Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review
Abstract
1. Introduction
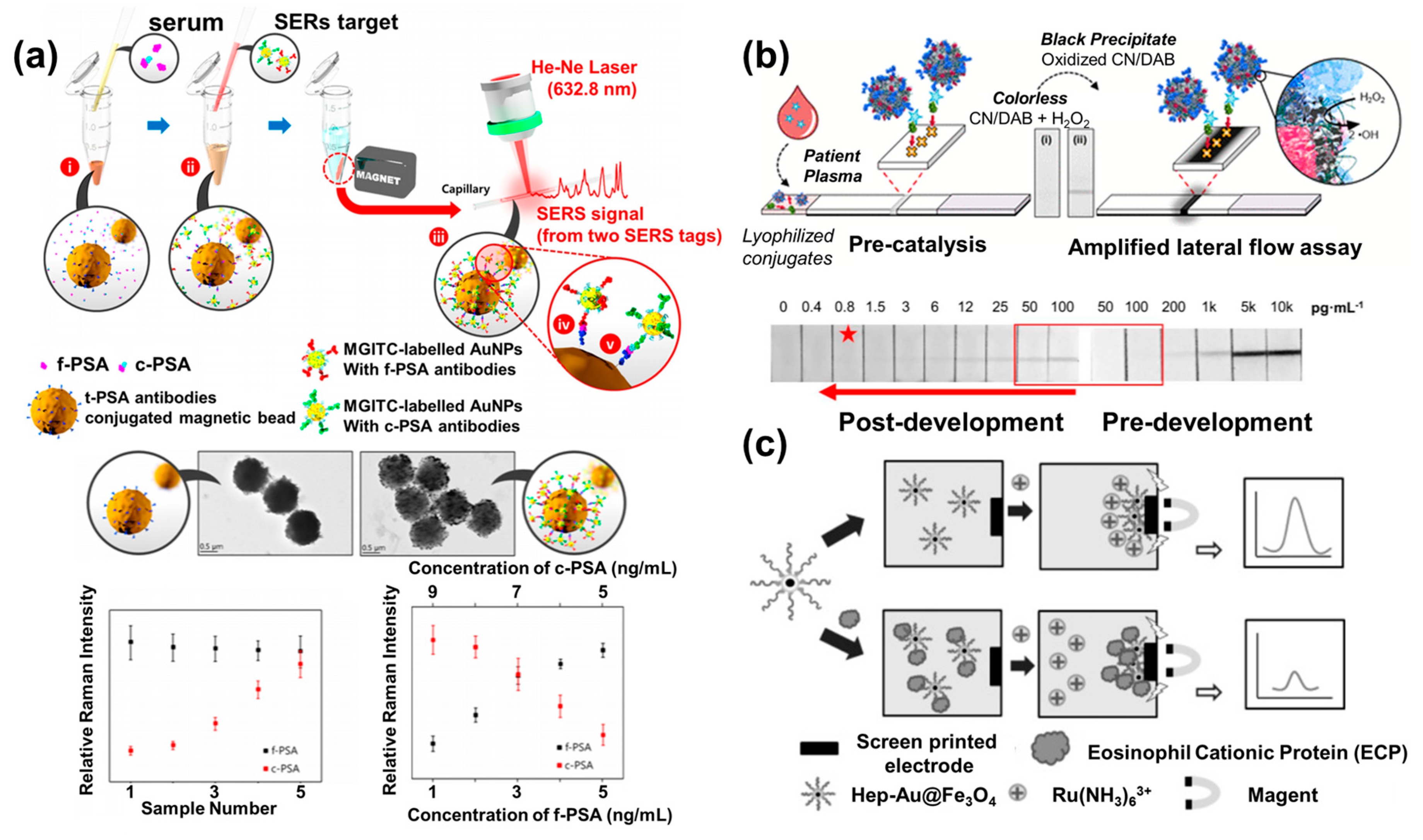
2. NP-based Biosensors
3. Carbon-Based Nanomaterials in Biosensors
4. Novel Nanomaterial-Based Biosensors
5. Hybrid Nanomaterial-based Biosensors
6. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Bohunicky, B.; Mousa, S.A. Biosensors: The new wave in cancer diagnosis. Nanotechnol. Sci. Appl. 2011, 4, 1–10. [Google Scholar]
- Shukla, S.; Hong, S.-Y.; Chung, S.H.; Kim, M. Rapid Detection Strategies for the Global Threat of Zika Virus: Current State, New Hypotheses, and Limitations. Front. Microbiol. 2016, 7, 1685. [Google Scholar] [CrossRef] [PubMed]
- Nunna, B.B.; Mandal, D.; Lee, J.U.; Singh, H.; Zhuang, S.; Misra, D.; Bhuyian, M.N.U.; Lee, E.S. Detection of cancer antigens (CA-125) using gold nano particles on interdigitated electrode-based microfluidic biosensor. Nano Converg. 2019, 6, 3. [Google Scholar] [CrossRef]
- Goode, J.A.; Rushworth, J.V.H.; Millner, P.A. Biosensor Regeneration: A Review of Common Techniques and Outcomes. Langmuir 2015, 31, 6267–6276. [Google Scholar] [CrossRef] [PubMed]
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent Advances in Biosensor Technology for Potential Applications—An Overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Wooley, R.; Ruth, M.; Glassner, D.; Sheehan, J. Process Design and Costing of Bioethanol Technology: A Tool for Determining the Status and Direction of Research and Development. Biotechnol. Prog. 1999, 15, 794–803. [Google Scholar] [CrossRef]
- North, J.R. Immunosensors: Antibody-based biosensors. Trends Biotechnol. 1985, 3, 7. [Google Scholar] [CrossRef]
- Jo, J.; Yoon, J.; Lee, T.; Cho, H.-Y.; Lee, J.-Y.; Choi, J.-W. H2O2 biosensor consisted of hemoglobin-DNA conjugate on nanoporous thin film electrode with electrochemical signal enhancement. Nano Converg. 2019, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Yemini, M.; Reches, M.; Gazit, E.; Rishpon, J. Peptide Nanotube-Modified Electrodes for Enzyme−Biosensor Applications. Anal. Chem. 2005, 77, 5155–5159. [Google Scholar] [CrossRef] [PubMed]
- Backmann, N.; Zahnd, C.; Huber, F.; Bietsch, A.; Pluckthun, A.; Lang, H.-P.; Guntherodt, H.-J.; Hegner, M.; Gerber, C. A label-free immunosensor array using single-chain antibody fragments. Proc. Natl. Acad. Sci. USA 2005, 102, 14587–14592. [Google Scholar] [CrossRef] [PubMed]
