Exploratory Full-Field Strain Analysis of Regenerated Bone Tissue from Osteoinductive Biomaterials
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
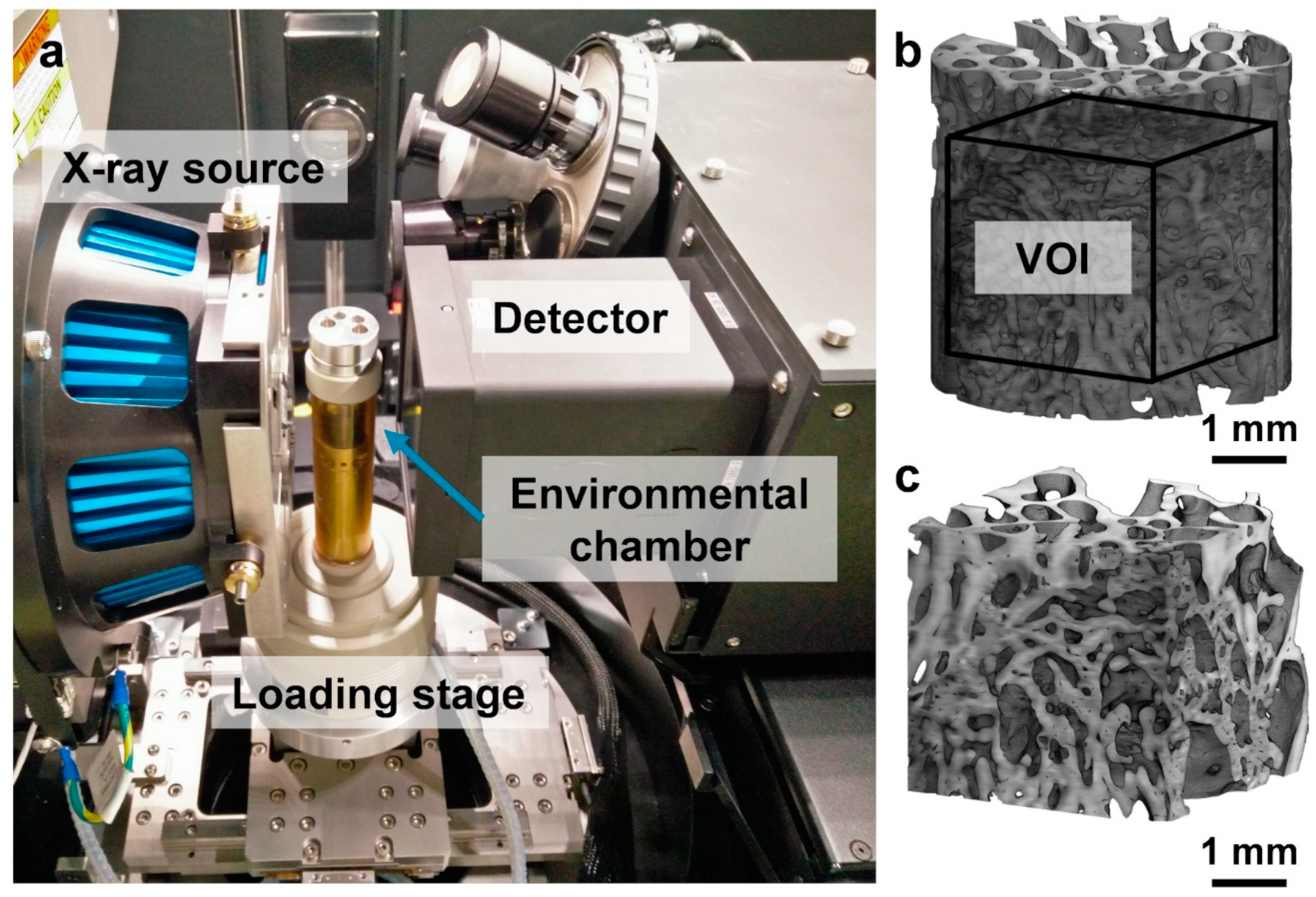
2.2. In Situ Mechanics and MicroCT Imaging
2.3. Mineral Density Distribution
2.4. Image Post-Processing
2.5. Morphometric Analysis
2.6. Digital Volume Correlation
2.7. Statistical Analysis
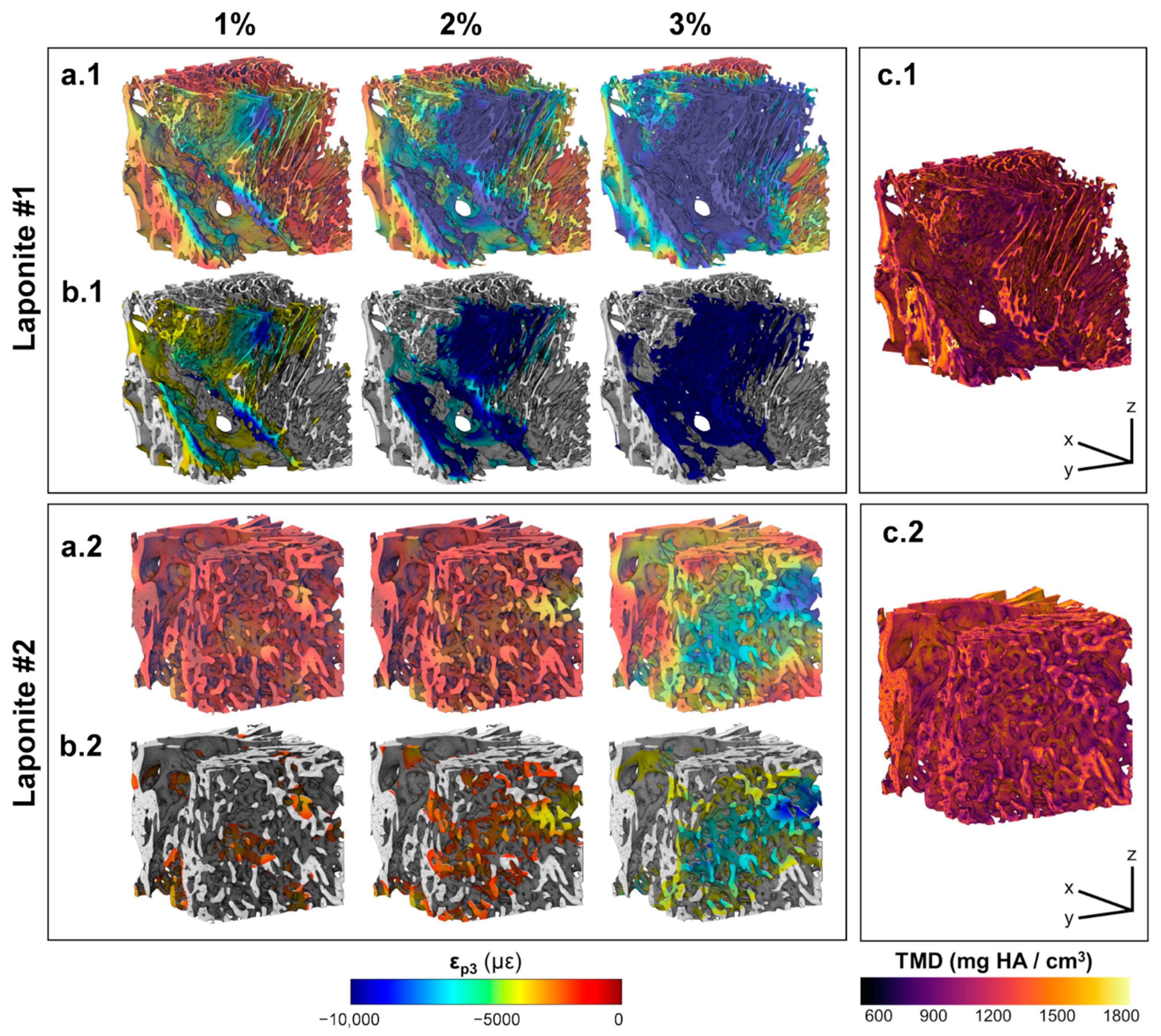
3. Results
3.1. In Situ Mechanical Testing and MicroCT Imaging
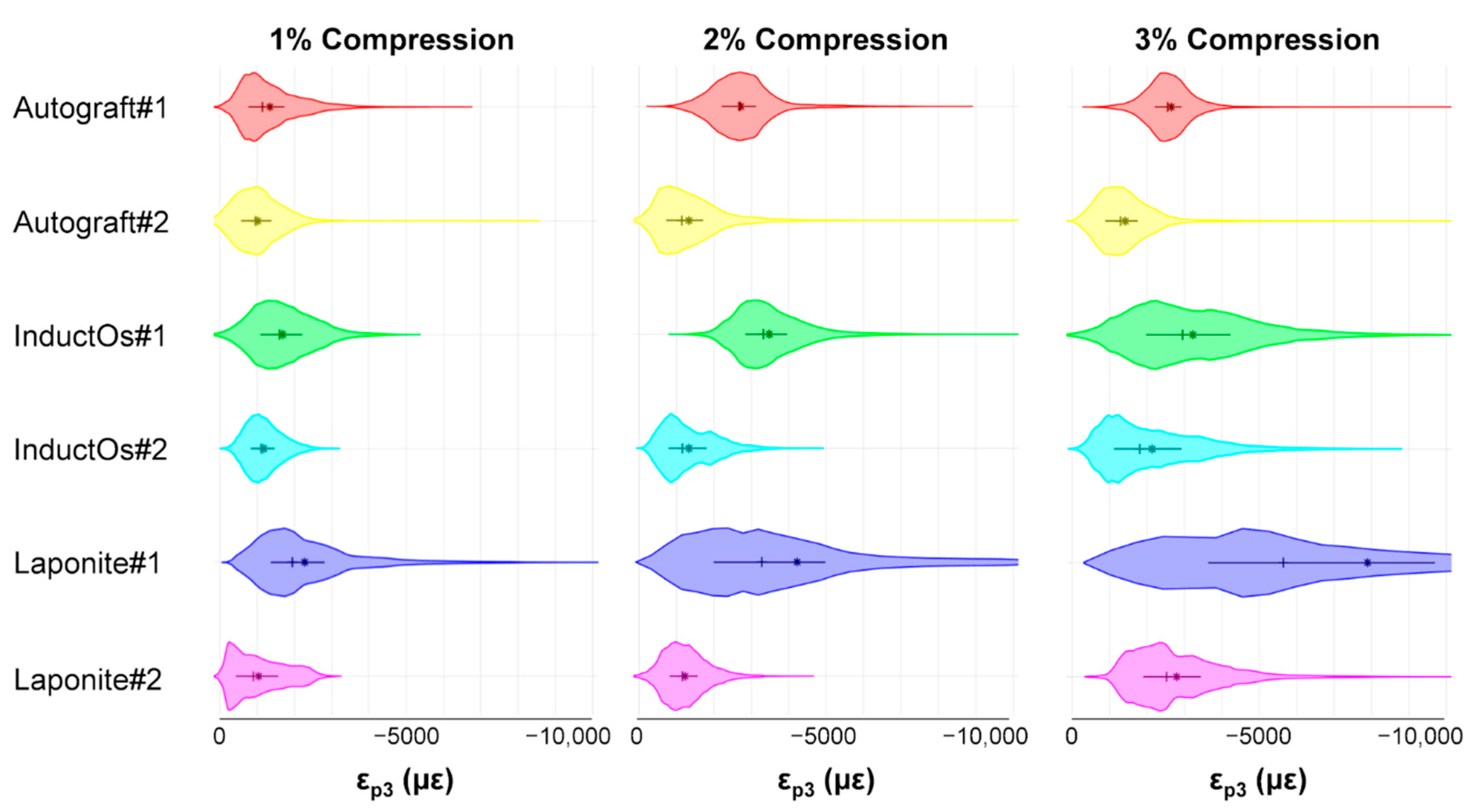
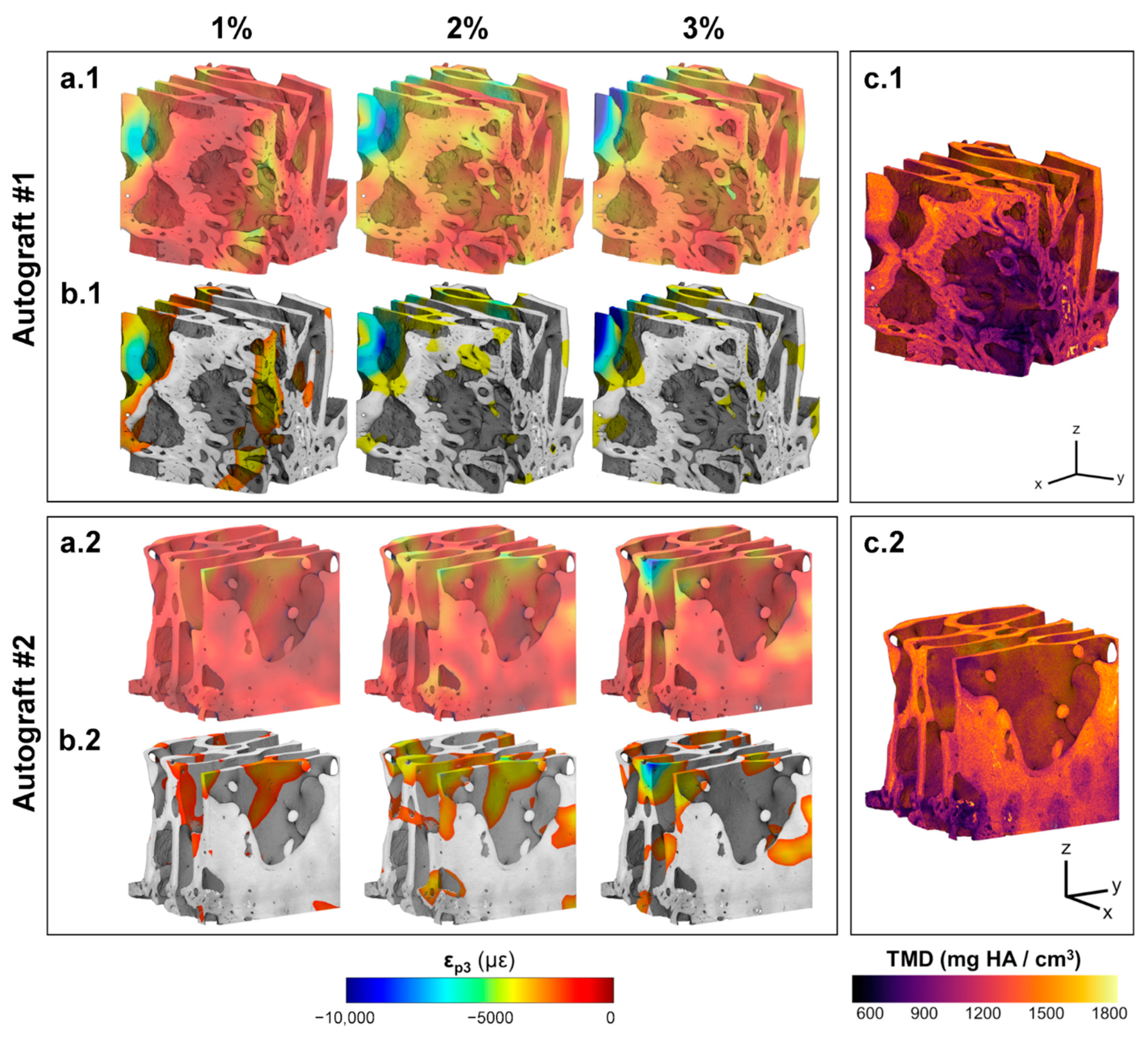
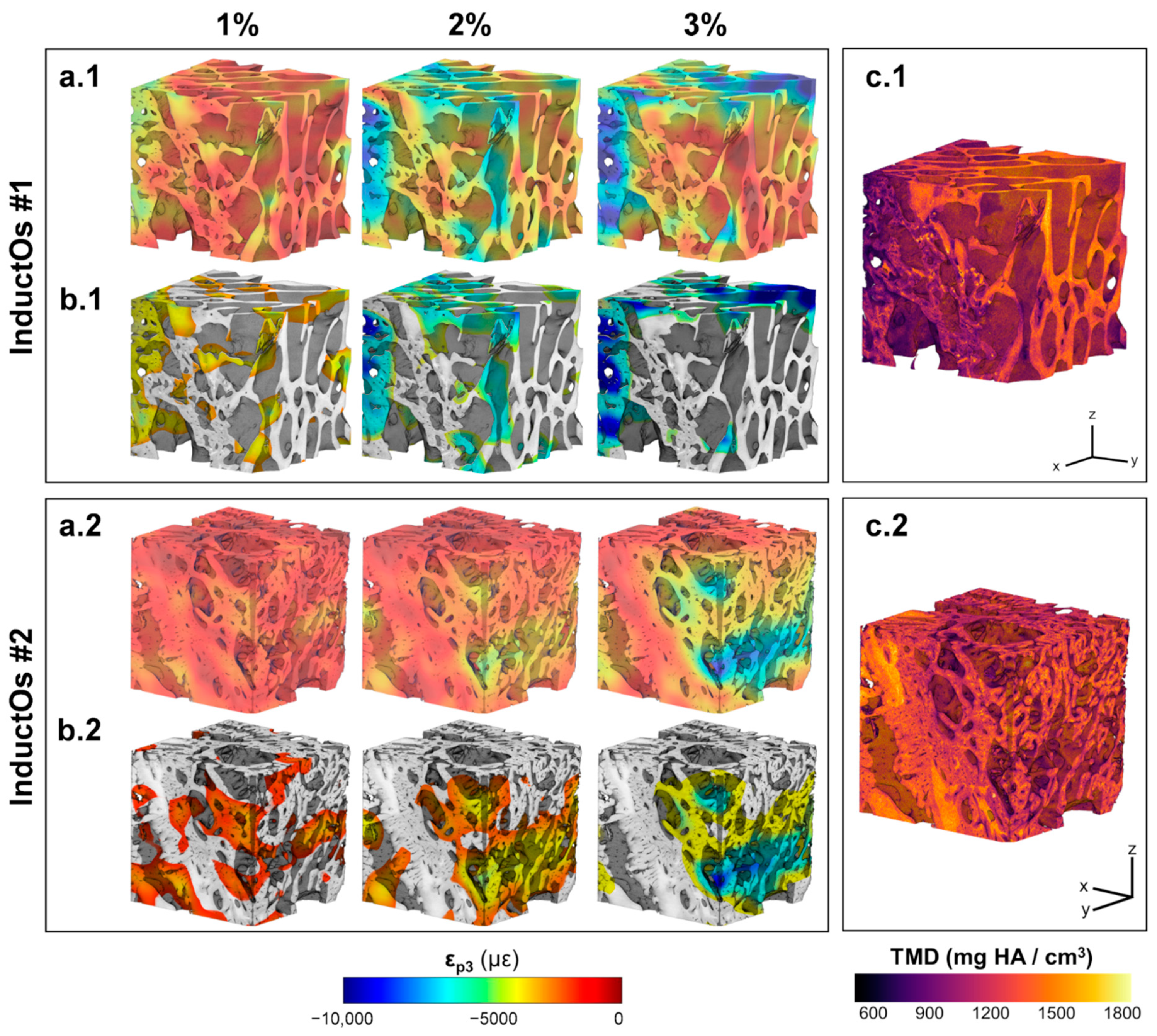
3.2. Digital Volume Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone injury and fracture healing biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar] [PubMed]
- Giannoudis, P.V.; Einhorn, T.A.; Marsh, D. Fracture healing: The diamond concept. Injury 2007, 38, 3–6. [Google Scholar] [CrossRef]
- Lasanianos, N.G.; Kanakaris, N.K.; Giannoudis, P.V. Current management of long bone large segmental defects. Orthop. Trauma 2010, 24, 149–163. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Bloemers, F.W.; Blokhuis, T.J.; Patka, P.; Bakker, F.C.; Wippermann, B.W.; Haarman, H.J.T.M. Autologous bone versus calcium-phosphate ceramics in treatment of experimental bone defects. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 66, 526–531. [Google Scholar] [CrossRef]
- Sen, M.K.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2007, 38, 2–7. [Google Scholar] [CrossRef]
- Schroeder, J.E.; Mosheiff, R. Tissue engineering approaches for bone repair: Concepts and evidence. Injury 2011, 42, 609–613. [Google Scholar] [CrossRef]
