A Comprehensive Review on Bio-Nanomaterials for Medical Implants and Feasibility Studies on Fabrication of Such Implants by Additive Manufacturing Technique
Abstract
1. Introduction
2. Nanocomposites Used for Biomedical Applications
2.1. Materials Compatible with Medical Implants
2.2. Nanocomposites with Metallic-Based Nanofillers
2.3. Nanocomposites with Ceramic Nanofillers
2.4. Nanocomposites with Carbon-Based Nanofillers
2.5. Nanocomposites with Cellulose-Based Nanofillers
3. Traditional Methods for Nanocomposite Synthesis for Biomedical Applications
3.1. Sol-Gel Technologies
3.2. Thermally Induced Phase Separation (Freeze-Drying)
3.3. Electrospinning
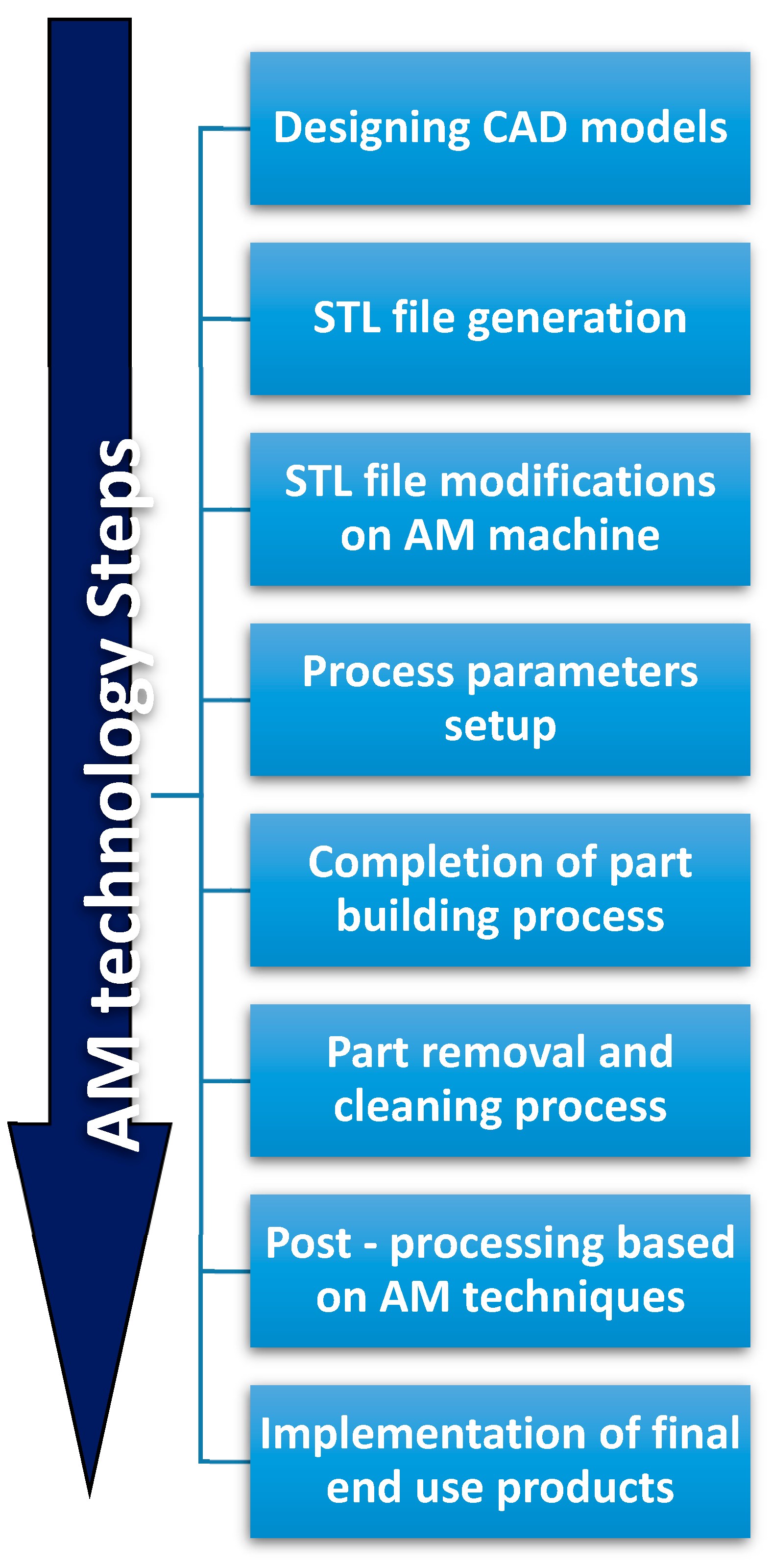
4. Rapid Prototyping and Additive Manufacturing Methods
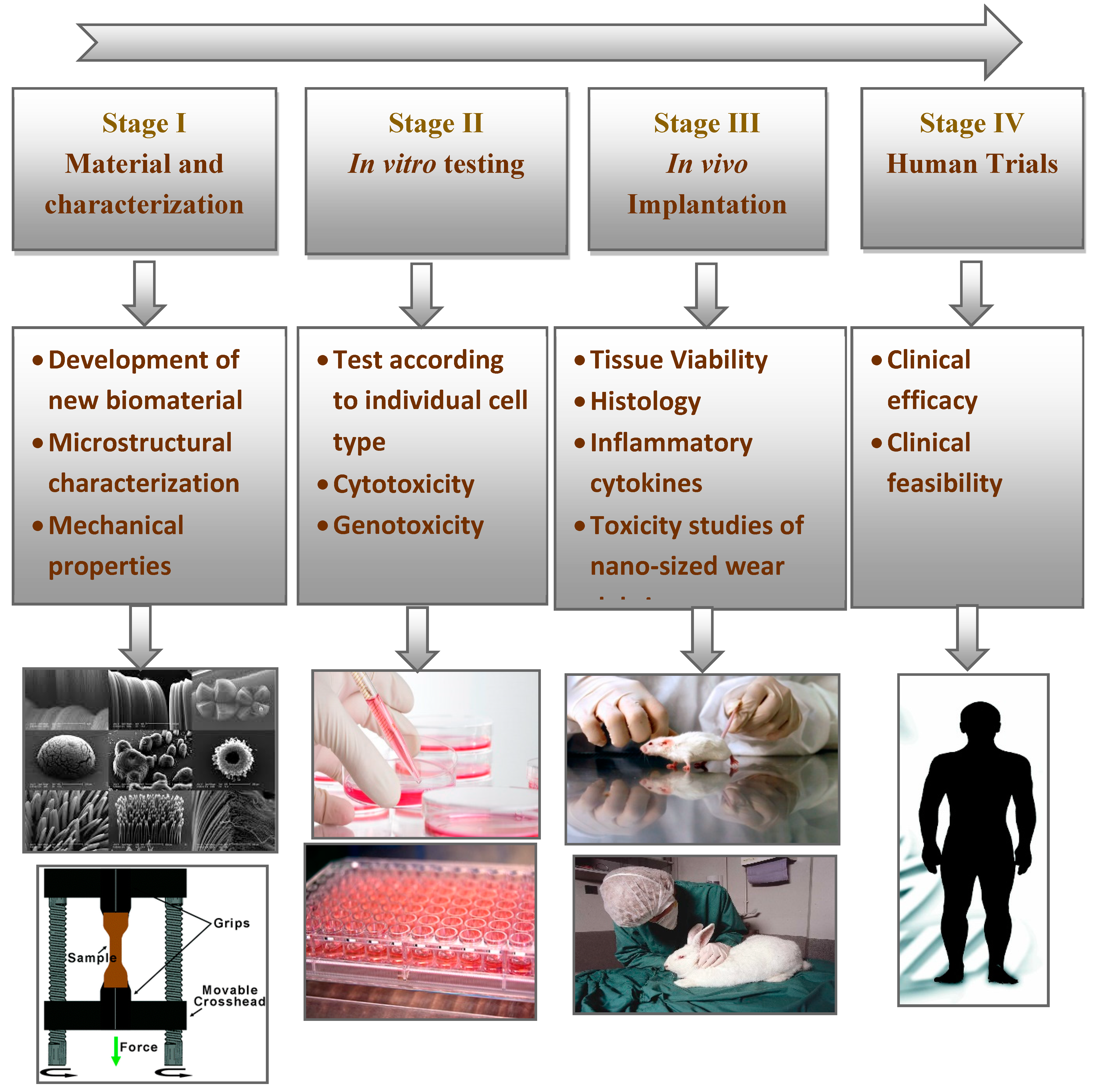
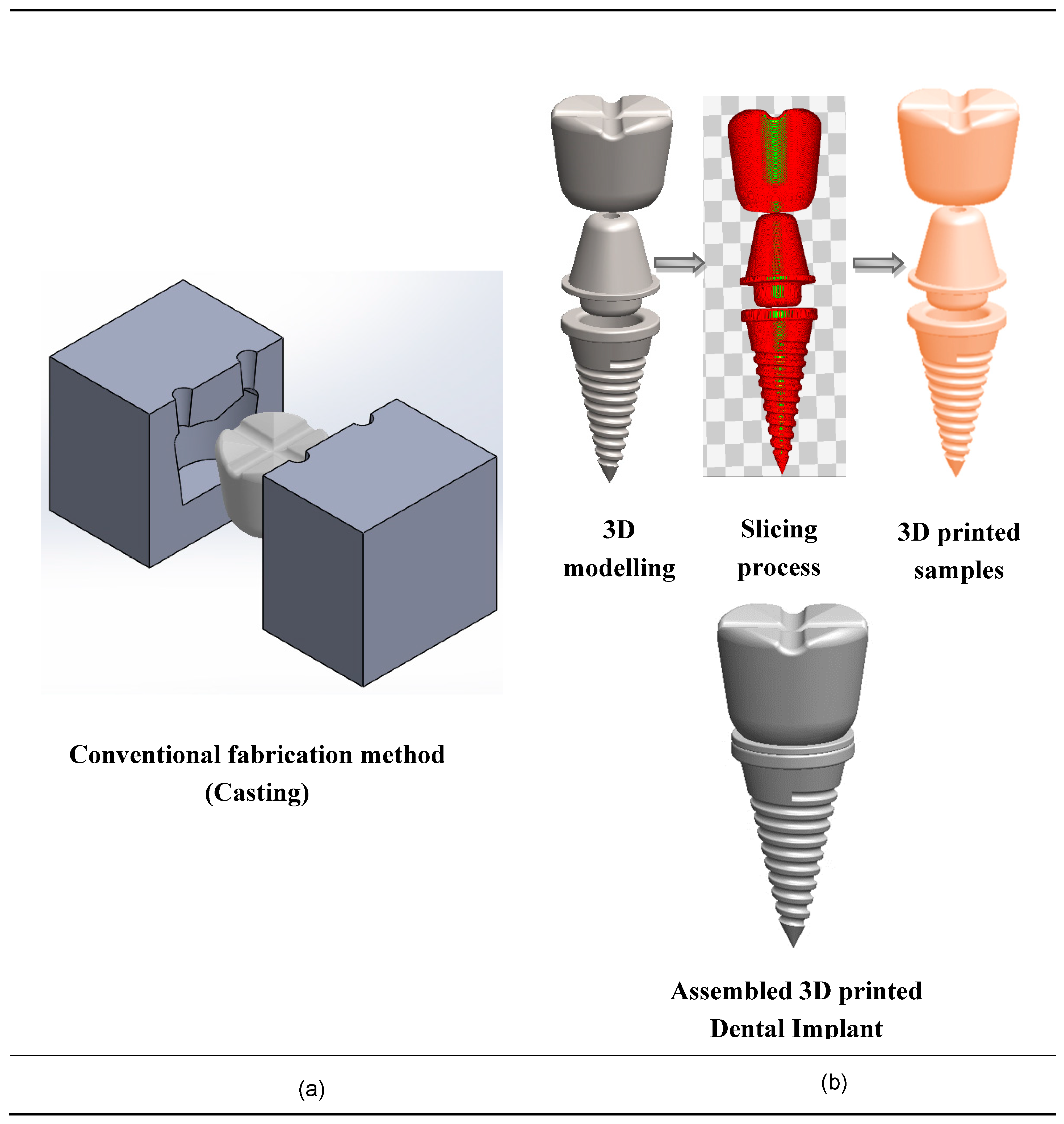
5. Additive Processing of Nanocomposites for Medical Implants
6. Current Limitations in Additive Manufacturing of Implantable Devices
7. Medical Industry Needs and Future Directions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Calais, T.; Bancaud, A.; Estève, A.; Rossi, C. Correlation between DNA Self-Assembly Kinetics, Microstructure, and Thermal Properties of Tunable Highly Energetic Al–CuO Nanocomposites for Micropyrotechnic Applications. ACS Appl. Nano Mater. 2018, 1, 4716–4725. [Google Scholar] [CrossRef]
- Blum, A.P.; Kammeyer, J.K.; Rush, A.M.; Callmann, C.E.; Hahn, M.E.; Gianneschi, N.C. Stimuli-Responsive Nanomaterials for Biomedical Applications. J. Am. Chem. Soc. 2015, 137, 2140–2154. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.; Moon, K.S. Nanomaterials for microelectronic and bio-packaging. In Nano-Bio-Electronic, Photonic and MEMS Packaging; Springer: Boston, MA, USA, 2010; pp. 1–17. [Google Scholar] [CrossRef]
- Boyett, M.R. ‘And the beat goes on’ The cardiac conduction system: The wiring system of the heart. Exp. Physiol. 2009, 94, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Jagur-Grodzinski, J. Polymers for tissue engineering, medical devices, and regenerative medicine. Concise general review of recent studies. Polym. Adv. Technol. 2006, 17, 395–418. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Dutra, G.V.S.; Neto, W.S.; Dutra, J.P.S.; Machado, F. Implantable medical devices and tissue engineering: An overview of manufacturing processes and the use of polymeric matrices for manufacturing and coating their surfaces. Curr. Med. Chem. 2018, 25. [Google Scholar] [CrossRef]
- Parthasarathy, J.; Starly, B.; Raman, S. A design for the additive manufacture of functionally graded porous structures with tailored mechanical properties for biomedical applications. J. Manuf. Process. 2011, 13, 160–170. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Quan, Z.; Wu, A.; Keefe, M.; Qin, X.; Yu, J.; Suhr, J.; Byun, J.H.; Kim, B.S.; Chou, T.W. Additive manufacturing of multi-directional preforms for composites: Opportunities and challenges. Mater. Today 2015, 18, 503–512. [Google Scholar] [CrossRef]
- Francis, V.; Jain, P.K. Advances in nanocomposite materials for additive manufacturing. Int. J. Rapid Manuf. 2015, 5, 215. [Google Scholar] [CrossRef]
- Carrow, J.K.; Gaharwar, A.K. Bioinspired Polymeric Nanocomposites for Regenerative Medicine. Macromol. Chem. Phys. 2015, 216, 248–264. [Google Scholar] [CrossRef]
- Hermawan, H.; Ramdan, D.; Djuansjah, J.R. Metals for Biomedical Applications; Ch. 17; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Klee, D.; Höcker, H. Polymers for Biomedical Applications: Improvement of the Interface Compatibility. Adv. Polym. Sci. 1999, 149, 1–57. [Google Scholar]
- Habibovic, P.; Barrère, F.; Blitterswijk, C.A.; Groot, K.; Layrolle, P. Biomimetic Hydroxyapatite Coating on Metal Implants. J. Am. Ceram. Soc. 2004, 85, 517–522. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139. [Google Scholar] [CrossRef]
- Pereira, I.H.; Ayres, E.; Patrício, P.S.; Góes, A.M.; Gomide, V.S.; Junior, E.P.; Oréfice, R.L. Photopolymerizable and injectable polyurethanes for biomedical applications: Synthesis and biocompatibility. Acta Biomater. 2010, 6, 3056–3066. [Google Scholar] [CrossRef]
- Guo, B.; Glavas, L.; Albertsson, A.-C. Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Schmidt, C.L.; Skarstad, P.M. The future of lithium and lithium-ion batteries in implantable medical devices. J. Power Sources 2001, 97–98, 742–746. [Google Scholar] [CrossRef]
- Wu, S.; Weng, Z.; Liu, X.; Yeung, K.W.K.; Chu, P.K. Functionalized TiO2 Based Nanomaterials for Biomedical Applications. Adv. Funct. Mater. 2014, 24, 5464–5481. [Google Scholar] [CrossRef]