- Hamidi-Asl, E.; Palchetti, I.; Hasheminejad, E.; Mascini, M. A review on the electrochemical biosensors for determination of microRNAs. Talanta 2013, 115, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Chae, E.-J.; Park, S.-j.; Choi, J.-W. Label-free detection of γ-aminobutyric acid based on silicon nanowire biosensor. Nano Converg. 2019, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kim, G.Y.; Kim, S.M.; Hong, K.; Kim, Y.; Park, C.; Sohn, H.; Min, J. Label-free localized surface plasmon resonance biosensor composed of multi-functional DNA 3 way junction on hollow Au spike-like nanoparticles (HAuSN) for avian influenza virus detection. Colloid Surf. B Biointerfaces 2019, 182, 110341. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Tang, L.; Song, D.; Song, S.; Ma, W.; Xu, L.; Kuang, H.; Wu, X.; Liu, L.; Chen, X.; et al. A SERS-active sensor based on heterogeneous gold nanostar core–silver nanoparticle satellite assemblies for ultrasensitive detection of aflatoxinB1. Nanoscale 2016, 8, 1873–1878. [Google Scholar] [CrossRef]
- Thudi, L.; Jasti, L.S.; Swarnalatha, Y.; Fadnavis, N.W.; Mulani, K.; Deokar, S.; Ponrathnam, S. Adsorption induced enzyme denaturation: The role of protein surface in adsorption induced protein denaturation on allyl glycidyl ether (AGE)—Ethylene glycol dimethacrylate (EGDM) copolymers. Colloid Surf. B Biointerfaces 2012, 90, 184–190. [Google Scholar] [CrossRef]
- Zhuang, J.; Young, A.P.; Tsung, C.-K. Integration of Biomolecules with Metal-Organic Frameworks. Small 2017, 13, 1700880. [Google Scholar] [CrossRef]
- Wu, J.-F.; Xu, M.-Q.; Zhao, G.-C. Graphene-based modified electrode for the direct electron transfer of Cytochrome c and biosensing. Electrochem. Commun. 2010, 12, 175–177. [Google Scholar] [CrossRef]
- Jia, N.; Liu, L.; Zhou, Q.; Wang, L.; Yan, M.; Jiang, Z. Bioelectrochemistry and enzymatic activity of glucose oxidase immobilized onto the bamboo-shaped CNx nanotubes. Electrochim. Acta 2005, 51, 611–618. [Google Scholar] [CrossRef]
- Doughty, A.; Hoover, A.; Layton, E.; Murray, C.; Howard, E.; Chen, W. Nanomaterial Applications in Photothermal Therapy for Cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Lou, X.W.D. SnO2—Based Nanomaterials: Synthesis and Application in Lithium-Ion Batteries. Small 2013, 9, 1877–1893. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, N.; Cui, Y. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Wei, Q.; Yang, D.; Fan, M.; Harris, H.G. Applications of Nanomaterial-Based Membranes in Pollution Control. Crit. Rev. Environ. Sci. Technol. 2013, 43, 2389–2438. [Google Scholar] [CrossRef]
- Zhang, G.; Khan, A.A.; Wu, H.; Chen, L.; Gu, Y.; Gu, N. The Application of Nanomaterials in Stem Cell Therapy for Some Neuro-logical Diseases. Curr. Drug Targets 2018, 19, 279–298. [Google Scholar] [CrossRef]
- Venkatesh, C.; Clear, O.; Major, I.; Lyons, J.G.; Devine, D.M. Faster Release of Lumen-Loaded Drugs than Matrix-Loaded Equivalent in Polylactic Acid/Halloysite Nanotubes. Materials 2019, 12, 1830. [Google Scholar] [CrossRef]
- Lei, J.; Ju, H. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef]