- Banwart, J.C.; Asher, M.A.; Hassanein, R.S. Iliac Crest Bone Graft Harvest Donor Site Morbidity: A Statistical Evaluation. Spine 1995, 20, 1055–1060. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Roy, M. Recent Advances in Bone Tissue Engineering Scaffolds. Trends Biotechnol. 2013, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Bishop, T.; Valerio, I.L.; Fisher, J.P.; Dean, D. The potential impact of bone tissue engineering in the clinic. Regen. Med. 2016, 11, 571–587. [Google Scholar] [CrossRef]
- Faßbender, M.; Minkwitz, S.; Strobel, C.; Schmidmaier, G.; Wildemann, B. Stimulation of bone healing by sustained bone morphogenetic protein 2 (BMP-2) delivery. Int. J. Mol. Sci. 2014, 15, 8539–8552. [Google Scholar] [CrossRef]
- Spector, J.A.; Luchs, J.S.; Mehrara, B.J.; Greenwald, J.A.; Smith, L.P.; Longaker, M.T. Expression of bone morphogenetic proteins during membranous bone healing. Plast. Reconstr. Surg. 2001, 107, 124–134. [Google Scholar] [CrossRef]
- Garrison, K.R.; Shemilt, I.; Donell, S.; Ryder, J.J.; Mugford, M.; Harvey, I.; Song, F.; Alt, V. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Boerckel, J.D.; Kolambkar, Y.M.; Dupont, K.M.; Uhrig, B.A.; Phelps, E.A.; Stevens, H.Y.; García, A.J.; Guldberg, R.E. Effects of protein dose and delivery system on BMP-mediated bone regeneration. Biomaterials 2011, 32, 5241–5251. [Google Scholar] [CrossRef]
- Geiger, M.; Li, R.H.; Friess, W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629. [Google Scholar] [CrossRef]
- Gibbs, D.M.R.; Black, C.R.M.; Hulsart-Billstrom, G.; Shi, P.; Scarpa, E.; Oreffo, R.O.C.; Dawson, J.I. Bone induction at physiological doses of BMP through localization by clay nanoparticle gels. Biomaterials 2016, 99, 16–23. [Google Scholar] [CrossRef]
- Carragee, E.J.; Chu, G.; Rohatgi, R.; Hurwitz, E.L.; Weiner, B.K.; Yoon, S.T.; Comer, G.; Kopjar, B. