is at 1153 cm
−1, and the peak at 1095 cm
−1 is formed by PO· symmetrical stretching vibration [
19]. The characteristic peak at 2955 cm
−1 is consistent with the stretching vibration frequency of –CH
2– [
20], and characteristic peak coincides with the characteristic peak of aluminum diethylphosphinate. The phenolic resin system with aluminum diethylphosphinate and melamine added at the same time has these absorption peak. In addition, the other characteristic peaks are consistent with the pure phenolic resin. The results were confirmed that the aluminum diethylphosphinate and melamine were synthesized successfully.
3.3. Modified Phenolic Resin Flame-Retardant Properties
The flame-retardant properties of different resin systems were tested by LOI and the UL-94 vertical burning test [
25].
Table 5 and
Table 6 presents the LOI values and UL-94 test results of the samples.
According to
Table 5, we can seen that the LOI value of pure phenolic resin is 30.0%, which is inherently flame-retardant, and almost no dripping is formed in the vertical burning test. Compared with that of pure phenolic resin, the LOI was 33.1% when the amount addition reached 10% by weight, and passed the UL-94 V-0 level in the vertical burning test. Continuing to increase the amount of flame retardant, when the addition amount reaches 15 wt%, the LOI of phenolic resin composites is 34.6%, and in the vertical burning test, can pass the UL-94 V-0 level. However, when the aluminum diethylphosphinate was 20 wt %, the flame-retardant capacity of phenolic resin decreased with the increase of the aluminum diethylphosphinate. It can be seen that when the addition amount of aluminum diethylphosphinate is 15%, the LOI and UL-94 test results of the phenolic resin composite system are the best, which can improve the flame-retardant performance of the phenolic resin. This highlights that most of the decomposition products of aluminum diethylphosphinate are volatilized into the gas phase during combustion, and may produce free radicals such as PO· in the gas phase, and capture free radicals produced by combustion to inhibit combustion [
26,
27]. When melamine was added to the phenolic resin, the LOI increased first and then decreased with the addition amount. When the addition reached 4 wt %, the LOI reached a maximum of 32.1%. In the vertical burning test, it can pass the UL-94 V-0 level. Then, with the increase of the amount of addition, the LOI began to decrease, showing that adding a suitable amount of melamine can improve the flame-retardant properties of the phenolic resin.
The flame-retardant properties of aluminum diethylphosphinate and melamine were simultaneously added to the phenolic resin, as shown in
Table 6. When the added amount of aluminum diethylphosphinate is 10 wt % and melamine is 4 wt %, the LOI of the phenolic resin composite system reaches a maximum value of 35.8%, and can pass the UL-94 V-0 grade. Compared with adding melamine alone, there is a significant improvement. With the addition of aluminum diethylphosphinate into phenolic resin, the phosphorus-containing group is thermally oxidized and decomposed during combustion to form phosphoric acid-promoting charcoal, suggesting that aluminum diethylphosphinate and melamine can play a synergistic role in the phenolic resin composite system. The synergistic effect of the nitrogen-phosphorus ion improves the flame retardancy. When the added amount of the two flame retardants is increased, the LOI value is decreased, indicating the addition amount has exceeded the appropriate range. If too much flame retardant is added, the flame retardancy of the phenolic resin system is lowered.
3.4. Cone Calorimeter Test (CCT) of Modified Phenolic Resin
The cone calorimeter test (CCT) can test the combustion properties of composites under real fire conditions, and it is one of the most important tests to study the flame retardant properties of composites [
28]. The matching results of CCT were summarized in
Table 7, including peak heat release rate (p
HRR), time corresponding to pHRR (T
pHRR), time to sustained ignition (TTI), total heat release (THR) and amount of carbon residue [
29].
