Incommensurately Modulated Crystal Structure and Photoluminescence Properties of Eu2O3- and P2O5-Doped Ca2SiO4 Phosphor
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Constituent Phases and Crystal Structure of IC-Phase
3.2. PL Properties Associated with Phase Compositions
4. Conclusions
- The β- and α’L-phases coexisted for the specimen annealed at 1473 K. With increasing annealing temperature, the relative amount of the IC-phase with respect to the β-phase steadily increased. The specimen annealed at 1773 K was composed exclusively of the IC-phase.
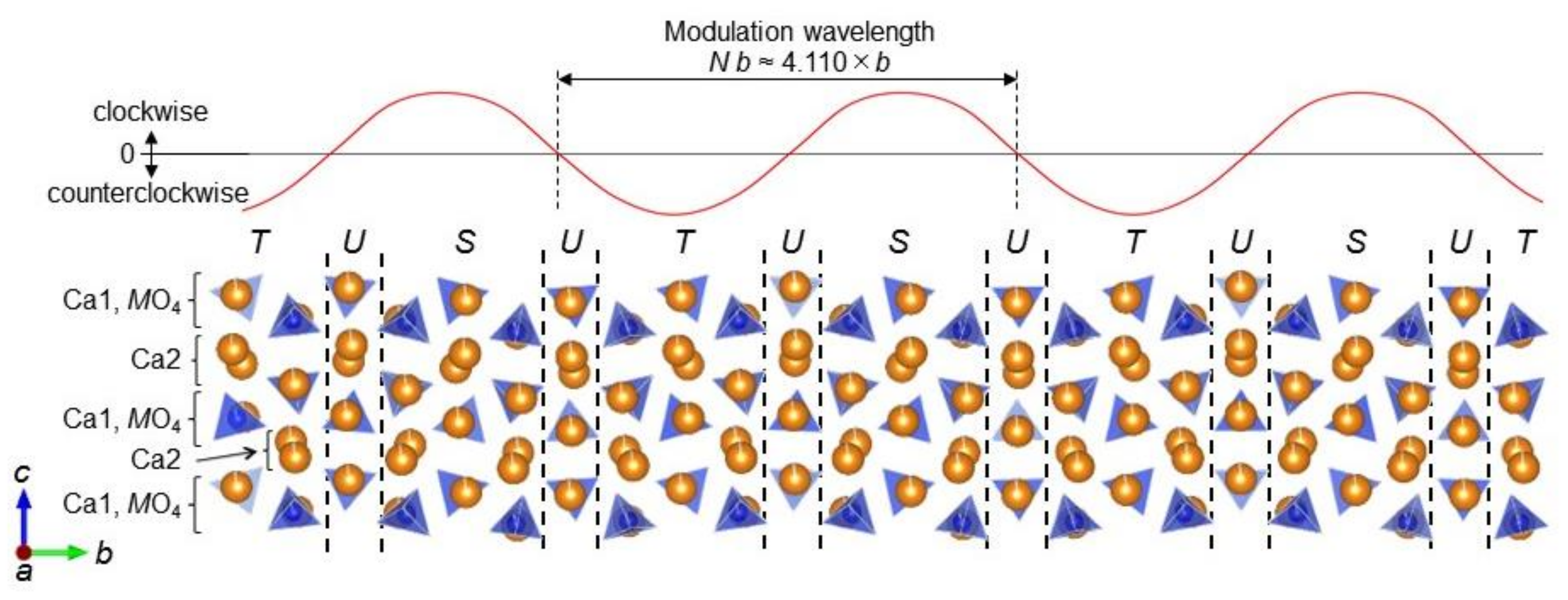
- The incommensurately modulated crystal structure was determined using a (3 + 1)-dimensional description based on the superspace group Pnma(0β0)00s. It was composed of β-phase like layers (S, S’’, T, and T’’) and the interlayer (U). The S and S’’ layers included the [MO4] tetrahedra (M = Si or P) tilted clockwise when viewed along [00]. Those of T and T’’ contained the tetrahedra tilted counterclockwise. The interlayer U was characterized by a slightly tilted tetrahedra. The incommensurate modulation, with modulation wavevector of 0.2433(2) × b*, was induced by the long-range stacking order of these layers.