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J. Bone Joint Surg. Am. 2013, 95, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Bettencourt, A. Bone regeneration: Biomaterials as local delivery systems with improved osteoinductive properties. Mater. Sci. Eng. C 2018, 82, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Walsh, W.R.; Oliver, R.A.; Christou, C.; Lovric, V.; Walsh, E.R.; Prado, G.R.; Haider, T. Critical size bone defect healing using collagen-calcium phosphate bone graft materials. PLoS ONE 2017, 12, e0168883. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Jun, S.H.; Lee, E.J.; Jang, T.S.; Kim, H.E.; Jang, J.H.; Koh, Y.H. Bone morphogenic protein-2 (BMP-2) loaded hybrid coating on porous hydroxyapatite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, A.; Eap, S.; Richert, L.; Lemoine, S.; Kalaskar, D.; Demoustier-Champagne, S.; Atmani, H.; Mély, Y.; Fioretti, F.; Schlatter, G.; et al. Osteogenetic properties of electrospun nanofibrous PCL scaffolds equipped with chitosan-based nanoreservoirs of growth factors. Macromol. Biosci. 2014, 14, 45–55. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Lu, X.; Li, M.; Liu, H.; Xie, C.; Meng, F.; Jiang, O.; Li, C.; Zhi, W. BMP-2 encapsulated polysaccharide nanoparticle modified biphasic calcium phosphate scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2015, 103, 1520–1532. [Google Scholar] [CrossRef]
- Peña Fernández, M.; Witte, F.; Tozzi, G. Applications of X-ray computed tomography for the evaluation of biomaterial-mediated bone regeneration in critical-sized defects. J. Microsc. 2019. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Nazarian, A.; Snyder, B.D.; Zurakowski, D.; Müller, R. Quantitative micro-computed tomography: A non-invasive method to assess equivalent bone mineral density. Bone 2008, 43, 302–311. [Google Scholar] [CrossRef]
- Nazarian, A.; Müller, R. Time-lapsed microstructural imaging of bone failure behavior. J. Biomech. 2004, 37, 55–65. [Google Scholar] [CrossRef]
- Bay, B.K.; Smith, T.S.; Fyhrie, D.P.; Saad, M. Digital volume correlation: Three-dimensional strain mapping using X-ray tomography. Exp. Mech. 1999, 39, 217–226. [Google Scholar] [CrossRef]