- Choi, A.H.; Ben-Nissan, B. Calcium Phosphate Nanocomposites for Biomedical and Dental Applications: Recent Developments. In Handbook of Composites from Renewable Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 423–450. [Google Scholar] [CrossRef]
- Smith, S.E.; Snider, C.L.; Gilley, D.R.; Grant, D.N.; Sherman, S.L.; Ulery, B.D.; Grant, D.A.; Grant, S.A. Homogenized Porcine Extracellular Matrix Derived Injectable Tissue Construct with Gold Nanoparticles for Musculoskeletal Tissue Engineering Applications. J. Biomater. Nanobiotechnol. 2017, 8, 125–143. [Google Scholar] [CrossRef]
- Bhowmick, S.; Koul, V. Assessment of PVA/silver nanocomposite hydrogel patch as antimicrobial dressing scaffold: Synthesis, characterization and biological evaluation. Mater. Sci. Eng. C 2016, 59, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Mandal, M.; Upadhyay, A.; Chattopadhyay, P.; Karak, N. Bio-based hyperbranched polyurethane/Fe3 O4 nanocomposites: Smart antibacterial biomaterials for biomedical devices and implants. Biomed. Mater. 2013, 8, 035003. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Raj, S.; Jain, S.; Chatterjee, K. Multifunctional biodegradable polymer nanocomposite incorporating graphene-silver hybrid for biomedical applications. Mater. Des. 2016, 108, 319–332. [Google Scholar] [CrossRef]
- Tomoaia, G.; Soritau, O.; Tomoaia-Cotisel, M.; Pop, L.B.; Pop, A.; Mocanu, A.; Horovitz, O.; Bobos, L.D. Scaffolds made of nanostructured phosphates, collagen and chitosan for cell culture. Powder Technol. 2013, 238, 99–107. [Google Scholar] [CrossRef]
- Kim, H.-L.; Jung, G.Y.; Yoon, J.H.; Han, J.S.; Park, Y.J.; Kim, D.G.; Zhang, M.; Kim, D.J. Preparation and characterization of nano-sized hydroxyapatite/alginate/chitosan composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 54, 20–25. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.; Shen, X.; Tong, H. Synthesis and characterization of chitosan–multiwalled carbon nanotubes/hydroxyapatite nanocomposites for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1843–1851. [Google Scholar] [CrossRef]
- Gouma, P.; Xue, R.; Goldbeck, C.P.; Perrotta, P.; Balázsi, C. Nano-hydroxyapatite—Cellulose acetate composites for growing of bone cells. Mater. Sci. Eng. C 2012, 32, 607–612. [Google Scholar] [CrossRef]
- Lee, E.J.; Kwak, H.; Kim, D.J. Ceramic Processing Research Mechanical properties of cellulose acetate/hydroxyapatite nanoparticle composite fiber by electro-spinning process. J. Ceram. Process. Res. 2015, 16, 330–334. [Google Scholar]
- Huang, Y.; Zhang, X.; Qiao, H.; Hao, M.; Zhang, H.; Xu, Z.; Zhang, X.; Pang, X.; Lin, H. Corrosion resistance and cytocompatibility studies of zinc-doped fluorohydroxyapatite nanocomposite coatings on titanium implant. Ceram. Int. 2016, 42, 1903–1915. [Google Scholar] [CrossRef]
- Kiran, A.; Kumar, T.S.; Sanghavi, R.; Doble, M.; Ramakrishna, S. Antibacterial and Bioactive Surface Modifications of Titanium Implants by PCL/TiO2 Nanocomposite Coatings. Nanomaterials 2018, 8, 860. [Google Scholar] [CrossRef] [PubMed]
- Khandan, A.; Abdellahi, M.; Ozada, N.; Ghayour, H. Study of the bioactivity, wettability and hardness behaviour of the bovine hydroxyapatite-diopside bio-nanocomposite coating. J. Taiwan Inst. Chem. Eng. 2016, 60, 538–546. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Zhang, Y.; Xu, M.; Shi, S.Q. The three-dimensional heterostructure synthesis of ZnO/cellulosic fibers and its application for rubber composites. Compos. Sci. Technol. 2019, 177, 10–17. [Google Scholar] [CrossRef]
- Jell, G.; Verdejo, R.; Safinia, L.; Shaffer, M.S.; Stevens, M.M.; Bismarck, A. Carbon nanotube-enhanced polyurethane scaffolds fabricated by thermally induced phase separation. J. Mater. Chem. 2008, 18, 1865. [Google Scholar] [CrossRef]
- Pan, L.; Pei, X.; He, R.; Wan, Q.; Wang, J. Multiwall carbon nanotubes/polycaprolactone composites for bone tissue engineering application. Colloids Surf. B Biointerfaces 2012, 93, 226–234. [Google Scholar] [CrossRef]
- Wei Tan Twomey, J.; Dongjie Guo Madhavan, K.; Min, L.i. Evaluation of Nanostructural, Mechanical, and Biological Properties of Collagen–Nanotube Composites. IEEE Trans. Nanobiosci. 2010, 9, 111–120. [Google Scholar] [CrossRef]
- Zhang, L.G.; Im, O.; Li, J.; Keidar, M.; Keidar, M. Biomimetic three-dimensional nanocrystalline hydroxyapatite and magnetically synthesized single-walled carbon nanotube chitosan nanocomposite for bone regeneration. Int. J. Nanomed. 2012, 7, 2087. [Google Scholar] [CrossRef]