- The PL intensity was the highest for the specimen consisting of both β- and α’L-phases. As the relative amount of the IC-phase increased with respect to the coexisting β-phase, the PL intensity steadily decreased. The eccentricity of the Eu3+ position in the crystal structures of the β- and IC-phases could be closely related to the PL intensities.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Crystal Structure Data
References
- Choi, S.-W.; Hong, S.-H. Characterization of Ca2SiO4: Eu2+ phosphor synthesized by polymeric precursor process. J. Am. Ceram. Soc. 2009, 92, 2025–2028. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Jo, D.S.; Senthil, K.; Tezuka, S.; Kakihana, M.; Toda, K.; Masaki, T.; Yoon, D.H. Synthesis of high efficient Ca2SiO4:Eu2+ green emitting phosphor by a liquid phase precursor method. J. Solid State Chem. 2012, 189, 68–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Li, Y.; Seo, H.J. Monitoring of hydroxyapatite conversion by luminescence intensity of Eu3+ ions during mineralization of Eu3+-doped β-Ca2SiO4. Opt. Mater. 2014, 37, 525–530. [Google Scholar] [CrossRef]
- Wei, F.; Jia, Q. Massive production of A2SiO4:Eu3+ and A2SiO4: Eu2+ (A = Ca, Sr, Ba) microspheres and luminescent properties. Superlattices Microstruct. 2015, 82, 11–17. [Google Scholar] [CrossRef]
- Sato, Y.; Kato, H.; Kobayashi, M.; Masaki, T.; Yoon, D.H.; Kakihana, M. Tailoring of deep-red luminescence in Ca2SiO4: Eu2+. Angew. Chem. Int. Ed. 2014, 53, 7756–7759. [Google Scholar] [CrossRef] [PubMed]
- Furuya, S.; Nakano, H.; Yokoyama, N.; Banno, H.; Fukuda, K. Enhancement of photoluminescence intensity and structural change by doping of P5+ ion for Ca2−x/2(Si1−xPx)O4: Eu2+ green phosphor. J. Alloy. Compd. 2016, 658, 147–151. [Google Scholar] [CrossRef]
- Nakano, H.; Yokoyama, N.; Banno, H.; Fukuda, K. Enhancement of PL intensity and formation of core-shell structure in annealed Ca2−x/2(Si1−xPx)O4:Eu2+ phosphor. Mater. Res. Bull. 2016, 83, 502–506. [Google Scholar] [CrossRef]
- Nakano, H.; Kamimoto, K.; Yokoyama, N.; Fukuda, K. The effect of heat treatment on the emission color of P-doped Ca2SiO4 phosphor. Materials 2017, 10, 1000. [Google Scholar] [CrossRef] [Green Version]
- Nakano, H.; Ozono, K.; Hayashi, H.; Fujihara, S. Synthesis and luminescent properties of a new Eu3+-doped Li1+x(Ta1−zNbz)1−xTixO3 Red phosphor. J. Am. Ceram. Soc. 2012, 95, 2795–2797. [Google Scholar] [CrossRef]
- Ichioka, H.; Furuya, S.; Asaka, T.; Nakano, H.; Fukuda, K. Crystal structures and enhancement of photoluminescence intensities by effective doping for lithium tantalate phosphors. Powder Diffr. 2015, 30, 326–332. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997. [Google Scholar]
- Fukuda, K.; Maki, I.; Ito, S. Thermoelastic behavior in Ca2SiO4 solid solutions. J. Am. Ceram. Soc. 1996, 79, 2925–2928. [Google Scholar] [CrossRef]
- Fukuda, K.; Maki, I.; Ito, S. Thermal hysteresis for the αL’ ↔ β transformations in strontium oxide-doped dicalcium silicates. J. Am. Ceram. Soc. 1996, 79, 2969–2970. [Google Scholar] [CrossRef]
- Fukuda, K.; Maki, I.; Ito, S.; Miyake, T. Structural change in phosphorus-bearing dicalcium silicates. J. Ceram. Soc. Jpn. 1997, 105, 117–121. [Google Scholar] [CrossRef] [Green Version]
- Jost, K.H.; Ziemer, B.; Seydel, R. Redetermination of the structure of β-dicalcium silicate. Acta Crystallogr. 1977, 33, 1696–1700. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Wang, S.; Liu, S.; Du, P.; Ye, Z.; Geng, X.; Cheng, X. Phase identification of γ- and β-Ca2SiO4 via the rear-earth fluorescence probe. J. Phys. Chem. C 2019, 123, 13877–13884. [Google Scholar] [CrossRef]
- Saalfeld, H.; Klaska, K.H. The crystal structure of 6 Ca2SiO4·1Ca3 (PO4)2. Z. Kristallogr.-Cryst. Mater. 1981, 155, 65–73. [Google Scholar] [CrossRef]
- Fukuda, K.; Maki, I. Transitional phase of Ca2SiO4 solid solution with incommensurate superstructure. J. Am. Ceram. Soc. 1989, 72, 2204–2207. [Google Scholar] [CrossRef]