- Bay, B.K. Methods and applications of digital volume correlation. J. Strain Anal. Eng. Des. 2008, 43, 745–760. [Google Scholar] [CrossRef]
- Gillard, F.; Boardman, R.; Mavrogordato, M.; Hollis, D.; Sinclair, I.; Pierron, F.; Browne, M. The application of digital volume correlation (DVC) to study the microstructural behaviour of trabecular bone during compression. J. Mech. Behav. Biomed. Mater. 2014, 29, 480–499. [Google Scholar] [CrossRef]
- Peña Fernández, M.; Cipiccia, S.; Dall’Ara, E.; Bodey, A.J.; Parwani, R.; Pani, M.; Blunn, G.W.; Barber, A.H.; Tozzi, G. Effect of SR-microCT radiation on the mechanical integrity of trabecular bone using in situ mechanical testing and digital volume correlation. J. Mech. Behav. Biomed. Mater. 2018, 88, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Christen, D.; Levchuk, A.; Schori, S.; Schneider, P.; Boyd, S.K.; Müller, R. Deformable image registration and 3D strain mapping for the quantitative assessment of cortical bone microdamage. J. Mech. Behav. Biomed. Mater. 2012, 8, 184–193. [Google Scholar] [CrossRef]
- Tozzi, G.; Danesi, V.; Palanca, M.; Cristofolini, L. Elastic Full-Field Strain Analysis and Microdamage Progression in the Vertebral Body from Digital Volume Correlation. Strain 2016, 52, 446–455. [Google Scholar] [CrossRef]
- Danesi, V.; Tozzi, G.; Cristofolini, L. Application of digital volume correlation to study the efficacy of prophylactic vertebral augmentation. Clin. Biomech. 2016, 39, 14–24. [Google Scholar] [CrossRef]
- Tozzi, G.; Zhang, Q.H.; Tong, J. 3D real-time micromechanical compressive behaviour of bone-cement interface: Experimental and finite element studies. J. Biomech. 2012, 45, 356–363. [Google Scholar] [CrossRef]
