Preparation of a Novel Flame Retardant Formulation for Cotton Fabric
Abstract
1. Introduction
2. Experimental
2.1. Materials
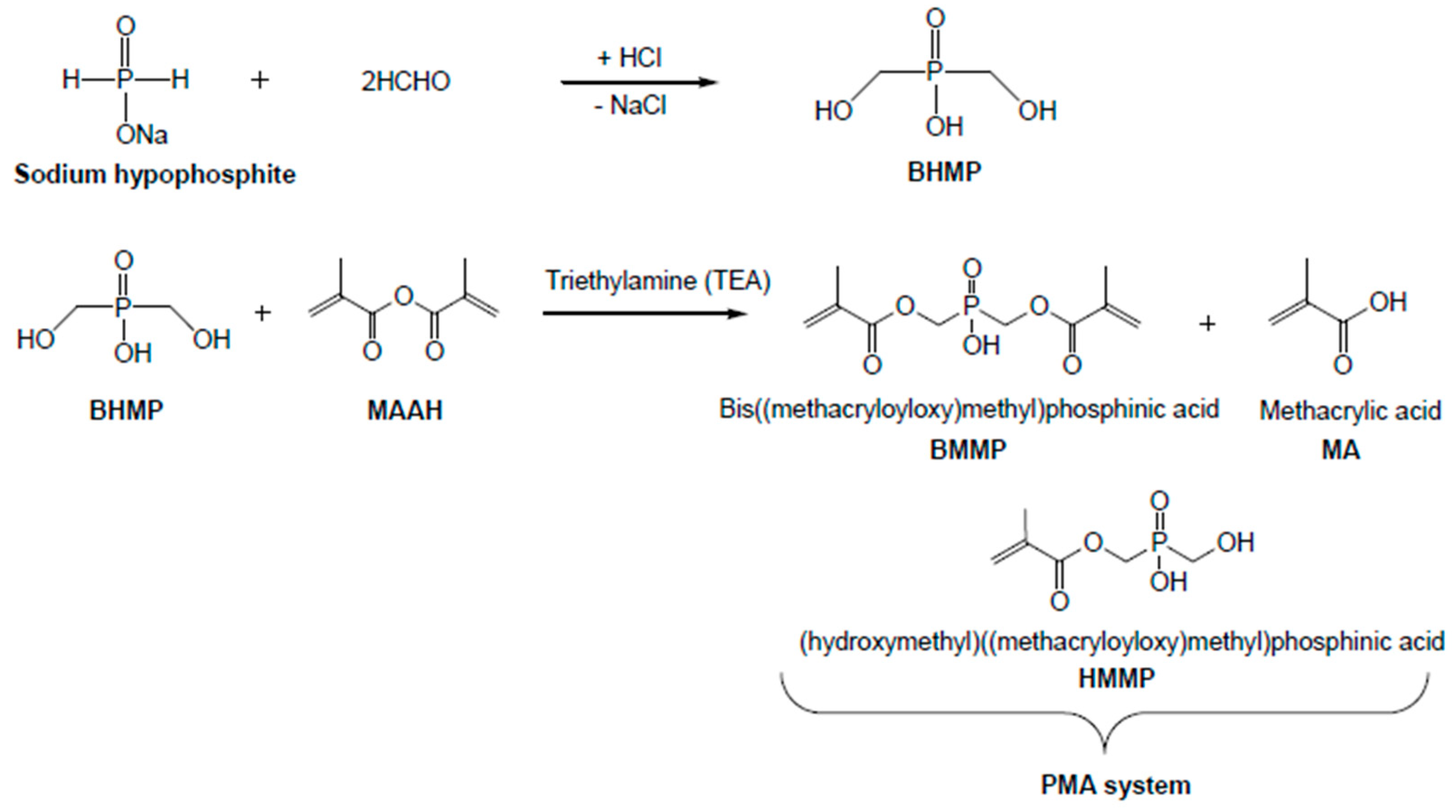
2.2. Preparation of the Phosphorus Containing Formulation
2.3. Fabric Treatment
2.4. Measurements
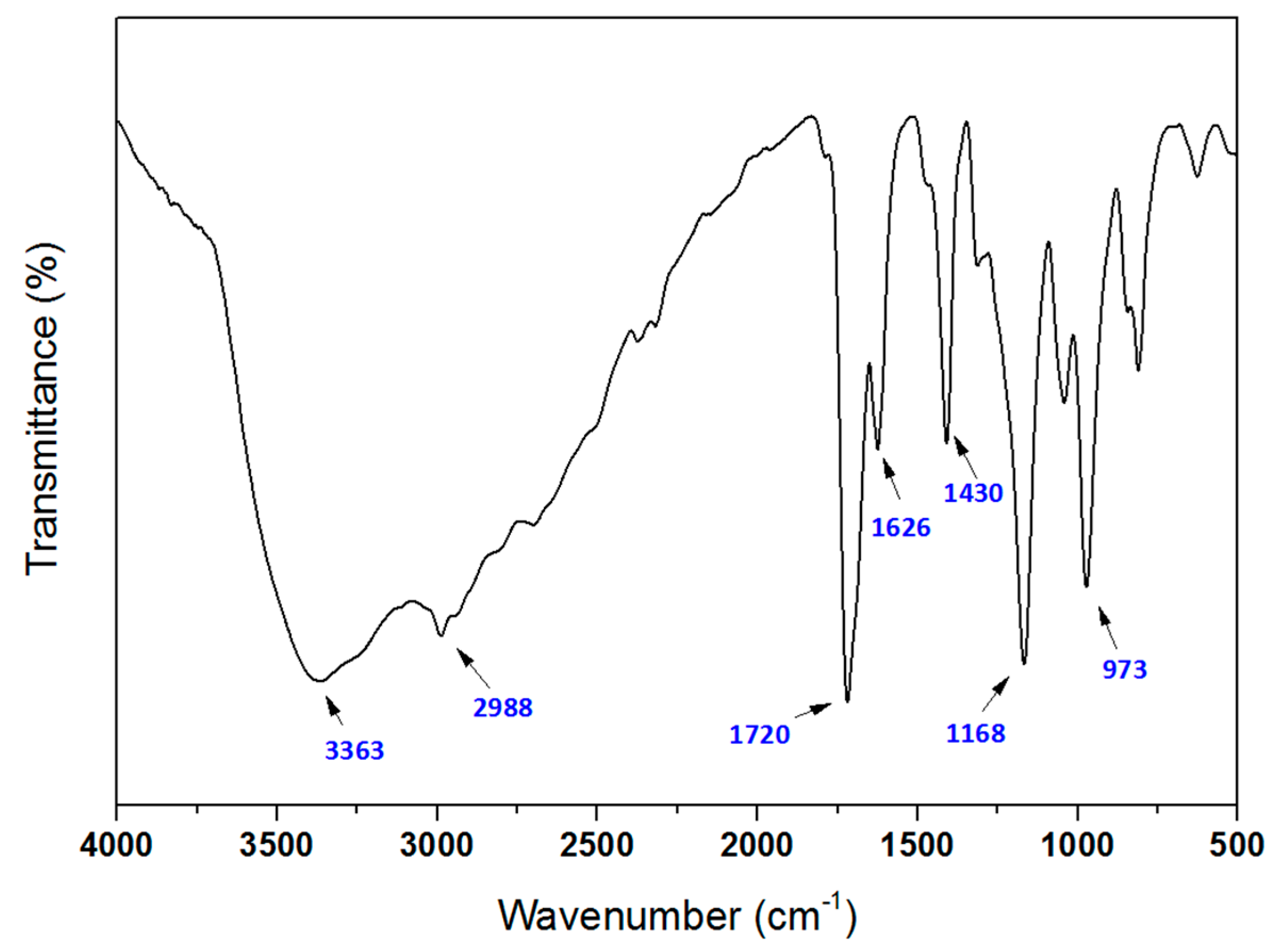
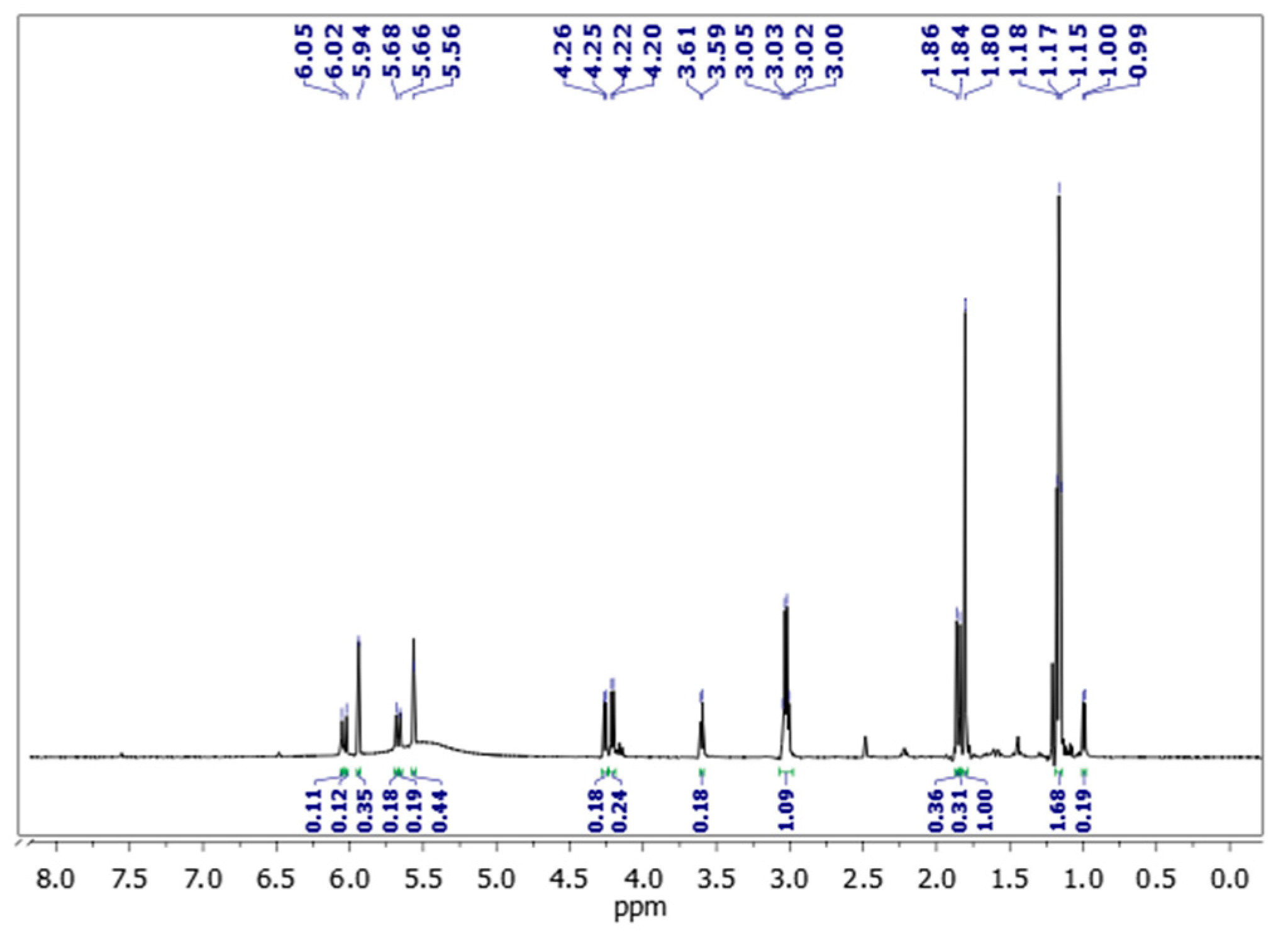
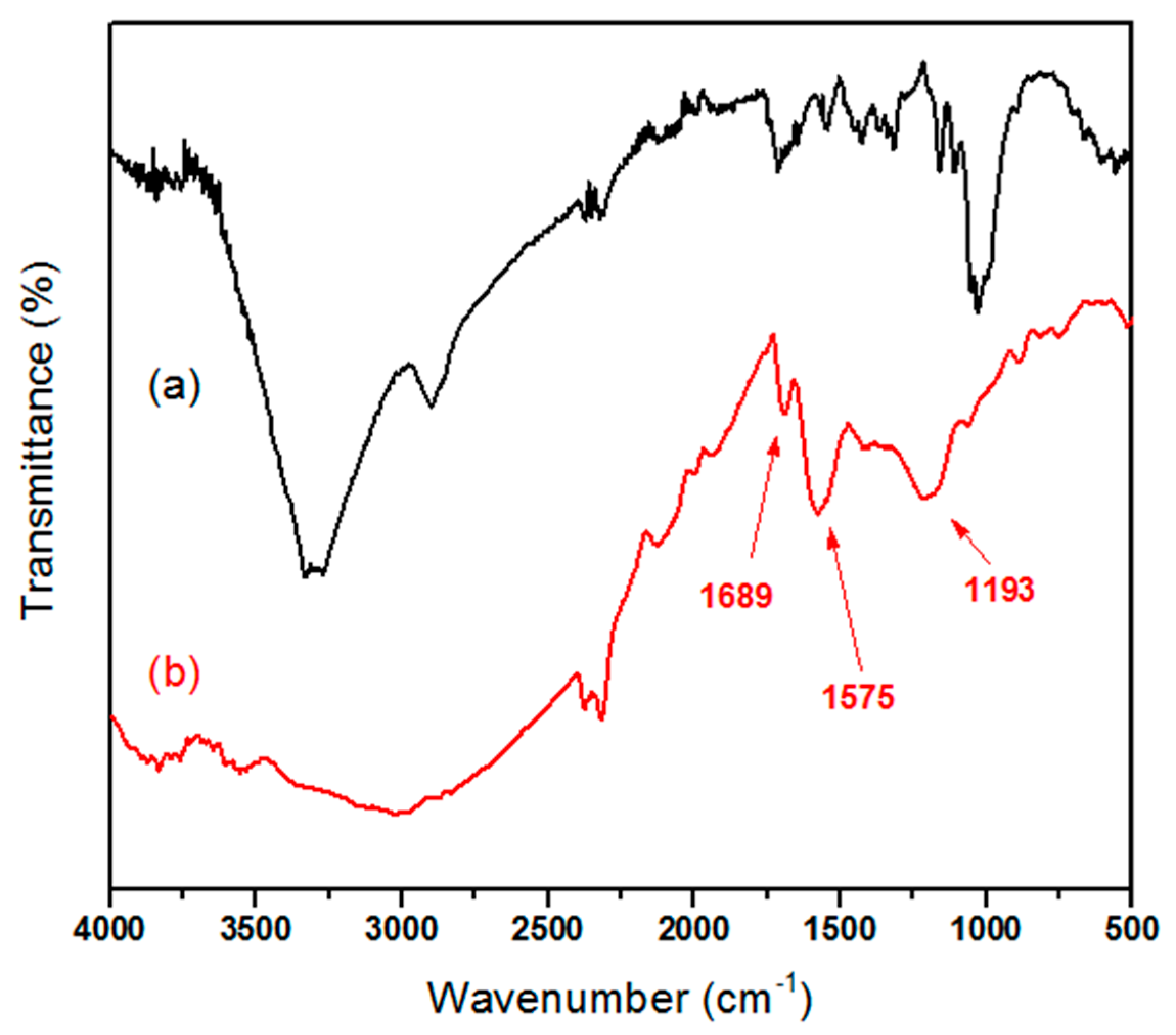
2.4.1. FTIR and 1H-NMR
2.4.2. Weight Gain
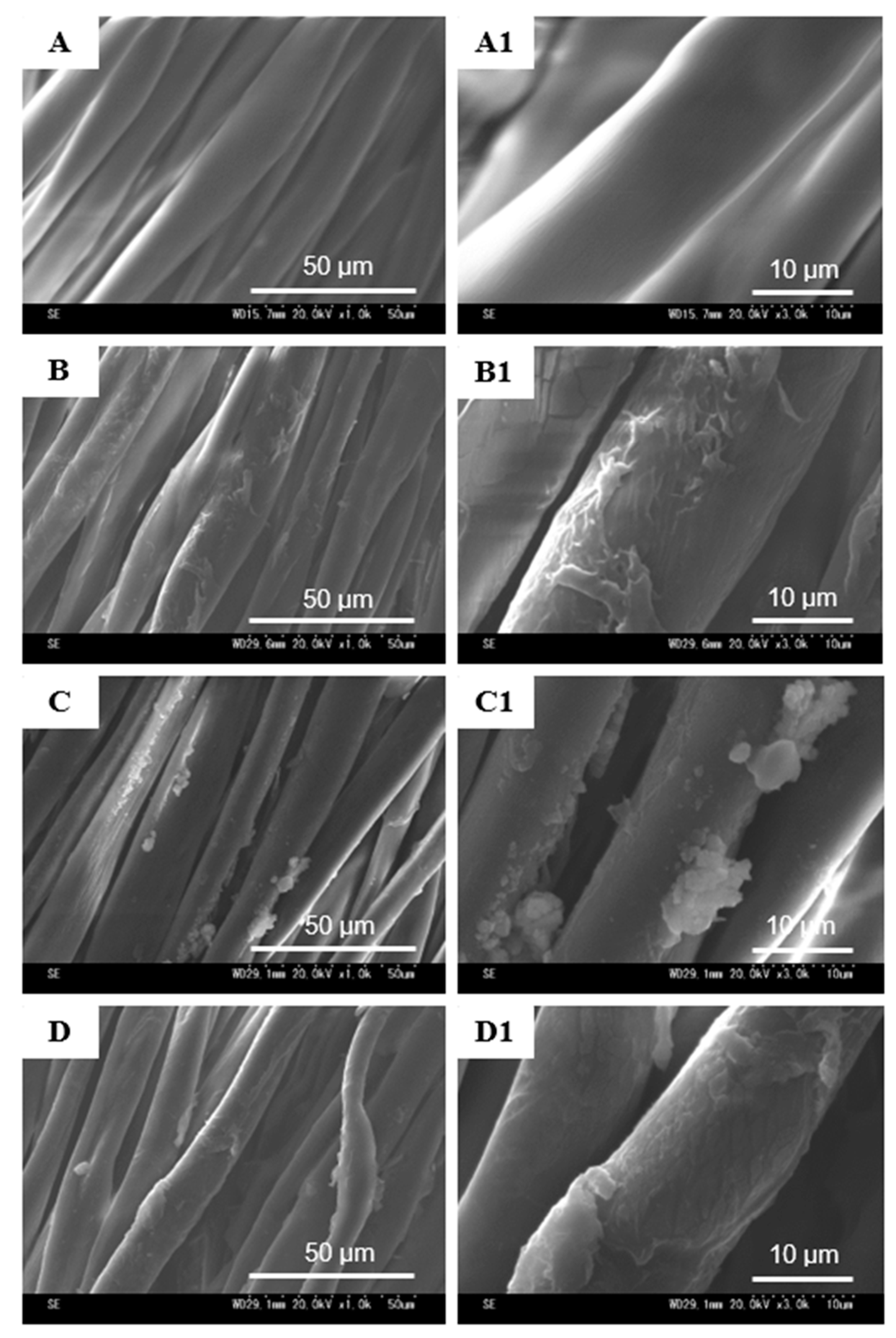
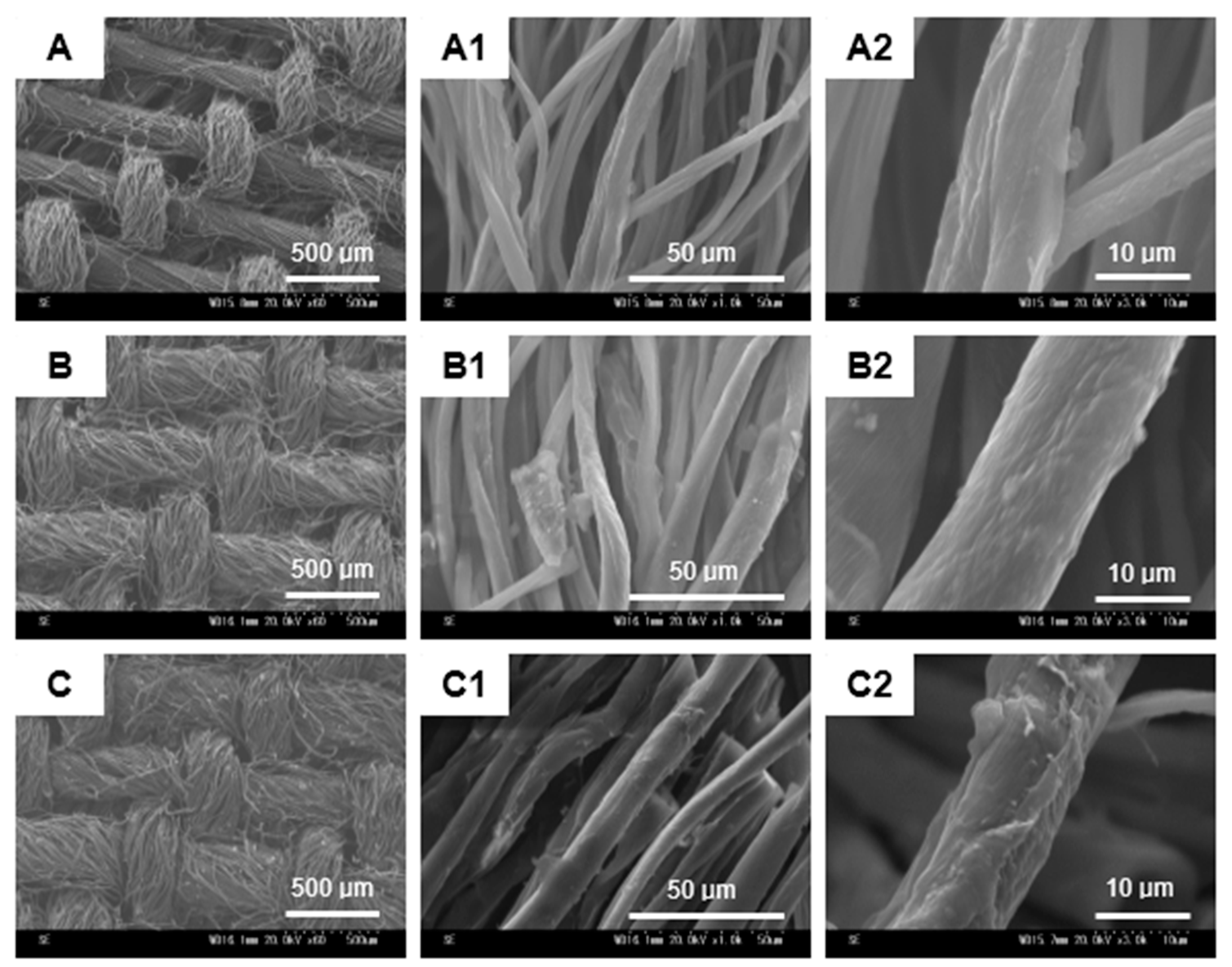
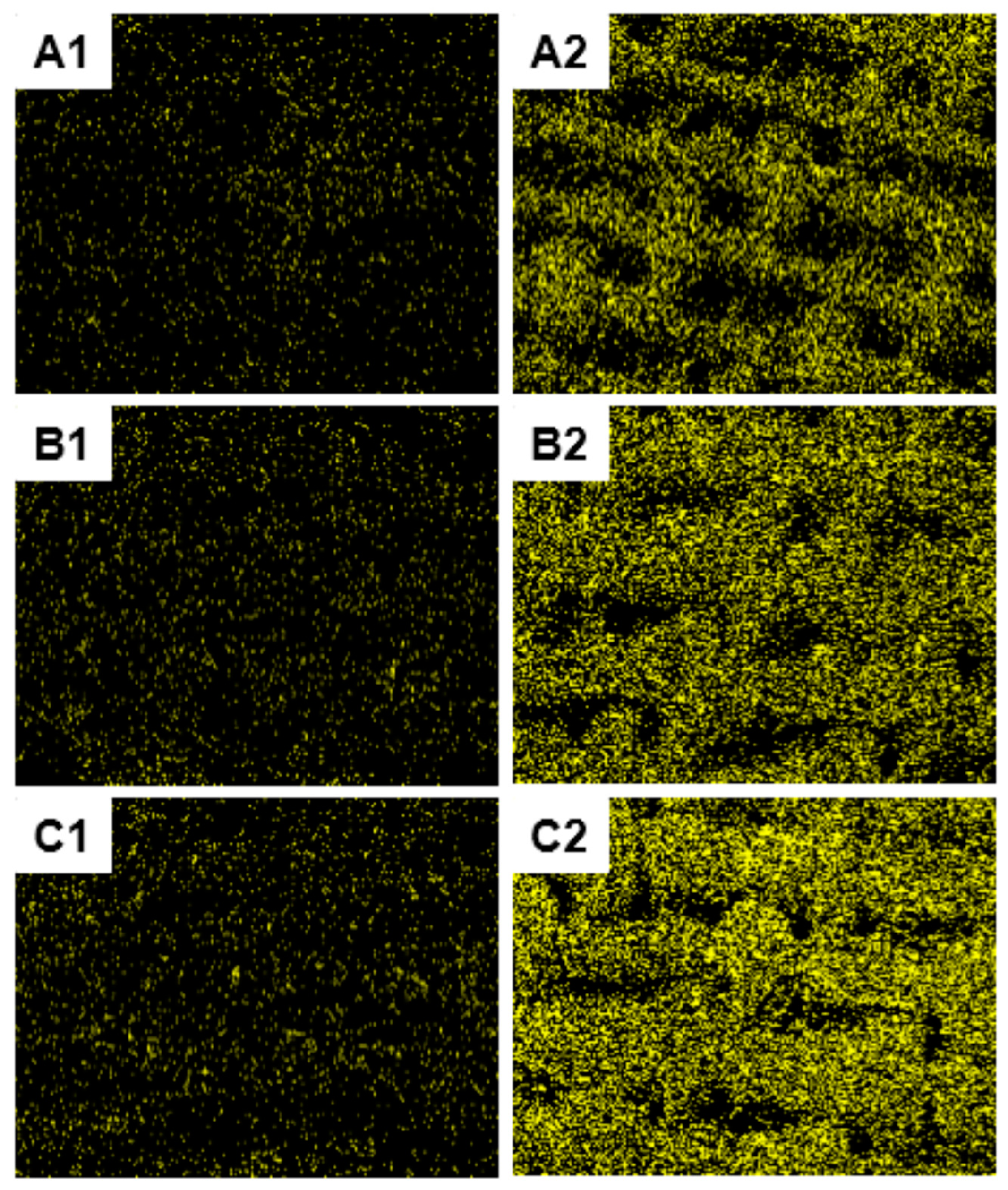
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Vertical Flame Test
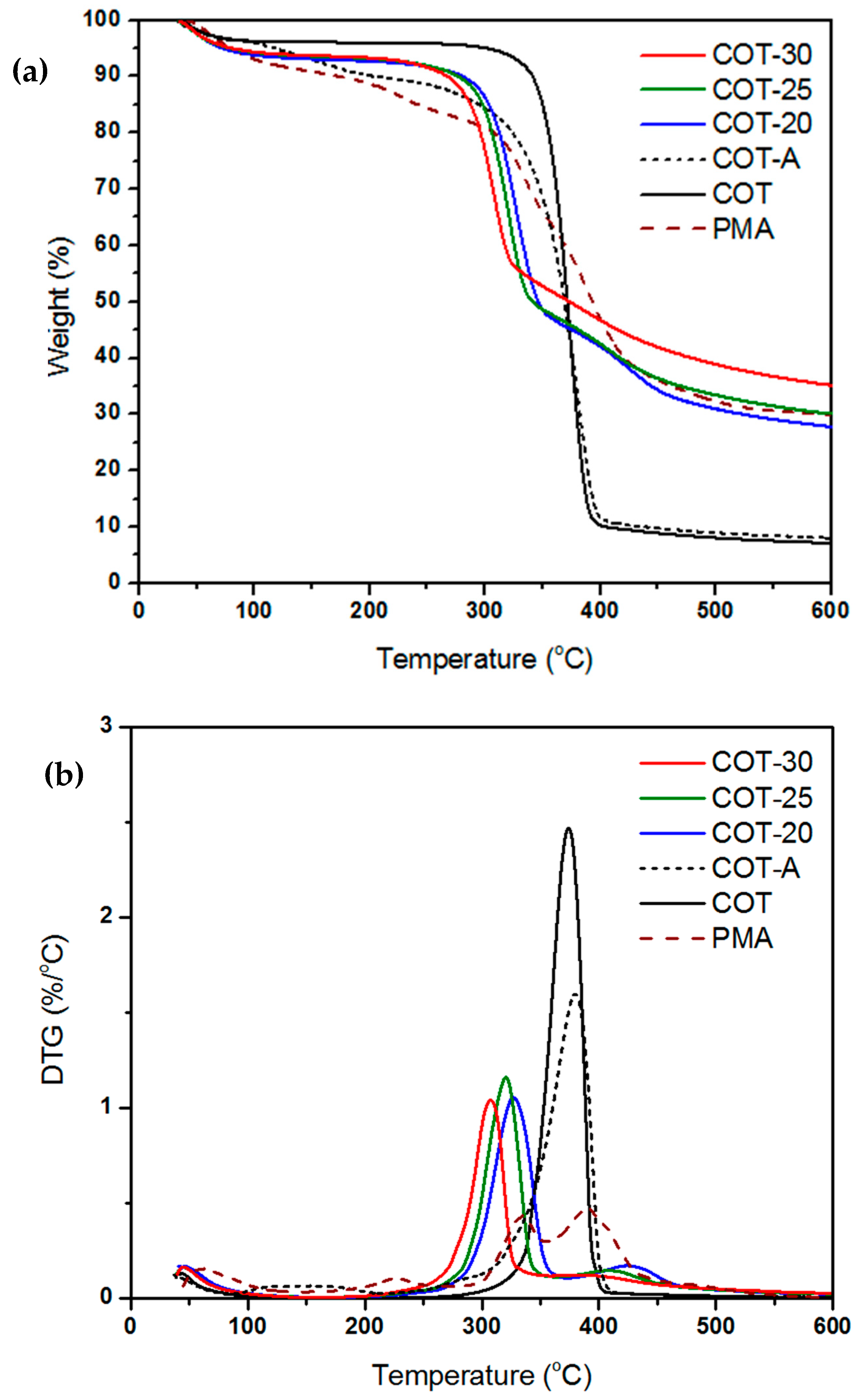
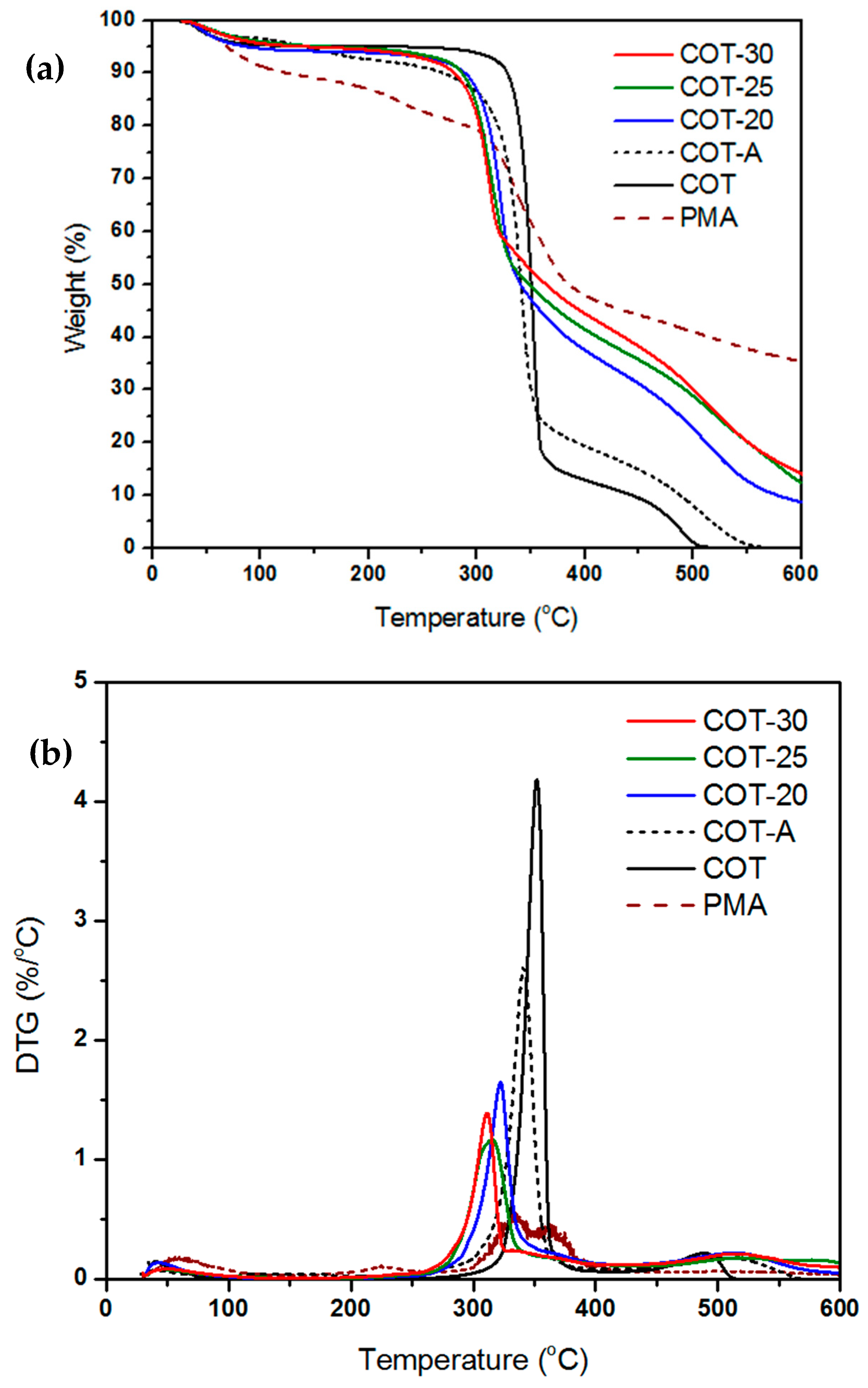
2.4.5. Thermogravimetric Analysis (TGA)
2.4.6. Durability Test
3. Results and Discussion
3.1. Synthesis of the PMA
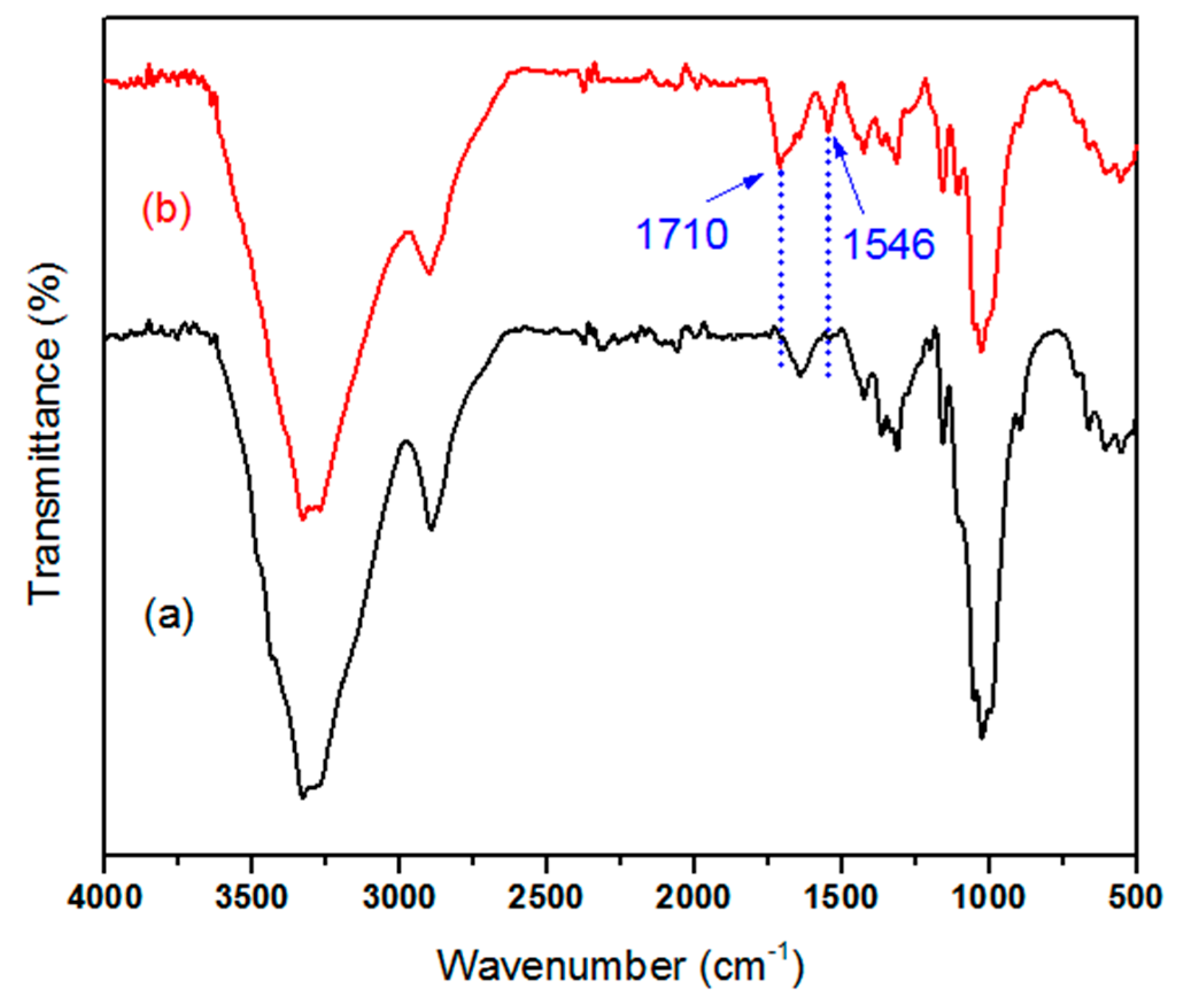
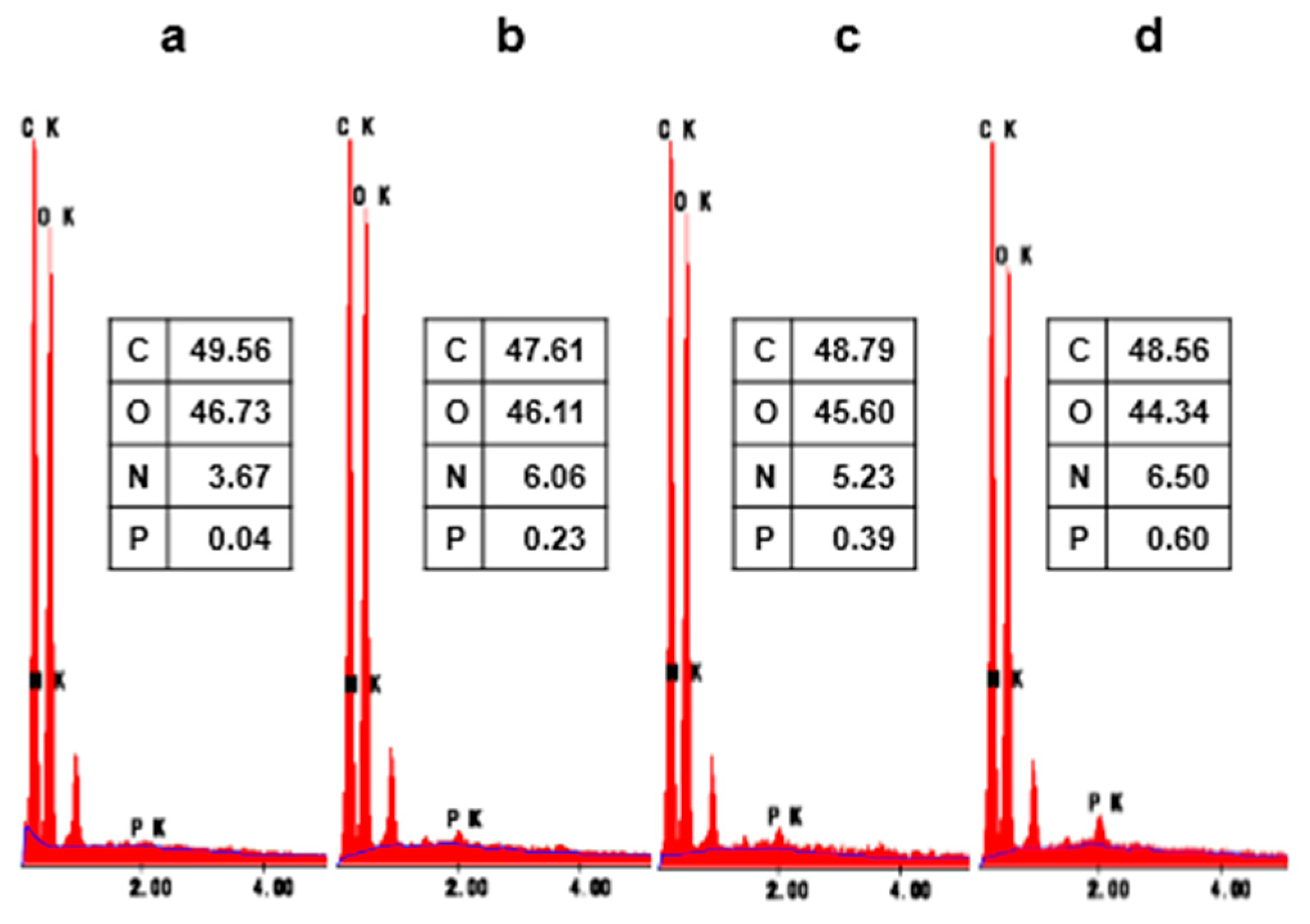
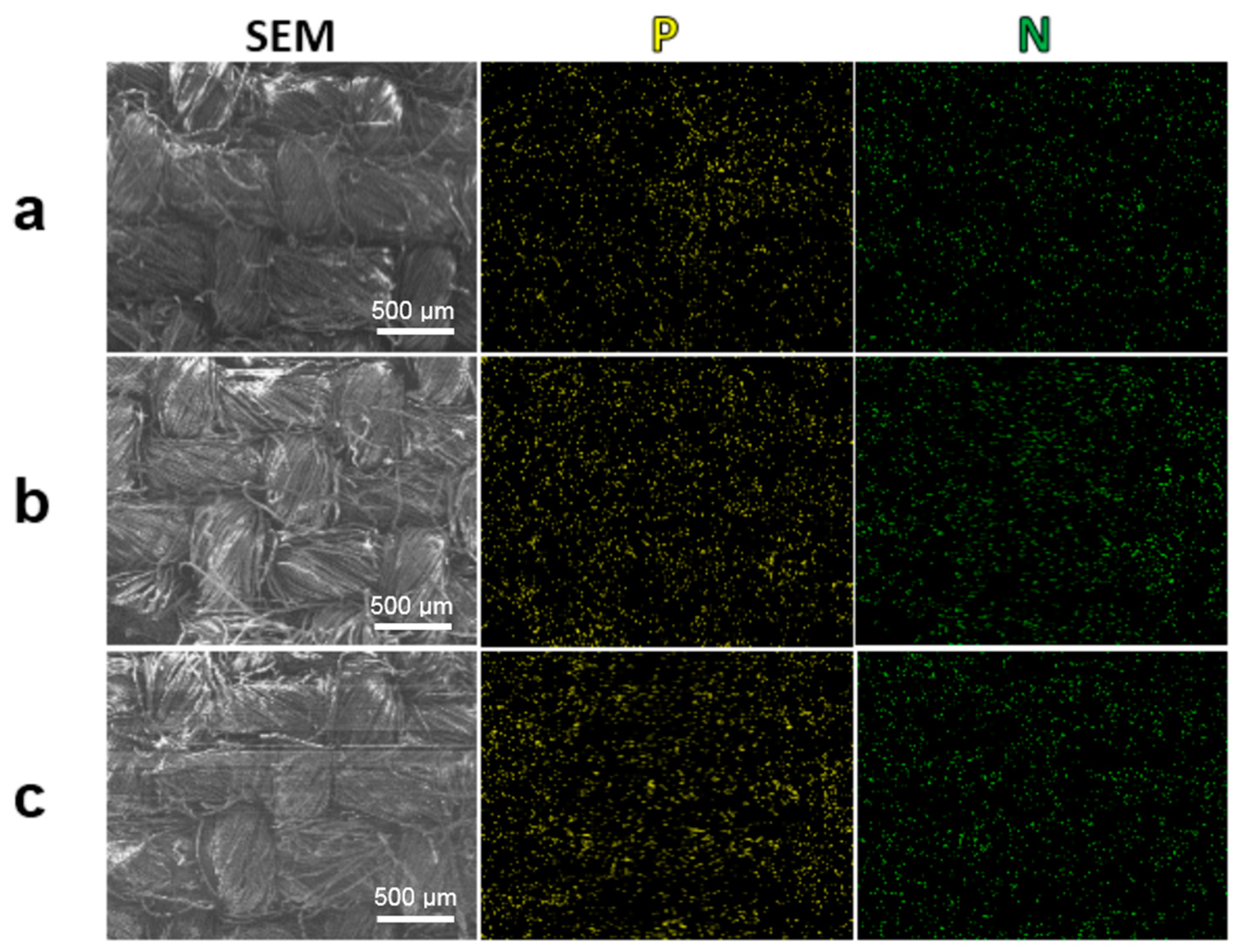
3.2. Surface Characterization of the Treated Cotton Fabrics