The HRR, THR, and mass loss plots of phenolic resin and its composites during combustion are shown in
Figure 5. As can be seen from
Figure 5 and
Table 7, the HRR of pure phenolic resin increases sharply after ignition and reaches a peak heat release rate of 304.4 kW/m
2 at 86 s, and THR was as high as 78.6 MJ/m
2, TTI was 26 s, and the carbon residue was 32.8%. This shows that pure phenolic resin has a high risk of burning. When 10 wt % aluminum diethylphosphinate was added to the phenolic resin, the HRR of the E-a2 group decreased to 245.6 kW/m
2, the time was extended to 104 s, and the THR decreased to 58.6 MJ/m
2. Compared with pure phenolic resin, HRR and THR decreased by 19.3% and 25.4%, respectively. TTI increased to 34 s, and increased by 8 s compared with pure phenolic resin. After adding 10 wt % aluminum diethylphosphinate and 3 wt % melamine to the phenolic resin, the HRR of the E-c1 group decreased to 196.2 kW/m
2 and the THR decreased to 51 MJ/m
2. Compared with the pure phenolic resin, the HRR decreased by 35.5% and 35.1%, respectively. The TTI increased to 42, and the pure phenolic resin increased by 16 s. From the mass loss image of
Figure 5c, it can be seen clearly that the mass loss curve of E-c1 is much more backward than pure phenolic resin. Compared with the pure phenolic resin residual carbon content of 32.2 wt %, the residual carbon content of E-c1 group is increased by 19.6%, which is 38.5 wt %. The simultaneous addition of aluminum diethylphosphinate and melamine is more effective than the phenolic resin system with only aluminum diethylphosphinate added. The simultaneous action of the two flame retardants can effectively improve the stability of the phenolic resin during combustion, reduce the heat release rate, heat released and the amount of residual carbon increase. Aluminum diethylphosphinate mainly produces flame retardant effect through the gas phase, while melamine can increase the amount of residual carbon and reduce the heat release rate.
By adding 4 wt % melamine to the phenolic resin, the HRR of the E-b2 group decreased to 286.6 kW/m2, and the time was extended to 90 s. Compared with pure phenolic resin, HRR and THR decreased by 5.8% and 20.2%, respectively. The TTI increased to 29 s. Compared with the phenolic resin system with aluminum diethylphosphinate and melamine added at the same time, the thermal stability was obviously reduced when burning. When 15 wt % aluminum diethylphosphinate and 5 wt % melamine were added to the phenolic resin, the HRR reached a maximum of 213.9 kW/m2 at 158 s, and THR values could reach 55.3 MJ/m2. Compared with pure phenolic resin, the pHRR and THR of E-c6 decreased by 29.7% and 29.6%, respectively. It can be seen that compared with the E-c1 group phenolic composite material, the combustion performance of the E-c6 composite material was not good, which means that the excessive flame-retardant combustion performance is not effective.
According to research on the causes of death of those killed in fires, about two-thirds of deaths are caused by the direct poisoning of CO generated in the fire. Although CO
2 is non-toxic, when it reaches a certain concentration, it will also cause harm to the human body. Therefore, it is particularly important to reduce the content of CO and CO
2. Figure 6 shows CO and CO
2 curves of all composites; the results are summarized in
Table 8, including peak concentration of CO (p
CO) and CO
2 (p
CO2), pink time of CO(Tp
CO) and CO
2 (Tp
CO2). The CO of pure phenolic resin increased sharply after ignition and reached the peak of 0.215% at 206 s, and CO
2 concentration was as high as 0.356% at 82 s. When 10 wt % aluminum diethylphosphinate was added to the phenolic resin, the maximum concentration of CO and CO
2 of the E-a2 group decreased to 0.201% at 152 s and 0.341%, respectively. Compared with pure phenolic resin, they decreased by 6.5% and 9%, respectively. By adding 10 wt % aluminum diethylphosphinate and 3 wt % melamine to the phenolic resin, the peak concentration of CO and CO
2 of the E-c1 group decreased to 0.196% and 0.222%, respectively. Compared with the pure phenolic resin, the content decreased by 8.8% and 37.6%, respectively. With the addition of 15 wt % aluminum diethylphosphinate and 5 wt % melamine to the phenolic resin, the concentration of CO and CO
2 of the E-c1 group decreased to 0.199% and 0.264%, respectively. Compared with the pure phenolic resin, they decreased by 7.4% and 0.134%, respectively. It is obvious that the CO and CO
2 concentrations were the minimum by adding 10 wt % aluminum diethylphosphinate to the phenolic resin, the hazard of the phenolic resin can be reduced.
3.5. Analysis of Char Residual
To further understand the flame-retardant effect of the modified phenolic resin, the residual char after the cone calorimeter test was analyzed.