- Peña Fernández, M.; Dall’Ara, E.; Bodey, A.J.; Parwani, R.; Barber, A.H.; Blunn, G.W.; Tozzi, G. Full-Field Strain Analysis of Bone–Biomaterial Systems Produced by the Implantation of Osteoregenerative Biomaterials in an Ovine Model. ACS Biomater. Sci. Eng. 2019, 5, 2543–2554. [Google Scholar] [CrossRef]
- Arrabal, P.M.; Visser, R.; Santos-Ruiz, L.; Becerra, J.; Cifuentes, M. Osteogenic molecules for clinical applications: Improving the BMP-collagen system. Biol. Res. 2013, 46, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.C.; Dawson, J.I. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Doube, M.; Klosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Meth. 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ross, S.M. Peirce’s criterion for the elimination of suspect experimental data. J. Eng. Technol. 2003, 20, 1–12. [Google Scholar]
- Madi, K.; Tozzi, G.; Zhang, Q.H.; Tong, J.; Cossey, A.; Au, A.; Hollis, D.; Hild, F. Computation of full-field displacements in a scaffold implant using digital volume correlation and finite element analysis. Med. Eng. Phys. 2013, 35, 1298–1312. [Google Scholar] [CrossRef]
- Peña, M.; Ara, E.D.; Kao, A.P.; Bodey, A.J.; Karali, A.; Blunn, G.W.; Barber, A.H.; Tozzi, G.; Peña Fernández, M.; Dall’Ara, E.; et al. Preservation of bone tissue integrity with temperature control for in situ SR-microCT experiments. Materials 2018, 11, 2155. [Google Scholar] [CrossRef]
- Peña Fernández, M.; Barber, A.H.; Blunn, G.W.; Tozzi, G. Optimisation of digital volume correlation computation in SR-microCT images of trabecular bone and bone-biomaterial systems. J. Microsc. 2018, 272, 213–228. [Google Scholar] [CrossRef]
- Palanca, M.; Cristofolini, L.; Dall’Ara, E.; Curto, M.; Innocente, F.; Danesi, V.; Tozzi, G. Digital volume correlation can be used to estimate local strains in natural and augmented vertebrae: An organ-level study. J. Biomech. 2016, 49, 3882–3890. [Google Scholar] [CrossRef]