3.3. Flame Retardant Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wakelyn, P.J.; Bertoniere, N.R.; French, A.D.; Thibodeaux, D.P.; Triplett, B.A.; Rousselle, M.; Goynes, W.R.; Edwards, J.V.; Hunter, L.; McAlister, D.D.; et al. Cotton Fiber Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Gaan, S.; Salimova, V.; Rupper, P.; Ritter, A.; Schmid, H. Flame Retardant Functional Textiles; Woodhead Publishing Limited: Cambridge, UK, 2011. [Google Scholar]
- Xing, W.; Jie, G.; Song, L.; Hu, S.; Lv, X.; Wang, X.; Hu, Y. Flame retardancy and thermal degradation of cotton textiles based on UV-curable flame retardant coatings. Thermochim. Acta 2011, 513, 75–82. [Google Scholar] [CrossRef]
- Placido, J.; Capareda, S. Production of silicon compounds and fulvic acids from cotton wastes biochar using chemical depolymerization. Ind. Crop. Prod. 2015, 67, 270–280. [Google Scholar] [CrossRef]
- Przybylak, M.; Maciejewski, H.; Dutkiewicz, A.; Wesolek, D.; Przybylak, M.W. Multifunctional, strongly hydrophobic and flame-retarded cotton fabrics modified with flame retardant agents and silicon compounds. Polym. Degrad. Stab. 2016, 128, 55–64. [Google Scholar] [CrossRef]