- Akasaka, T.; Yokoyama, A.; Matsuoka, M.; Hashimoto, T.; Abe, S.; Uo, M.; Watari, F. Adhesion of human osteoblast-like cells (Saos-2) to carbon nanotube sheets. Biomed. Mater. Eng. 2009, 19, 147–153. [Google Scholar] [CrossRef]
- Madanagopal, T.T.; Agarwalla, S.V.; Rosa, V. Carbon nanocomposites for implant dentistry and bone tissue engineering. Appl. Nanocomposite Mater. Dent. 2019, 47–63. [Google Scholar] [CrossRef]
- Kumar, A.M.; Suresh, B.; Ramakrishna, S.; Kim, K.-S. Biocompatible responsive polypyrrole/GO nanocomposite coatings for biomedical applications. RSC Adv. 2015, 5, 99866–99874. [Google Scholar] [CrossRef]
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of Cellulose Nanomaterials, Their Capabilities and Applications. JOM 2016, 68, 2383–2394. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Bakar, A.A.; Tahir, P.M.; Zaidul, I.S.M.; Jawaid, M. Cellulosic Nanocomposites from Natural Fibers for Medical Applications: A Review. In Handbook of Polymer Nanocomposites. Processing, Performance and Application; Springer: Berlin/Heidelberg, Germany, 2015; pp. 475–511. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Kim, B. Cellulose nanomaterials: Life cycle risk assessment, and environmental health and safety roadmap. Environ. Sci. Nano 2015, 2, 477–499. [Google Scholar] [CrossRef]
- Roman, M. Toxicity of Cellulose Nanocrystals: A Review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Kurtis, K.E. Innovations in cement-based materials: Addressing sustainability in structural and infrastructure applications. MRS Bull. 2015, 40, 1102–1109. [Google Scholar] [CrossRef]
- Ridi, F.; Fratini, E.; Baglioni, P. Cement: A two thousand year old nano-colloid. J. Colloid Interface Sci. 2011, 357, 255–264. [Google Scholar] [CrossRef]
- Mohammadkazemi, F.; Doosthoseini, K.; Ganjian, E.; Azin, M. Manufacturing of bacterial nano-cellulose reinforced fiber−cement composites. Constr. Build. Mater. 2015, 101, 958–964. [Google Scholar] [CrossRef]
- Schumann, D.A.; Wippermann, J.; Klemm, D.O.; Kramer, F.; Koth, D.; Kosmehl, H.; Wahlers, T.; Salehi-Gelani, S. Artificial vascular implants from bacterial cellulose: Preliminary results of small arterial substitutes. Cellulose 2009, 16, 877–885. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Mohan, T.; Spirk, S.; Kargl, R.; Doliška, A.; Vesel, A.; Salzmann, I.; Resel, R.; Ribitsch, V.; Stana-Kleinschek, K. Exploring the rearrangement of amorphous cellulose model thin films upon heat treatment. Soft Matter 2012, 8, 9807. [Google Scholar] [CrossRef]
- Favi, P.M.; Ospina, S.P.; Kachole, M.; Gao, M.; Atehortua, L.; Webster, T.J. Preparation and characterization of biodegradable nano hydroxyapatite–bacterial cellulose composites with well-defined honeycomb pore arrays for bone tissue engineering applications. Cellulose 2016, 23, 1263–1282. [Google Scholar] [CrossRef]
- de Sousa, R.B.; Vieira, E.G.; Meneguin, A.B.; Sábio, R.M.; Furtini, J.A.O.; da Silva Filho, E.C. Recent advances in methods of synthesis and applications of bacterial cellulose/calcium phosphates composites in bone tissue engineering. Int. J. Adv. Med. Biotechnol. 2018, 1, 11. [Google Scholar] [CrossRef]
- Goncalves, S.; Padrao, J.; Rodrigues, I.P.; Silva, J.P.; Sencadas, V.; Lanceros-Mendez, S.; Girão, H.; Dourado, F.; Rodrigues, L.R. Bacterial Cellulose As a Support for the Growth of Retinal Pigment Epithelium. Biomacromolecules 2015, 16, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]
- Millon, L.E.; Wan, W.K. The polyvinyl alcohol–bacterial cellulose system as a new nanocomposite for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 79, 245–253. [Google Scholar] [CrossRef]
- Butron Janices, A.; Llorente Zabala, O.; Fernández, J.; Meaurio Arrate, E.; Sarasua Fernández, J. Biodegradable Polyester/Crystalline Nanocellulose Nanocomposites for Biomedical Applications: Preparation and Characterization. In Proceedings of the XXXV Annual Congress of the Spanish Society of Biomedical Engineering: Book of Proceedings, Bilbao, Spain, 29 November–1 December 2018. [Google Scholar]
- Rashti, A.; Yahyaei, H.; Firoozi, S.; Ramezani, S.; Rahiminejad, A.; Karimi, R.; Farzaneh, K.; Mohseni, M.; Ghanbari, H. Development of novel biocompatible hybrid nanocomposites based on polyurethane-silica prepared by sol gel process. Mater. Sci. Eng. C 2016, 69, 1248–1255. [Google Scholar] [CrossRef]