- Friess, W.; Uludag, H.; Foskett, S.; Biron, R.; Sargeant, C. Characterization of absorbable collagen sponges as recombinant human bone morphogenetic protein-2 carriers. Int. J. Pharm. 1999, 185, 51–60. [Google Scholar] [CrossRef]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the delivery of growth factors and other therapeutic agents in tissue engineering approaches to bone regeneration. Front. Pharmacol. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cowin, S.C. The relationship between the elasticity tensor and the fabric tensor. Mech. Mater. 1985, 4, 137–147. [Google Scholar] [CrossRef]
- Zysset, P.K.; Curnier, A. An alternative model for anisotropic elasticity based on fabric tensors. Mech. Mater. 1995, 21, 243–250. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Niebur, G.L. Effects of Loading Orientation on the Morphology of the Predicted Yielded Regions in Trabecular Bone. Ann. Biomed. Eng. 2009, 37, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Öhman, C.; Baleani, M.; Perilli, E.; Dall’Ara, E.; Tassani, S.; Baruffaldi, F.; Viceconti, M. Mechanical testing of cancellous bone from the femoral head: Experimental errors due to off-axis measurements. J. Biomech. 2007, 40, 2426–2433. [Google Scholar] [CrossRef]
- Morgan, E.F.; Keaveny, T.M. Dependence of yield strain of human trabecular bone on anatomic site. J. Biomech. 2001, 34, 569–577. [Google Scholar] [CrossRef]
- Morgan, E.F.; Bayraktar, H.H.; Keaveny, T.M. Trabecular bone modulus–density relationships depend on anatomic site. J. Biomech. 2003, 36, 897–904. [Google Scholar] [CrossRef]
- Nazarian, A.; Muller, J.; Zurakowski, D.; Müller, R.; Snyder, B.D. Densitometric, morphometric and mechanical distributions in the human proximal femur. J. Biomech. 2007, 40, 2573–2579. [Google Scholar] [CrossRef]
- Linde, F.; Hvid, I.; Madsen, F. The effect of specimen geometry on the mechanicla behaviour of trabecular bone specimens. J. Biomech. 1992, 25, 359–368. [Google Scholar] [CrossRef]