- Giraud, S.; Bourbigot, S.; Rochery, M.; Vroman, I.; Tighzert, L.; Delobed, R. Flame Behavior of Cotton Coated with Polyurethane Containing Microencapsulated Flame Retardant Agent. J. Ind. Text. 2001, 31, 11–26. [Google Scholar] [CrossRef]
- Liu, W.; Chen, L.; Wang, Y.Z. A novel phosphorus-containing flame retardant for the formaldehyde-free treatment of cotton fabrics. Polym. Degrad. Stab. 2012, 97, 2487–2491. [Google Scholar] [CrossRef]
- Liang, S.; Matthias, N.; Neisius, N.S.; Gaan, S. Recent developments in flame retardant polymeric coatings. Progr. Org. Coat. 2013, 76, 1642–1665. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Flame Retardants for Plastics and Textiles; Carl Hanser Verlag GmbH Co KG.: Munich, Germany, 2015. [Google Scholar]
- Nguyen, C.T.; Kim, J.W. Thermal stabilities and flame retardancies of nitrogen-phosphorus flame retardants based on bisphosphoramidate. Polym. Degrad. Stab. 2008, 93, 1037–1043. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Kim, J.W. Synthesis of a Novel Nitrogen-Phosphorus Flame Retardant Based on Phosphoramidate and Its Application to PC, PBT, EVA, and ABS. Macromol. Res. 2008, 16, 620–625. [Google Scholar] [CrossRef]