- Saleh, T.A.; Al-Shalalfeh, M.M.; Al-Saadi, A.A. Graphene Dendrimer-stabilized silver nanoparticles for detection of methimazole using Surface-enhanced Raman scattering with computational assignment. Sci. Rep. 2016, 6, 32185. [Google Scholar] [CrossRef]
- Kurbanoglu, S.; Ozkan, S.A.; Merkoçi, A. Nanomaterials-based enzyme electrochemical biosensors operating through inhibition for biosensing applications. Biosens. Bioelectron. 2017, 89, 886–898. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, S.; Li, J.; Chen, L. Nanomaterial-based optical sensors for mercury ions. TrAC Trends Anal. Chem. 2016, 82, 175–190. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Sempionatto, J.R.; Barfidokht, A.; Mishra, R.K.; Campbell, A.S.; Hubble, L.J.; Wang, J. Wearable Bioelectronics: Enzyme-Based Body-Worn Electronic Devices. Acc. Chem. Res. 2018, 51, 2820–2828. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Lee, S.N.; Shin, M.K.; Kim, H.-W.; Choi, H.K.; Lee, T.; Choi, J.-W. Flexible electrochemical glucose biosensor based on GOx/gold/MoS2/gold nanofilm on the polymer electrode. Biosens. Bioelectron. 2019, 140, 111343. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.-F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Farka, Z.; Juřík, T.; Kovář, D.; Trnková, L.; Skládal, P. Nanoparticle-Based Immunochemical Biosensors and Assays: Recent Advances and Challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, Q. The role of nanomaterials in electroanalytical biosensors: A mini review. J. Electroanal. Chem. 2016, 781, 401–409. [Google Scholar] [CrossRef]
- El-Said, W.A.; Yoon, J.; Choi, J.-W. Nanostructured surfaces for analysis of anticancer drug and cell diagnosis based on electrochemical and SERS tools. Nano Converg. 2018, 5, 4. [Google Scholar] [CrossRef]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Kurmus, H.; Milas, J.; Arulrajah, A.; Horpibulsuk, S.; Abdul Kadir, A. Nanoparticles in Construction Materials and Other Applications, and Implications of Nanoparticle Use. Materials 2019, 12, 3052. [Google Scholar] [CrossRef]
- Lee, T.; Park, S.Y.; Jang, H.; Kim, G.H.; Lee, Y.; Park, C.; Mohammadniaei, M.; Lee, M.-H.; Min, J. Fabrication of Electrochemical Biosensor consisted of Multi-functional DNA Structure/porous Au Nanoparticle for Avian Influenza Virus (H5N1) in Human Serum. Mater. Sci. Eng. C 2019, 99, 511–519. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef]
- Zhao, B.; Huang, Q.; Dou, L.; Bu, T.; Chen, K.; Yang, Q.; Yan, L.; Wang, J.; Zhang, D. Prussian blue nanoparticles based lateral flow assay for high sensitive determination of clenbuterol. Sens. Actuator B Chem. 2018, 275, 223–229. [Google Scholar] [CrossRef]