- Ribeiro, M.; Ferraz, M.P.; Monteiro, F.J.; Fernandes, M.H.; Beppu, M.M.; Mantione, D.; Sardon, H. Antibacterial silk fibroin/nanohydroxyapatite hydrogels with silver and gold nanoparticles for bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 231–239. [Google Scholar] [CrossRef]
- Kim, H.-W.; Kim, H.-E.; Salih, V.; Knowles, J.C. Hydroxyapatite and titania sol-gel composite coatings on titanium for hard tissue implants; Mechanical andin vitro biological performance. J. Biomed. Mater. Res. 2005, 72, 1–8. [Google Scholar] [CrossRef]
- Cai, X.; Tong, H.; Shen, X.; Chen, W.; Yan, J.; Hu, J. Preparation and characterization of homogeneous chitosan–polylactic acid/hydroxyapatite nanocomposite for bone tissue engineering and evaluation of its mechanical properties. Acta Biomater. 2009, 5, 2693–2703. [Google Scholar] [CrossRef]
- Deville, S. Ice-Templated Materials: Polymers, Ceramics, Metals and Their Composites; Springer: Cham, Switzerland, 2017; pp. 253–350. [Google Scholar] [CrossRef]
- Niu, X.; Feng, Q.; Wang, M.; Guo, X.; Zheng, Q. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J. Control. Release 2009, 134, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, P.X. Poly(α-hydroxyl acids)/hydroxyapatite porous composites for bone-tissue engineering. I. Preparation and morphology. J. Biomed. Mater. Res. 1999, 44, 446–455. [Google Scholar] [CrossRef]
- Todo, M.; Kagawa, T. Improvement of fracture energy of HA/PLLA biocomposite material due to press processing. J. Mater. Sci. 2008, 43, 799–801. [Google Scholar] [CrossRef]
- Chronakis, I.S. Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—A review. J. Mater. Process. Technol. 2005, 167, 283–293. [Google Scholar] [CrossRef]
- Aznar-Cervantes, S.; Roca, M.I.; Martinez, J.G.; Meseguer-Olmo, L.; Cenis, J.L.; Moraleda, J.M.; Otero, T.F. Fabrication of conductive electrospun silk fibroin scaffolds by coating with polypyrrole for biomedical applications. Bioelectrochemistry 2012, 85, 36–43. [Google Scholar] [CrossRef]
- Kai, D.; Prabhakaran, M.P.; Jin, G.; Ramakrishna, S. Polypyrrole-contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. J. Biomed. Mater. Res. Part A 2011, 99, 376–385. [Google Scholar] [CrossRef]
- Asran, A.S.; Henning, S.; Michler, G.H. Polyvinyl alcohol–collagen–hydroxyapatite biocomposite nanofibrous scaffold: Mimicking the key features of natural bone at the nanoscale level. Polymer 2010, 51, 868–876. [Google Scholar] [CrossRef]
- Puperi, D.S.; Kishan, A.; Punske, Z.E.; Wu, Y.; Cosgriff-Hernandez, E.; West, J.L.; Grande-Allen, K.J. Electrospun Polyurethane and Hydrogel Composite Scaffolds as Biomechanical Mimics for Aortic Valve Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1546–1558. [Google Scholar] [CrossRef]
- Xue, Y.; Ravishankar, P.; Zeballos, M.A.; Sant, V.; Balachandran, K.; Sant, S. Valve leaflet-inspired elastomeric scaffolds with tunable and anisotropic mechanical properties. Polym. Adv. Technol. 2020, 31, 94–106. [Google Scholar] [CrossRef]
- Ravichandran, R.; Venugopal, J.R.; Mukherjee, S.; Sundarrajan, S.; Ramakrishna, S. Elastomeric Core/Shell Nanofibrous Cardiac Patch as a Biomimetic Support for Infarcted Porcine Myocardium. Tissue Eng. Part A 2015, 21, 1288–1298. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies; Springer: New York City, NY, USA, 2014. [Google Scholar]
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The status, challenges, and future of additive manufacturing in engineering. Comput. Des. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Berman, B. 3-D printing: The new industrial revolution. Bus. Horiz. 2012, 55, 155–162. [Google Scholar] [CrossRef]
- Greenhalgh, S.; Schreuders, P. Implementing emerging technologies in interior design education: a case study utilizing rapid prototyping. UNIversitas 2012, 7, 1–13. [Google Scholar]
- Thomas, S.; Potts, J. Industry Sustainability Under Technological Evolution: A Case Study of the Overshooting Hypothesis in Sports. Procedia Eng. 2015, 112, 562–567. [Google Scholar] [CrossRef]
- Velu, R.; Raspall, F.; Singamneni, S. 3D printing technologies and composite materials for structural applications. Green Compos. Automot. Appl. 2019, 171–196. [Google Scholar] [CrossRef]
- Williams, C.B.; Mistree, F.; Rosen, D.W. A Functional Classification Framework for the Conceptual Design of Additive Manufacturing Technologies. J. Mech. Des. 2011, 133, 121002. [Google Scholar] [CrossRef]
- Wang, Q.; Mitsumura, N.; Chen, Q.; Sarkar, A.; Kurokawa, H.; Sekiguchi, K.; Sugiyama, K. Investigation of condensation reaction during phenol liquefaction of waste woody materials. Int. J. Sustain. Dev. Plan. 2014, 9, 658–668. [Google Scholar] [CrossRef]