- Fukuda, K.; Maki, I.; Ito, S.; Yoshida, H.; Aoki, K. Structure and microtexture changes in phosphorous-bearing Ca2SiO4 solid solutions. J. Am. Ceram. Soc. 1994, 77, 2615–2619. [Google Scholar] [CrossRef]
- Hiramatsu, Y.; Michiue, Y.; Funahashi, S.; Hirosaki, N.; Banno, H.; Urushihara, D.; Asaka, T.; Fukuda, K. Crystal structure and photoluminescence properties of an incommensurate phase in EuO- and P2O5-doped Ca2SiO4. Inorg. Chem. 2019, 58, 6155–6160. [Google Scholar] [CrossRef]
- Smaalen, S.V. Incommensurate Crystallography; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Janssen, T.; Chapuis, G.; de Boisseu, M. Aperiodic Crystals; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Brindley, G.W. The effect of grain or particle size on X-ray reflections from mixed powders and alloys, considered in relation to the quantitative determination of crystalline substances by X-ray methods. Philos. Mag. 1945, 36, 347–369. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Udagawa, S.; Urabe, K.; Yano, T.; Takada, K.; Natsume, M. Studies on the Dusting of Ca2SiO4—The Crystal Structure of α’L-Ca2SiO4. Proc. Jpn. Cem. Eng. Assoc. 1979, 33, 35–37. [Google Scholar]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system JANA 2006: General features. Z. Kristallogr.-Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Momma, K.; Isumi, F. VESTA 3 for three-demensional visualization of crystal, Volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Balic-Zunic, T.; Vickovic, I. IVTON—A program for the calculation of geometrical aspects of crystal structures and some crystal chemical applications. J. Appl. Crystallogr. 1996, 29, 305–306. [Google Scholar] [CrossRef]



| Phase | β | α’L | IC | |
|---|---|---|---|---|
| K | ||||
| 1473 | 70.7 mol % | 29.3 mol % | - | |
| 1573 | 18.9 mol % | - | 81.1 mol % | |
| 1673 | 8.5 mol % | - | 91.5 mol % | |
| 1773 | - | - | 100 mol % | |
| Chemical Formula | (Ca1.950Eu3+0.013☐0.037)(Si0.940P0.060)O4 |
|---|---|
| Crystal system | Orthorhombic |
| Superspace group | Pnma(0β0)00s |
| a/nm | 0.68004(2) |
| b/nm | 0.54481(2) |
| c/nm | 0.93956(3) |
| Modulation wavevector | 0.2433(2) × b* |
| V/nm3 | 0.34810(2) |
| Z | 4 |
| Dx/Mgm−3 | 3.289 |
| Site | x | y | z | Uiso (×10−2 nm2) | sof |
|---|---|---|---|---|---|
| Ca1 | 0.1680(3) | 1/4 | 0.4243(4) | 0.0270(10) | Ca/Eu: 0.9750/0.0065 |
| Ca2 | 0.4935(3) | 1/4 | 0.7106(2) | 0.0139(7) | Ca/Eu: 0.9750/0.0065 |
| M | 0.2240(4) | 1/4 | 0.0755(6) | 0.0134(10) | Si/P: 0.940/0.060 |
| O1 | 0.3213(12) | 1/4 | 0.9305(9) | 0.025(3) | 1 |
| O2 | 0.2994(8) | 0.0092(12) | 0.1471(7) | 0.0181(18) | 1 |
| O3 | −0.0059(10) | 1/4 | 0.0731(13) | 0.038(3) | 1 |
| Site | t | n | Ca–O Max. (nm) | Eccentricity (nm) | Layer |
|---|---|---|---|---|---|
| Ca1 | 0.189 | 7 | 0.280 | 0.054 | U |
| Ca1 | 0.189 | 6 | 0.267 | 0.105 | U |
| Ca1 | 0.689 | 8 | 0.294 | 0.041 | U |
| Ca1 | 0.689 | 7 | 0.273 | 0.031 | U |
| Ca1 | 0.439 | 7 | 0.280 | 0.041 | S |
| Ca1 | 0.939 | 7 | 0.280 | 0.041 | T |
| Ca2 | 0.189 | 7 | 0.259 | 0.009 | U |
| Ca2 | 0.689 | 6 | 0.258 | 0.040 | U |
| Ca2 | 0.439 | 8 | 0.279 | 0.023 | S |
| Ca2 | 0.939 | 8 | 0.279 | 0.023 | T |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, H.; Ando, S.; Kamimoto, K.; Hiramatsu, Y.; Michiue, Y.; Hirosaki, N.; Fukuda, K. Incommensurately Modulated Crystal Structure and Photoluminescence Properties of Eu2O3- and P2O5-Doped Ca2SiO4 Phosphor. Materials 2020, 13, 58. https://doi.org/10.3390/ma13010058
Nakano H, Ando S, Kamimoto K, Hiramatsu Y, Michiue Y, Hirosaki N, Fukuda K. Incommensurately Modulated Crystal Structure and Photoluminescence Properties of Eu2O3- and P2O5-Doped Ca2SiO4 Phosphor. Materials. 2020; 13(1):58. https://doi.org/10.3390/ma13010058
Chicago/Turabian StyleNakano, Hiromi, Shota Ando, Konatsu Kamimoto, Yuya Hiramatsu, Yuichi Michiue, Naoto Hirosaki, and Koichiro Fukuda. 2020. "Incommensurately Modulated Crystal Structure and Photoluminescence Properties of Eu2O3- and P2O5-Doped Ca2SiO4 Phosphor" Materials 13, no. 1: 58. https://doi.org/10.3390/ma13010058