- Guo, P.; Sikdar, D.; Huang, X.; Si, K.J.; Xiong, W.; Gong, S.; Yap, L.W.; Premaratne, M.; Cheng, W. Plasmonic core–shell nanoparticles for SERS detection of the pesticide thiram: Size- and shape-dependent Raman enhancement. Nanoscale 2015, 7, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Theiss, J.; Pavaskar, P.; Echternach, P.M.; Muller, R.E.; Cronin, S.B. Plasmonic Nanoparticle Arrays with Nanometer Separation for High-Performance SERS Substrates. Nano Lett. 2010, 10, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Wang, C.; Rong, Z.; Ding, H.; Li, H.; Li, S.; Shao, N.; Dong, P.; Xiao, R.; et al. Facile Synthesis of Au-Coated Magnetic Nanoparticles and Their Application in Bacteria Detection via a SERS Method. ACS Appl. Mater. Interfaces 2016, 8, 19958–19967. [Google Scholar] [CrossRef]
- Lee, T.; Yoo, S.-Y.; Chung, Y.-H.; Min, J.; Choi, J.-W. Signal Enhancement of Electrochemical Biomemory Device Composed of Recombinant Azurin/Gold Nanoparticle. Electroanalysis 2011, 23, 2023–2029. [Google Scholar] [CrossRef]
- Tian, L.; Qian, K.; Qi, J.; Liu, Q.; Yao, C.; Song, W.; Wang, Y. Gold nanoparticles superlattices assembly for electrochemical biosensor detection of microRNA-21. Biosens. Bioelectron. 2018, 99, 564–570. [Google Scholar] [CrossRef]
- Sanaeifar, N.; Rabiee, M.; Abdolrahim, M.; Tahriri, M.; Vashaee, D.; Tayebi, L. A novel electrochemical biosensor based on Fe3O4 nanoparticles-polyvinyl alcohol composite for sensitive detection of glucose. Anal. Biochem. 2017, 519, 19–26. [Google Scholar] [CrossRef]
- Cheng, Z.; Choi, N.; Wang, R.; Lee, S.; Moon, K.C.; Yoon, S.-Y.; Chen, L.; Choo, J. Simultaneous Detection of Dual Prostate Specific Antigens Using Surface-Enhanced Raman Scattering-Based Immunoassay for Accurate Diagnosis of Prostate Cancer. ACS Nano 2017, 11, 4926–4933. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum Nanocatalyst Amplification: Redefining the Gold Standard for Lateral Flow Immunoassays with Ultrabroad Dynamic Range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef]
- Bo, B.; Zhang, T.; Jiang, Y.; Cui, H.; Miao, P. Triple Signal Amplification Strategy for Ultrasensitive Determination of miRNA Based on Duplex Specific Nuclease and Bridge DNA–Gold Nanoparticles. Anal. Chem. 2018, 90, 2395–2400. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Wu, L.-P.; Chou, T.-T.; Hsieh, Y.-Z. Functional magnetic nanoparticles–assisted electrochemical biosensor for eosinophil cationic protein in cell culture. Sens. Actuator B Chem. 2018, 257, 672–677. [Google Scholar] [CrossRef]
- Ramnani, P.; Saucedo, N.M.; Mulchandani, A. Carbon nanomaterial-based electrochemical biosensors for label-free sensing of environmental pollutants. Chemosphere 2016, 143, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dai, Z. Carbon nanomaterials-based electrochemical biosensors: An overview. Nanoscale 2015, 7, 6420–6431. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, T.; Hasebe, Y.; Zhang, Z.; Tao, D. Carbon Black-Carbon Nanotube Co-Doped Polyimide Sensors for Simultaneous Determination of Ascorbic Acid, Uric Acid, and Dopamine. Materials 2018, 11, 1691. [Google Scholar] [CrossRef]