- Masood, S.; Song, W. Development of new metal/polymer materials for rapid tooling using Fused deposition modelling. Mater. Des. 2004, 25, 587–594. [Google Scholar] [CrossRef]
- Huang, Y.; Leu, M.C.; Mazumder, J.; Donmez, A. Additive Manufacturing: Current State, Future Potential, Gaps and Needs, and Recommendations. J. Manuf. Sci. Eng. 2015, 137, 014001. [Google Scholar] [CrossRef]
- Jovane, F.; Yoshikawa, H.; Alting, L.; Boer, C.R.; Westkamper, E.; Williams, D.; Tseng, M.; Seliger, G.; Paci, A.M. The incoming global technological and industrial revolution towards competitive sustainable manufacturing. CIRP Ann. 2008, 57, 641–659. [Google Scholar] [CrossRef]
- Mani, M.; Lyons, K.W.; Gupta, S.K. Sustainability Characterization for Additive Manufacturing. J. Res. Natl. Inst. Stand. Technol. 2014, 119, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.; Paloheimo, K.-S.; Tuomi, J.; Wolff, J.; Mäkitie, A. Accuracy of medical models made by additive manufacturing (rapid manufacturing). J. Cranio Maxillofac. Surg. 2013, 41, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef] [PubMed]
- Lohfeld, S.; Barron, V.; McHugh, P.E. Biomodels of Bone: A Review. Ann. Biomed. Eng. 2005, 33, 1295–1311. [Google Scholar] [CrossRef] [PubMed]
- Rengier, F.; Mehndiratta, A.; Von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- AlAli, A.B.; Griffin, M.F.; Butler, P.E. Three-Dimensional Printing Surgical Applications. Eplasty 2015, 15, e37. [Google Scholar]
- D’Ancona, G.; Amaducci, A.; Rinaudo, A.; Pasta, S.; Follis, F.; Pilato, M.; Baglini, R. Haemodynamic predictors of a penetrating atherosclerotic ulcer rupture using fluid–structure interaction analysis. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 576–578. [Google Scholar] [CrossRef]
- Scardulla, F.; Bellavia, D.; D’Acquisto, L.; Raffa, G.M.; Pasta, S. Particle image velocimetry study of the celiac trunk hemodynamic induced by continuous-flow left ventricular assist device. Med. Eng. Phys. 2017, 47, 47–54. [Google Scholar] [CrossRef]
- Gibson, I.; Cheung, L.K.; Chow, S.P.; Cheung, W.L.; Beh, S.L.; Savalani, M.; Lee, S.H. The use of rapid prototyping to assist medical applications. Rapid Prototyp. J. 2006, 12, 53–58. [Google Scholar] [CrossRef]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Material processing of hydroxyapatite and titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: A review. Mater. Des. 2014, 55, 165–175. [Google Scholar] [CrossRef]
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Lantada, A.D.; Morgado, P.L. Rapid Prototyping for Biomedical Engineering: Current Capabilities and Challenges. Annu. Rev. Biomed. Eng. 2012, 14, 73–96. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D printing in pharmaceutical and medical applications. Pharm. Res. 2018, 35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dodziuk, H. Applications of 3D printing in healthcare. Kardiochirurgia Torakochirurgia Pol. 2016, 13, 283–293. [Google Scholar] [CrossRef]
- Yablokova, G.; Speirs, M.; Van Humbeeck, J.; Kruth, J.P.; Schrooten, J.; Cloots, R.; Boschini, F.; Lumay, G.; Luyten, J. Rheological behavior of β-Ti and NiTi powders produced by atomization for SLM production of open porous orthopedic implants. Powder Technol. 2015, 283, 199–209. [Google Scholar] [CrossRef]
- Parthasarathy, J.; Starly, B.; Raman, S.; Christensen, A. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM). J. Mech. Behav. Biomed. Mater. 2010, 3, 249–259. [Google Scholar] [CrossRef]
- Demir, A.G.; Previtali, B. Additive manufacturing of cardiovascular CoCr stents by selective laser melting. Mater. Des. 2017, 119, 338–350. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; de Carvalho Zavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Kharmandayan, P.; Calderoni, D.; Maciel Filho, R. Customised titanium implant fabricated in additive manufacturing for craniomaxillofacial surgery. Virtual Phys. Prototyp. 2014, 9, 115–125. [Google Scholar] [CrossRef]
- Johansson, F. Mechanical Properties of Trabecular Structures Produced by SLM, as a Function of the Trabecular Morphology. Master’s Thesis, Jönköping University, Jönköping, Sweden, May 2017. [Google Scholar]
- Progressing Orthopedic Implants with Additive Manufacturing—3D Printing Media Network. Available online: https://www.3dprintingmedia.network/progressing-orthopedic-implants-additive-manufacturing/ (accessed on 9 June 2019).