- Hai, V.T.; Nguyen, C.T.; Lee, K.H.; Kim, J.W. Thermal stability and flame retardancy of novel phloroglucinol based organo phosphorus compound. Polym. Degrad. Stab. 2010, 95, 1092–1098. [Google Scholar]
- Yuan, H.; Xing, W.; Zhang, P.; Song, L.; Hu, Y. Functionalization of Cotton with UV-Cured Flame Retardant Coatings. Ind. Eng. Chem. Res 2012, 51, 5394–5401. [Google Scholar] [CrossRef]
- Edwards, B.; El-Shafei, A.; Hauser, P.; Malshe, P. Towards flame retardant cotton fabrics by atmospheric pressure plasma-induced graft polymerization: Synthesis and application of novel phosphoramidate monomers. Surf. Coat. Technol. 2012, 209, 73–79. [Google Scholar] [CrossRef]
- Cheema, H.A.; El-Shafei, A.; Hauser, P.J. Conferring flame retardancy on cotton using novel halogen-free flame retardant bifunctional monomers: Synthesis, characterizations and applications. Carbohydr. Polym. 2013, 92, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sui, X.; Li, Y.; Li, J.; Xu, H.; Zhong, Y.; Zhang, L.; Mao, Z. Durable flame retardant finishing of cotton fabrics with organosilic on functionalized cyclotriphosphazene. Polym. Degrad. Stab. 2016, 128, 22–28. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, C.Q.; Zhao, T. Heat release properties and flammability of the nylon/cotton blend fabric treated with a crosslinkable organophosphorus flame retardant system. J. Anal. Appl. Pyrolysis 2014, 110, 205–212. [Google Scholar] [CrossRef]
- Li, X.H.; Chen, H.Y.; Wang, W.T.; Liu, Y.Q.; Zhao, P.H. Synthesis of a formaldehyde-free phosphorus–nitrogen flame retardant with multiple reactive groups and its application in cotton fabrics. Polym. Degrad. Stab. 2015, 120, 193–202. [Google Scholar] [CrossRef]
- Dong, C.H.; Lu, Z.; Wang, P.; Zhu, P.; Li, X.C.; Sui, S.Y.; Zhang, L.; Liu, J. Flammability and thermal properties of cotton fabrics modified with a novel flame retardant containing triazine and phosphorus components. Text. Res. J. 2017, 87, 1367–1376. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S.; Malucelli, G. Recent Advances for Flame Retardancy of Textiles Based on Phosphorus Chemistry. Polymers 2016, 8, 319. [Google Scholar] [CrossRef]
- Ivanov, B.E.; Karpova, T.I. Synthesis and properties of hydroxymethyl phosphonic and bishydroxymethylphosphinic acids. Russ. Chem. Bull. 1964, 13, 1140–1142. [Google Scholar] [CrossRef]