- Harrison, N.M.; McHugh, P.E. Comparison of trabecular bone behavior in core and whole bone samples using high-resolution modeling of a vertebral body. Biomech. Model. Mechanobiol. 2010, 9, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Un, K.; Bevill, G.; Keaveny, T.M. The effects of side-artifacts on the elastic modulus of trabecular bone. J. Biomech. 2006, 39, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P.J.; Lanyon, L.E. Mechanical strain and bone cell function: A review. Osteoporos. Int. 2002, 13, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, H.H.; Morgan, E.F.; Niebur, G.L.; Morris, G.E.; Wong, E.K.; Keaveny, T.M. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 2004, 37, 27–35. [Google Scholar] [CrossRef]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar]
- Carter, D.R. Mechanical loading history and skeletal biology. J. Biomech. 1987, 20, 1095–1109. [Google Scholar] [CrossRef]
- Salisbury Palomares, K.T.; Gleason, R.E.; Mason, Z.D.; Cullinane, D.M.; Einhorn, T.A.; Gerstenfeld, L.C.; Morgan, E.F. Mechanical stimulation alters tissue differentiation and molecular expression during bone healing. J. Orthop. Res. 2009, 27, 1123–1132. [Google Scholar] [CrossRef]
- Epari, D.R.; Duda, G.N.; Thompson, M.S. Mechanobiology of bone healing and regeneration: In vivo models. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1543–1553. [Google Scholar] [CrossRef]
- Betts, D.C.; Müller, R. Mechanical regulation of bone regeneration: Theories, models, and experiments. Front. Endocrinol. (Lausanne) 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Öhman, C.; Dall’Ara, E.; Baleani, M.; Jan, S.V.S.; Viceconti, M. The effects of embalming using a 4% formalin solution on the compressive mechanical properties of human cortical bone. Clin. Biomech. 2008, 23, 1294–1298. [Google Scholar] [CrossRef]