- Melchels, F.P.; Domingos, M.A.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- De Leon, A.C.; Chen, Q.; Palaganas, N.B.; Palaganas, J.O.; Manapat, J.; Advincula, R.C. High performance polymer nanocomposites for additive manufacturing applications. React. Funct. Polym. 2016, 103, 141–155. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, Y.; Qi, L. Bioinspired colloidal materials with special optical, mechanical, and cell-mimetic functions. J. Mater. Chem. B 2013, 1, 251–264. [Google Scholar] [CrossRef]
- Yao, H.-B.; Fang, H.-Y.; Wang, X.-H.; Yu, S.-H. Hierarchical assembly of micro-/nano-building blocks: Bio-inspired rigid structural functional materials. Chem. Soc. Rev. 2011, 40, 3764. [Google Scholar] [CrossRef] [PubMed]
- Valo, H.; Peltonen, L.; Vehviläinen, S.; Karjalainen, M.; Kostiainen, R.; Laaksonen, T.; Hirvonen, J. Electrospray Encapsulation of Hydrophilic and Hydrophobic Drugs in Poly(L-lactic acid) Nanoparticles. Small 2009, 5, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.-F.; Du, Z.; Chua, C.-K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Anctil, A.; Babbitt, C.W.; Raffaelle, R.P.; Landi, B.J. Material and Energy Intensity of Fullerene Production. Environ. Sci. Technol. 2011, 45, 2353–2359. [Google Scholar] [CrossRef]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of Nanoparticles in Biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Gunn, J.; Zhang, M. Polyblend nanofibers for biomedical applications: Perspectives and challenges. Trends Biotechnol. 2010, 28, 189–197. [Google Scholar] [CrossRef]
- Baiguera, S.; Del Gaudio, C.; Lucatelli, E.; Kuevda, E.; Boieri, M.; Mazzanti, B.; Bianco, A.; Macchiarini, P. Electrospun gelatin scaffolds incorporating rat decellularized brain extracellular matrix for neural tissue engineering. Biomaterials 2014, 35, 1205–1214. [Google Scholar] [CrossRef]
- Li, B.; Chen, F.; Wang, X.; Ji, B.; Wu, Y. Isolation and identification of antioxidative peptides from porcine collagen hydrolysate by consecutive chromatography and electrospray ionization–mass spectrometry. Food Chem. 2007, 102, 1135–1143. [Google Scholar] [CrossRef]
- Fuchs, S.; Mozes, E.; Maoz, A.; Sela, M. Thymus independence of a collagen-like synthetic polypeptide and of collagen, and the need for thymus and bone marrow-cell cooperation in the immune response to gelatin. J. Exp. Med. 1974, 139, 148–158. [Google Scholar] [CrossRef] [PubMed]
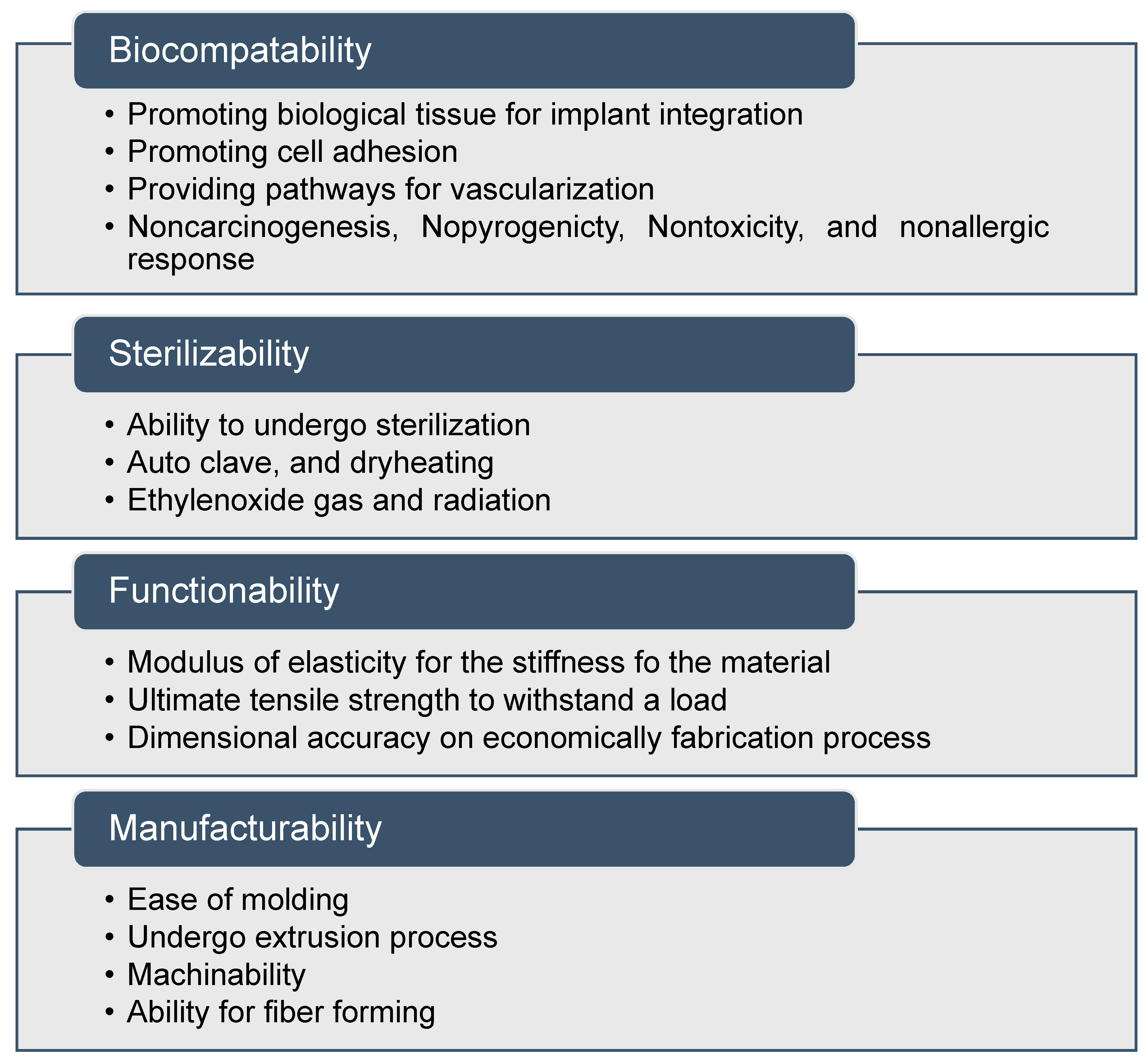
- Biomaterials for Surgical Operation-Shuko Suzuki, Yoshito Ikada-Google Books. Available online: https://books.google.com.sg/books?hl=en&lr=&id=xk45_vmi44sC&oi=fnd&pg=PR3&dq=biocompatibility+features,+sterilizability,+functionability+and+manufacturability+to+finally+achieve+as+medically+graded+materia&ots=Z2nSvcuyn9&sig=LDcbztNtiSp6XdTosDFVpbn7QBw#v= (accessed on 9 June 2019).
- Microbial Adhesion on Biomaterials and the Sources of Human Beta-Defensin-3 in Septic Joint Implant Loosening. Available online: https://helda.helsinki.fi/handle/10138/178484 (accessed on 9 June 2019).