- Testing of Textiles-Determination of the Burning Behaviour-Vertically Method, Ignition by Application of Flame to Base of Specimen; DIN 53906:1974-02; Beuth Verlag: Berlin, Germany, 1974.
- Textiles-Tests for Colour Fastness-Part C10: Colour Fastness to Washing with Soap or Soda and Soda; IS/ISO 105-C10:2006; International Organization for Standardization: Geneva, Switzerland, 2006.
- Jimenez, M.; Guin, T.; Bellayer, S.; Dupretz, R.; Bourbigot, S.; Grunlan, J.C. Microintumescent mechanism of flame-retardant water-based chitosan–ammonium polyphosphate multilayer nanocoating on cotton fabric. J. Appl. Polym. Sci. 2016, 133, 43783. [Google Scholar] [CrossRef]
- Price, D.; Horrocks, A.R.; Akalin, M.; Faroq, A.A. Influence of flame retardants on the mechanism of pyrolysis of cotton (celluIose) fabrics in air. J. Anal. Appl. Pyrolysis 1997, 40, 511–524. [Google Scholar] [CrossRef]
- Qi, X.; Qu, J.; Zhang, H.B.; Yang, D.Z.; Yu, Y.H.; Chi, C.; Yu, Z.Z. FeCl Intercalated Few-Layer Graphene for High Lithium-Ion Storage Performance. J. Mater. Chem. A 2015, 3, 15489–15504. [Google Scholar] [CrossRef]