- Wieding, J.; Mick, E.; Wree, A.; Bader, R. Influence of three different preservative techniques on the mechanical properties of the ovine cortical bone. Acta Bioeng. Biomech. 2015, 17, 137–146. [Google Scholar] [PubMed]


| Specimen | Stiffness (N/mm) | Avg-TMD (mgHA/cm3) | Peak-TMD (mgHA/cm3) | BV/TV (%) | Tb.Th (µm) | Tb.Sp (µm) |
|---|---|---|---|---|---|---|
| Autograft #1 | 249.3 | 1207.8 | 1279.5 | 46.1 | 222 ± 71 | 484 ± 168 |
| Autograft #2 | 212.1 | 1247.9 | 1314.8 | 33.9 | 267 ± 72 | 736 ± 246 |
| InductOs #1 | 547.6 | 1107.2 | 1185.4 | 52.2 | 233 ± 68 | 477 ± 183 |
| InductOs #2 | 322.6 | 1183.1 | 1232.5 | 54.6 | 147 ± 41 | 338 ± 198 |
| Laponite #1 | 131.9 | 1152.3 | 1220.7 | 22.4 | 107 ± 47 | 466 ± 282 |
| Laponite #2 | 363.0 | 1112.5 | 1185.4 | 45.4 | 141 ± 35 | 339 ± 189 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña Fernández, M.; Black, C.; Dawson, J.; Gibbs, D.; Kanczler, J.; Oreffo, R.O.C.; Tozzi, G. Exploratory Full-Field Strain Analysis of Regenerated Bone Tissue from Osteoinductive Biomaterials. Materials 2020, 13, 168. https://doi.org/10.3390/ma13010168
Peña Fernández M, Black C, Dawson J, Gibbs D, Kanczler J, Oreffo ROC, Tozzi G. Exploratory Full-Field Strain Analysis of Regenerated Bone Tissue from Osteoinductive Biomaterials. Materials. 2020; 13(1):168. https://doi.org/10.3390/ma13010168
Chicago/Turabian StylePeña Fernández, Marta, Cameron Black, Jon Dawson, David Gibbs, Janos Kanczler, Richard O. C. Oreffo, and Gianluca Tozzi. 2020. "Exploratory Full-Field Strain Analysis of Regenerated Bone Tissue from Osteoinductive Biomaterials" Materials 13, no. 1: 168. https://doi.org/10.3390/ma13010168
APA StylePeña Fernández, M., Black, C., Dawson, J., Gibbs, D., Kanczler, J., Oreffo, R. O. C., & Tozzi, G. (2020). Exploratory Full-Field Strain Analysis of Regenerated Bone Tissue from Osteoinductive Biomaterials. Materials, 13(1), 168. https://doi.org/10.3390/ma13010168