- Abe, F.; Osakada, K.; Kitamura, Y.; Matsumoto, M.; Shiomi, M. Manufacturing of titanium parts for medical purposes by selective laser melting. Proc. Rapid Prototyping 2000, 12, 288–293. [Google Scholar]
- Hieu, L.C.; Bohez, E.; Vander Sloten, J.; Phien, H.N.; Vatcharaporn, E.; Binh, P.H.; An, P.V.; Oris, P. Design for medical rapid prototyping of cranioplasty implants. Rapid Prototyp. J. 2003, 9, 175–186. [Google Scholar] [CrossRef]
- Teixeira, L.N.; Ravagnani, C.; Peitl, O.; Zanotto, E.D. In vitro osteogenesis on a highly bioactive glass-ceramic. J. Biomed. Mater. Res. Part A 2006, 28–31. [Google Scholar] [CrossRef]
- Warnke, P.H.; Douglas, T.; Wollny, P.; Sherry, E.; Steiner, M.; Galonska, S.; Becker, S.T. Rapid Prototyping: Porous Titanium Alloy Scaffolds Produced by Selective Laser Melting for Bone Tissue Engineering. Tissue Eng. Part C Methods 2009, 15, 115–124. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Medina, F.; Lopez, H.; Martinez, E.; Machado, B.I.; Hernandez, D.H.; Martinez, L.; Lopez, M.I.; Wicker, R.B.; et al. Next-generation biomedical implants using additive manufacturing of complex cellular and functional mesh arrays. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1999–2032. [Google Scholar] [CrossRef]
- Salmi, M.; Tuomi, J.; Paloheimo, K.S.; Björkstrand, R.; Paloheimo, M.; Salo, J.; Kontio, R.; Mesimäki, K.; Mäkitie, A.A. Patient-specific reconstruction with 3D modeling and DMLS additive manufacturing. Rapid Prototyp. J. 2012, 18, 209–214. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; Maciel Filho, R.; de Carvalho Zavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Calderoni, D.R.; Kharmandayan, P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J. Cranio Maxillofac. Surg. 2014, 42, 1877–1884. [Google Scholar] [CrossRef]
- Das, K.; Bose, S.; Bandyopadhyay, A.; Karandikar, B.; Gibbins, B.L. Surface coatings for improvement of bone cell materials and antimicrobial activities of Ti implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 455–460. [Google Scholar] [CrossRef]
- Elloumi-Hannachi, I.; Yamato, M.; Okano, T. Cell sheet engineering: A unique nanotechnology for scaffold-free tissue reconstruction with clinical applications in regenerative medicine. J. Intern. Med. 2010, 267, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Matsumoto, T. Synthesis and modification of apatite nanoparticles for use in dental and medical applications. Jpn. Dent. Sci. Rev. 2015, 51, 85–95. [Google Scholar] [CrossRef]
- Chudinova, E.; Surmeneva, M.; Koptioug, A.; Sharonova, A.; Loza, K.; Surmenev, R. Surface modification of additive manufactured Ti6Al4V alloy with Ag nanoparticles: Wettability and surface morphology study. IOP Conf. Ser. Mater. Sci. Eng. 2016, 116, 012004. [Google Scholar] [CrossRef]
- Van Hengel, I.A.; Riool, M.; Fratila-Apachitei, L.E.; Witte-Bouma, J.; Farrell, E.; Zadpoor, A.A.; Zaat, S.A.; Apachitei, I. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials 2017, 140, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Heil, J.; Reifferscheid, G.; Waldmann, P.; Leyhausen, G.; Geurtsen, W. Genotoxicity of dental materials. Mutat. Res. Toxicol. 1996, 368, 181–194. [Google Scholar] [CrossRef]
- Murray, P.E.; García Godoy, C.; García Godoy, F. How Is the Biocompatibilty of Dental Biomaterials Evaluated? Med Oral Patol. Oral Cir. Bucal 2007, 12, 258–266. [Google Scholar]
- Quan, R.; Tang, Y.; Huang, Z.; Xu, J.; Wu, X.; Yang, D. Study on the genotoxicity of HA/ZrO2 composite particles in vitro. Mater. Sci. Eng. C 2013, 33, 1332–1338. [Google Scholar] [CrossRef]
- Anderson, J.M. Biological Responses to Materials. Annu. Rev. Mater. Res. 2001, 31, 81–110. [Google Scholar] [CrossRef]
- Matsuo, K.; Irie, N. Osteoclast–osteoblast communication. Arch. Biochem. Biophys. 2008, 473, 201–209. [Google Scholar] [CrossRef]
- Wieslander, A.P.; Nordin, M.K.; Hansson, B.; Baldetorp, B.; Kjellstrand, P.T.T. In Vitro Toxicity of Biomaterials Determined with Cell Density, Total Protein, Cell Cycle Distribution and Adenine Nucleotides. Biomater. Artif. Cells Immobil. Biotechnol. 1993, 21, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Sharma, A.; Basu, B. In vitro cytocompatibility assessment of amorphous carbon structures using neuroblastoma and Schwann cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Afzal, M.A.F.; Kesarwani, P.; Reddy, K.M.; Kalmodia, S.; Basu, B.; Balani, K. Functionally graded hydroxyapatite-alumina-zirconia biocomposite: Synergy of toughness and biocompatibility. Mater. Sci. Eng. C 2012, 32, 1164–1173. [Google Scholar] [CrossRef]
- Wilson, J.R.; Mills, J.G.; Prather, I.D.; Dimitrijevich, S.D. A toxicity index of skin and wound cleansers used on in vitro fibroblasts and keratinocytes. Adv. Skin Wound Care 2005, 18, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, P.; Cheng, X.; Xie, Y.; Liang, C.; Li, C.; Xu, S. Selective laser sintering fabrication of nano-hydroxyapatite/poly-ε-caprolactone scaffolds for bone tissue engineering applications. Int. J. Nanomed. 2013, 8, 4197–4213. [Google Scholar]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef]
- Olakanmi, E.O. Selective laser sintering/melting (SLS/SLM) of pure Al, Al–Mg, and Al–Si powders: Effect of processing conditions and powder properties. J. Mater. Process. Technol. 2013, 213, 1387–1405. [Google Scholar] [CrossRef]
- Chen, Q.; Mangadlao, J.D.; Wallat, J.; De Leon, A.; Pokorski, J.K.; Advincula, R.C. 3D Printing Biocompatible Polyurethane/Poly(lactic acid)/Graphene Oxide Nanocomposites: Anisotropic Properties. ACS Appl. Mater. Interfaces 2017, 9, 4015–4023. [Google Scholar] [CrossRef]
- Reddy, U.M.; Goldenberg, R.; Silver, R.; Smith, G.C.; Pauli, R.M.; Wapner, R.J.; Gardosi, J.; Pinar, H.; Grafe, M.; Kupferminc, M.; et al. Stillbirth classification-developing an international consensus for research: Executive summary of a National Institute of Child Health and Human Development workshop. Obstet. Gynecol. 2009, 114, 901–914. [Google Scholar] [CrossRef]
- Seeds, J.W.; Cefalo, R.C.; Herbert, W.N.P. Amniotic band syndrome. Am. J. Obstet. Gynecol. 1982, 144, 243–248. [Google Scholar] [CrossRef]
- Sayuk, A. Design and Implementation of a Low Cost Hand for Prosthetic Applications. Master’s Thesis, University of Coimbra, Coimbra, Spain, September 2015. [Google Scholar]
- De Almeida Corveira, J.A. Design and development of a Soft Body-Actuated 3D printed prosthetic hand. Master’s Thesis, University of Coimbra, Coimbra, Spain, September 2017. [Google Scholar]
- Tong, Y.; Kucukdeger, E.; Halper, J.; Cesewski, E.; Karakozoff, E.; Haring, A.P.; McIlvain, D.; Singh, M.; Khandelwal, N.; Meholic, A.; et al. Low-cost sensor-integrated 3D-printed personalized prosthetic hands for children with amniotic band syndrome: A case study in sensing pressure distribution on an anatomical human-machine interface (AHMI) using 3D-printed conformal electrode arrays. PLoS ONE 2019, 14, e0214120. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Lee, M.Y.; Wang, S.H. Digital denture manufacturing-An integrated technologies of abrasive computer tomography, CNC machining and rapid prototyping. Int. J. Adv. Manuf. Technol. 2006, 31, 41–49. [Google Scholar] [CrossRef]