- Gaan, S.; Rupper, P.; Salimova, V.; Heuberger, M.; Rabe, S.; Vogel, F. Thermal decomposition and burning behavior of cellulose treated with ethyl ester phosphoramidates: Effect of alkyl substituent on nitrogen atom. Polym. Degrad. Stab. 2009, 94, 1125–1134. [Google Scholar] [CrossRef]



| Sample | Composition FR(g)/Acetone (L) | Weight Gain after Washing 10 Times (%) |
|---|---|---|
| COT-20 | 380 | 20.73 |
| COT-25 | 440 | 25.70 |
| COT-30 | 500 | 30.27 |
| Substance | Signal | Group |
|---|---|---|
| BMMP | + 1.86 (s) ppm + 4.20 (d) ppm + 5.66 (s) and 6.02 (s) ppm | C–CH3 P–CH2–OC(O) C=CH2 |
| HMMP | + 1.84 (s) ppm + 3.59 (d) ppm + 4.25 (d) ppm + 5.68 (s) and 6.05 (s) ppm | C–CH3 P–CH2–OH P–CH2–OC(O) C=CH2 |
| MA | + 1.80 (s) ppm + 5.94 (s) and 5.56 (s) ppm | C–CH3 C=CH2 |
| Sample | COT | COT-20 | COT-25 | COT-30 | COT-A |
|---|---|---|---|---|---|
 |  |  |  |  | |
| After flame time (s) | 18 | 25 | 0 | 0 | 18 |
| After glow time (s) | 14 | 0 | 0 | 0 | 5 |
| Char length (mm) | 150 | 150 | 100 | 90 | 150 |
| DIN 53906 | Fail | Fail | Pass | Pass | Fail |