- Buiculescu, R.; Stefanakis, D.; Androulidaki, M.; Ghanotakis, D.; Chaniotakis, N.A. Controlling carbon nanodot fluorescence for optical biosensing. Analyst 2016, 141, 4170–4180. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Yook, J.-G. Graphene Nanomaterials-Based Radio-Frequency/Microwave Biosensors for Biomaterials Detection. Materials 2019, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, X.; Wu, Q.; Yi, J.; Zhang, G. Carbon Nanodots-Based Fluorescent Turn-On Sensor Array for Biothiols. Anal. Chem. 2017, 89, 7084–7089. [Google Scholar] [CrossRef]
- Fang, D.; Gao, G.; Shen, J.; Yu, Y.; Zhi, J. A reagentless electrochemical biosensor based on thionine wrapped E. coli and chitosan-entrapped carbon nanodots film modified glassy carbon electrode for wastewater toxicity assessment. Electrochim. Acta 2016, 222, 303–311. [Google Scholar] [CrossRef]
- Mao, Y.; Cui, S.; Li, W.; Fan, X.; Liu, Y.; Xu, S.; Luo, X. A lab-on-a-carbon nanodot sensor array for simultaneous pattern recognition of multiple antibiotics. Sens. Actuator B Chem. 2019, 296, 126694. [Google Scholar] [CrossRef]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-Based Materials for Biosensors: A Review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, S.; Min, D.-H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Salimian, R. Ultrasensitive detection of cancer biomarkers using conducting polymer/electrochemically reduced graphene oxide-based biosensor: Application toward BRCA1 sensing. Sens. Actuator B Chem. 2018, 266, 160–169. [Google Scholar] [CrossRef]
- García-Mendiola, T.; Bravo, I.; López-Moreno, J.M.; Pariente, F.; Wannemacher, R.; Weber, K.; Popp, J.; Lorenzo, E. Carbon nanodots based biosensors for gene mutation detection. Sens. Actuator B Chem. 2018, 256, 226–233. [Google Scholar] [CrossRef]
- Lu, L.; Zhou, L.; Chen, J.; Yan, F.; Liu, J.; Dong, X.; Xi, F.; Chen, P. Nanochannel-Confined Graphene Quantum Dots for Ultrasensitive Electrochemical Analysis of Complex Samples. ACS Nano 2018, 12, 12673–12681. [Google Scholar] [CrossRef]
- Ghrera, A.S.; Pandey, C.M.; Malhotra, B.D. Multiwalled carbon nanotube modified microfluidic-based biosensor chip for nucleic acid detection. Sens. Actuator B Chem. 2018, 266, 329–336. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Tan, C.; Zhang, X.; Lu, Q.; Sindoro, M.; Huang, X.; Huang, W.; Wang, L.; Zhang, H. Two-dimensional transition metal dichalcogenide nanomaterials for biosensing applications. Mater. Chem. Front. 2017, 1, 24–36. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Gadhari, N.S.; Li, X.; Rao, Z.; Navale, S.T.; Shen, Y.; Patil, V.R.; Huang, Y. Recent advances in MXene–based electrochemical sensors and biosensors. TrAC Trends Anal. Chem. 2019, 120, 115643. [Google Scholar] [CrossRef]