- Kowsari, K.; Zhang, B.; Panjwani, S.; Chen, Z.; Hingorani, H.; Akbari, S.; Fang, N.X.; Ge, Q. Photopolymer formulation to minimize feature size, surface roughness, and stair-stepping in digital light processing-based three-dimensional printing. Addit. Manuf. 2018, 24, 627–638. [Google Scholar] [CrossRef]
- Reiser, A.; Lindén, M.; Rohner, P.; Marchand, A.; Galinski, H.; Sologubenko, A.S.; Wheeler, J.M.; Zenobi, R.; Poulikakos, D.; Spolenak, R. Multi-metal electrohydrodynamic redox 3D printing at the submicron scale. Nat. Commun. 2019, 10, 1853. [Google Scholar] [CrossRef]
- Petrovic, V.; Vicente Haro Gonzalez, J.; Jordá Ferrando, O.; Delgado Gordillo, J.; Ramón Blasco Puchades, J.; Portolés Griñan, L. Additive layered manufacturing: sectors of industrial application shown through case studies. Inter. Prod. Res. 2011, 49, 1061–1079. [Google Scholar] [CrossRef]
- Campbell, I.; Bourell, D.; Gibson, I. Additive manufacturing: rapid prototyping comes of age. Rapid Prototyp. J. 2012, 18, 255–258. [Google Scholar] [CrossRef]
- Vayre, B.; Vignat, F.; Villeneuve, F. Metallic additive manufacturing: State-of-the-art review and prospects. Mech. Ind. 2012, 13, 89–96. [Google Scholar] [CrossRef]
- Spierings, A.; Levy, G.; Labhart, L.; Wegener, K. Production of functional parts using SLM—Opportunities and limitations. Innov. Dev. Virtual Phys. Prototyp. 2012, 785–790. [Google Scholar] [CrossRef]
- Scipioni Bertoli, U.; Wolfer, A.J.; Matthews, M.J.; Delplanque, J.-P.R.; Schoenung, J.M. On the limitations of Volumetric Energy Density as a design parameter for Selective Laser Melting. Mater. Des. 2017, 113, 331–340. [Google Scholar] [CrossRef]
- Jayakumar, A.; Singamneni, S.; Ramos, M.; Al-Jumaily, A.M.; Pethaiah, S.S. Manufacturing the gas diffusion layer for PEM fuel cell using a novel 3D printing technique and critical assessment of the challenges encountered. Materials 2017, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schubert, C.; Van Langeveld, M.C.; Donoso, L.A. Innovations in 3D printing: A 3D overview from optics to organs. Br. J. Ophthalmol. 2014, 98, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P. Regional knowledge capabilities, embeddedness of firms and industry organisation: Bioscience megacentres and economic geography. Eur. Plan. Stud. 2004, 12, 625–641. [Google Scholar] [CrossRef]
- Liebenau, J. Medical Science and Medical Industry: The Formation of the American Pharmaceutical Industry; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Gebhardt, A.; Schmidt, F.M.; Hötter, J.S.; Sokalla, W.; Sokalla, P. Additive Manufacturing by Selective Laser Melting: The realizer desktop machine and its application for the dental industry. Phys. Procedia 2010, 5, 543–549. [Google Scholar] [CrossRef]
- Mihalko, W.M. Additive Manufacturing of Arthroplasty Implants. 3D Print. Orthop. Surg. 2019, 49–53. [Google Scholar] [CrossRef]
- Bradley, E.H.; Elkins, B.R.; Herrin, J.; Elbel, B. Health and social services expenditures: Associations with health outcomes. BMJ Qual. Saf. 2011, 20, 826–831. [Google Scholar] [CrossRef]

| Year | Implant | Materials | Micro/Nano | AM Method | Outcome Summary |
|---|---|---|---|---|---|
| Microsized Materials | |||||
| 2000 [124] | Proposed for bone and dental | Titanium powder 200 μm and 60 μm | Micro | SLM | Fabricated dental crowns and bones with high strength and density |
| 2003 [125] | Bone | PMMA | Micro | Proposed | Proposed the cost reduction Cranioplasty implants fabricated from AM using CT scanning image |
| 2007 [126,127] | Bone | HA powder 2.78 μm | Micro | 3DP Ink jet | Extensive bone ingrowth formation in 3D printed HA scaffolds |
| Bone | Titanium alloy (Ti-6Al-4V) | Micro | SLM | Scaffolds are biocompatible, and pore width influences pore overgrowth, resistance to compressive force, and porosity. | |
| 2010 [128] | Tibial Knee stems, hip stems and intermedullary rods | Titanium alloys (Ti-6Al-4V) 100 μm | Micro | EBM | The array of cellular, reticular mesh manufactured in monolithic form has potential for unique bone compatibility |
| 2012 [129] | Facial bone (orbital area) | Titanium (Ti64 Al4V-ELI) 30 μm | Micro | DMLS | The method enables exact fitting of implants, designed with low mass and therefore sensitive to hot and cold temperature |
| 2013 [89] | Skull bone | polymer | Micro | SLS & Poly Jet | Fabricated skulls using Poly Jet and SLS, the accuracy of Poly Jet was higher than SLS or 3DP using novel measuring technique |
| 2014 [130] | Bone (Cranial head) | Titanium (Ti64 ELI) | Micro | DMLS | Protocol developed and created an anatomic bio model of the bone defect for surgical planning and, finally, the design and manufacture of the patient-specific implant. |
| Nanosized Materials | |||||
| 2008 [131] | Proposed for bone and dental | Titania nanotube | Nano | Proposed | Silver-treated Titania nanotube surface provides antibacterial properties to prevent implants against postoperative infections |
| 2009 [132] | Endoscopic transplantation (oral muscular cells) | Poly(N-isopropylacrylamide) (PIPPAm) | Nano | EBM | Nanoscale thermo responsive surface to untimely reconstruct multifunctional three-dimensional tissues in vitro to regenerate a defective tissue |
| 2015 [133] | Proposed for bone and dental | HA 100nm | Nano | Proposed | Synthesized HAp exhibits excellent biocompatibility, |
| 2016 [134] | Bone grafting (Hip/Knee) | AgNPs- coated Ti6Al4V | Nano coating | EBM | Higher surface energy is observed for AgNPs-coated Ti6Al4V surfaces (70.17 mN/m) compared with uncoated ones (49.07 mN/m). |
| 2017 [135] | Bone | AgNPs- coated Titanium (Ti-6Al-4V) | Nano coating | SLM | Antimicrobial assays consistently showed strong antimicrobial activity of the developed implants against MRSA including released activity, surface antimicrobial activity and prevention of biofilm formation. |
| 2018 [136] | orthopedic | Silver nanoparticles (AgNPs) | Nano | Proposed | AgNP release, exploration of suitable size, shape, as well as the novel method of surface modification, such as 3DP technology |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velu, R.; Calais, T.; Jayakumar, A.; Raspall, F. A Comprehensive Review on Bio-Nanomaterials for Medical Implants and Feasibility Studies on Fabrication of Such Implants by Additive Manufacturing Technique. Materials 2020, 13, 92. https://doi.org/10.3390/ma13010092
Velu R, Calais T, Jayakumar A, Raspall F. A Comprehensive Review on Bio-Nanomaterials for Medical Implants and Feasibility Studies on Fabrication of Such Implants by Additive Manufacturing Technique. Materials. 2020; 13(1):92. https://doi.org/10.3390/ma13010092
Chicago/Turabian StyleVelu, Rajkumar, Theo Calais, Arunkumar Jayakumar, and Felix Raspall. 2020. "A Comprehensive Review on Bio-Nanomaterials for Medical Implants and Feasibility Studies on Fabrication of Such Implants by Additive Manufacturing Technique" Materials 13, no. 1: 92. https://doi.org/10.3390/ma13010092
APA StyleVelu, R., Calais, T., Jayakumar, A., & Raspall, F. (2020). A Comprehensive Review on Bio-Nanomaterials for Medical Implants and Feasibility Studies on Fabrication of Such Implants by Additive Manufacturing Technique. Materials, 13(1), 92. https://doi.org/10.3390/ma13010092