| Sample | Nitrogen | Oxygen | ||
|---|---|---|---|---|
| Onset (°C) | wt% of Char at 600°C | Onset (°C) | wt% of Char at 600°C | |
| PMA | 320 | 30.0 | 316 | 35.4 |
| COT | 343 | 7.1 | 331 | 0.2 |
| COT-20 | 299 | 27.8 | 299 | 8.7 |
| COT-25 | 294 | 30.1 | 293 | 12.4 |
| COT-30 | 283 | 35.2 | 285 | 14.1 |
| COT-A | 326 | 8.0 | 310 | 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.K.; Sakai, W.; Nguyen, C. Preparation of a Novel Flame Retardant Formulation for Cotton Fabric. Materials 2020, 13, 54. https://doi.org/10.3390/ma13010054
Nguyen HK, Sakai W, Nguyen C. Preparation of a Novel Flame Retardant Formulation for Cotton Fabric. Materials. 2020; 13(1):54. https://doi.org/10.3390/ma13010054
Chicago/Turabian StyleNguyen, Hung Kim, Wataru Sakai, and Congtranh Nguyen. 2020. "Preparation of a Novel Flame Retardant Formulation for Cotton Fabric" Materials 13, no. 1: 54. https://doi.org/10.3390/ma13010054
APA StyleNguyen, H. K., Sakai, W., & Nguyen, C. (2020). Preparation of a Novel Flame Retardant Formulation for Cotton Fabric. Materials, 13(1), 54. https://doi.org/10.3390/ma13010054