- Barua, S.; Dutta, H.S.; Gogoi, S.; Devi, R.; Khan, R. Nanostructured MoS2—Based Advanced Biosensors: A Review. ACS Appl. Nano Mater. 2018, 1, 2–25. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Wang, Q.; Xiao, M.; Zhong, D.; Sun, W.; Zhang, G.; Zhang, Z. Ultrasensitive Monolayer MoS2 Field-Effect Transistor Based DNA Sensors for Screening of Down Syndrome. Nano Lett. 2019, 19, 1437–1444. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. TrAC Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Liu, H.; Duan, C.; Yang, C.; Shen, W.; Wang, F.; Zhu, Z. A novel nitrite biosensor based on the direct electrochemistry of hemoglobin immobilized on MXene-Ti3C2. Sens. Actuator B Chem. 2015, 218, 60–66. [Google Scholar] [CrossRef]
- Lopez-Iscoa, P.; Ojha, N.; Aryal, U.; Pugliese, D.; Boetti, N.G.; Milanese, D.; Petit, L. Spectroscopic Properties of Er3+—Doped Particles—Containing Phosphate Glasses Fabricated Using the Direct Doping Method. Materials 2019, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Zhang, Q. Recent Advances on Functionalized Upconversion Nanoparticles for Detection of Small Molecules and Ions in Biosystems. Adv. Sci. 2018, 5, 1700609. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Xu, X.; Wang, X.; Zhou, Q.; Ding, W.; Chen, B.; Guo, X.; Hao, Y.; Chen, G. Point of care upconversion nanoparticles-based lateral flow assay quantifying myoglobin in clinical human blood samples. Sens. Actuator B Chem. 2019, 282, 309–316. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, S.; Pineda-Gómez, R.; Ibarlucea, B.; Ma, J.; Lohe, M.R.; Akbar, T.F.; Baraban, L.; Cuniberti, G.; Feng, X. Electrochemically Exfoliated High-Quality 2H-MoS2 for Multiflake Thin Film Flexible Biosensors. Small 2019, 15, 1901265. [Google Scholar] [CrossRef]
- Kumar, S.; Lei, Y.; Alshareef, N.H.; Quevedo-Lopez, M.A.; Salama, K.N. Biofunctionalized two-dimensional Ti3C2 MXenes for ultrasensitive detection of cancer biomarker. Biosens. Bioelectron. 2018, 121, 243–249. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.; Duan, C.; Xiao, D.; Tang, Y.; Zhu, J. An organ-like titanium carbide material (MXene) with multilayer structure encapsulating hemoglobin for a mediator-free biosensor. J. Electrochem. Soc. 2015, 162, B16–B21. [Google Scholar] [CrossRef]