Figure 7 and
Figure 8 are digital photographs of phenolic resin and composite materials before and after combustion under the same thermal radiation conditions in a cone calorimeter test.
As can be seen from the figures, pure phenolic resin is completely burned, and only a few carbon residues were left, and the residual carbon is obviously loose, light and thin. However, the carbon layer of E-a2 and E-b2 composites has a significant degree of expansion and an increase in the amount of carbon residue, as shown in
Table 9. The thicknesses of the residual carbon layers in the E-0, E-a2, E-b2 and E-c1 groups were 2.5, 5.5, 5 and 5.8 mm, respectively. Compared with pure phenolic resin, the thickness of the residual carbon layer increased significantly. When aluminum diethylphosphinate and melamine are added together, the carbon residue of E-c1 is significantly increased compared to pure phenolic resin. The results show that the nitrogen-phosphorus ion obviously increases the amount of carbon residue and the strength of carbon layer after phenolic resin combustion.
In order to explore the microstructure of the carbon layer of the phenolic composite, the carbon layer after the combustion of the pure phenolic resin and phenolic resin composites was analyzed by SEM. It can be observed from
Figure 9a that the pure phenolic resin had a relatively porous hole and a large crack on the surface of the carbon layer after the CCT test, which is attributed to the rapid volatilization of the flammable volatile gas produced by the combustion on the pure phenolic resin surface. With the addition of aluminum diethylphosphinate, as shown in
Figure 9b, the carbon layer of the phenolic resin composite system still had large pores, and the carbon layer was rather loose, which did not block oxygen and heat. This is because aluminum diethylphosphinate generates volatile diethyl hypophosphorous acid during degradation, which acts as a trapping free radical in the gas phase and blocks the combustion process. With the volatilization of diethyl hypophosphorous acid, the phosphorus content inside the phenolic resin substrate decreases rapidly, resulting in a decrease in the char forming ability of the system. Therefore, the effect of the condensed phase flame-retardant mechanism is not obvious [
30], which can be explained through TGA. Therefore, the final residual amount of phenolic composite system with aluminum diethylphosphinate at 800 °C in TGA analysis has a small increase compared with that of pure phenolic resin material. When the phenolic system was introduced into melamine, as shown in
Figure 9c, the carbon layer of the phenolic composite material did not have pores and cracks such as pure phenolic resin, and became smoother and finer. This is because melamine reacts with formaldehyde after it is added. The formation of melamine formaldehyde and melamine formaldehyde disturbs the intermolecular rigid structure of phenolic resin, and forms an irregular cross-linked interpenetrating network structure with phenolic resin, which hinders the decomposition of phenolic resin and needs higher energy in decomposition [
31]. It can be explained that in the TGA analysis the final carbon residue of the phenolic composite system with melamine introduced at 800 °C was higher than that of the pure phenolic resin material. The carbon layer of the phenolic system with aluminum diethylphosphinate and melamine added at the same time, as shown in
Figure 9d, was more compact, continuous and dense, and there were almost no large cracks and holes. This shows that the combined action of the two can play a good role in heat insulation and oxygen insulation, and can slow down the mass and heat transfer and the release of combustible volatiles, so as to prevent the further combustion of phenolic resin composite materials.
The FTIR spectra of the char residues after the cone calorimeter test are shown in
Figure 10. By comparing with the spectra of the
Figure 1, it can be seen that the peaks at 3469 cm
−1 and 3419 cm
−1 (NH
2 groups), 2955 cm
−1 (–CH
2 groups), 1153 cm
−1 (P=C groups) disappear, and some new peaks are formed during combustion, as shown in
Figure 10, indicating that the cross-linking reaction occurred between the phenolic resin and two flame retardants. The strong characteristic peaks in the range of 3500–3100 cm
−1 are attributed to the stretching vibration of –OH and –NH– groups. The peaks at 1632, 1400 and 997 cm
−1 are due to the C=C absorption in carbonization reaction, P-N groups and P–O–C groups, respectively. After the addition of aluminum diethylphosphinate, a new peak is observed at 1247 cm
−1 (P=O groups) and 781 cm
−1 (Al–O groups) in
Figure 10. Additionally, the char rich in P–O–P groups, P-O-C groups, P-N groups and C=C groups exhibits a more intumescent and compact structure, which gives an illustration of the lower heat release and gas emissions during the combustion process.