- He, H.; Liu, B.; Wen, S.; Liao, J.; Lin, G.; Zhou, J.; Jin, D. Quantitative Lateral Flow Strip Sensor Using Highly Doped Upconversion Nanoparticles. Anal. Chem. 2018, 90, 12356–12360. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, J.; Choi, H. Magnetic Particle Filled Elastomeric Hybrid Composites and Their Magetorheological Response. Materials 2018, 11, 1040. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, C.; Wang, M.; Guo, Q.; Wang, B.; Luo, W.; Wang, Y.; Zhang, C.; Zhou, L.; Zhang, D.; et al. Efficient Photocatalytic Degradation of Malachite Green in Seawater by the Hybrid of Zinc-Oxide Nanorods Grown on Three-Dimensional (3D) Reduced Graphene Oxide(RGO)/Ni Foam. Materials 2018, 11, 1004. [Google Scholar] [CrossRef]
- Zhang, S.; Pelligra, C.I.; Feng, X.; Osuji, C.O. Directed Assembly of Hybrid Nanomaterials and Nanocomposites. Adv. Mater. 2018, 30, 1705794. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, M.; Lischner, J.; Meng, S.; Prezhdo, O.V. Coexistence of Different Charge-Transfer Mechanisms in the Hot-Carrier Dynamics of Hybrid Plasmonic Nanomaterials. Nano Lett. 2019, 19, 3187–3193. [Google Scholar] [CrossRef]
- Wang, B.B.; Zhong, X.X.; Ming, B.M.; Zhu, M.K.; Chen, Y.A.; Cvelbar, U.; Ostrikov, K. Structure and photoluminescence properties of MoO3−/graphene nanoflake hybrid nanomaterials formed via surface growth. Appl. Surf. Sci. 2019, 480, 1054–1062. [Google Scholar] [CrossRef]
- Nugroho, F.A.A.; Darmadi, I.; Cusinato, L.; Susarrey-Arce, A.; Schreuders, H.; Bannenberg, L.J.; da Silva Fanta, A.B.; Kadkhodazadeh, S.; Wagner, J.B.; Antosiewicz, T.J.; et al. Metal–polymer hybrid nanomaterials for plasmonic ultrafast hydrogen detection. Nat. Mater. 2019, 18, 489–495. [Google Scholar] [CrossRef]
- Zhu, J.; Wen, M.; Wen, W.; Du, D.; Zhang, X.; Wang, S.; Lin, Y. Recent progress in biosensors based on organic-inorganic hybrid nanoflowers. Biosens. Bioelectron. 2018, 120, 175–187. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, T.; Bapurao, G.B.; Jo, J.; Oh, B.-K.; Choi, J.-W. Electrochemical H2O2 biosensor composed of myoglobin on MoS2 nanoparticle-graphene oxide hybrid structure. Biosens. Bioelectron. 2017, 93, 14–20. [Google Scholar] [CrossRef]
- Wang, P.; Cao, L.; Chen, Y.; Wu, Y.; Di, J. Photoelectrochemical Biosensor Based on Co3O4 Nanoenzyme Coupled with PbS Quantum Dots for Hydrogen Peroxide Detection. ACS Appl. Nano Mater. 2019, 2, 2204–2211. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Sun, M.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Hybrid Nanoparticle Pyramids for Intracellular Dual MicroRNAs Biosensing and Bioimaging. Adv. Mater. 2017, 29, 1606086. [Google Scholar] [CrossRef]
- Rabie, H.; Zhang, Y.; Pasquale, N.; Lagos, M.J.; Batson, P.E.; Lee, K. NIR Biosensing of Neurotransmitters in Stem Cell-Derived Neural Interface Using Advanced Core–Shell Upconversion Nanoparticles. Adv. Mater. 2019, 31, 1806991. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, J. Hollow carbon sphere supported Ag nanoparticles for promoting electrocatalytic performance of dopamine sensing. Sens. Actuator B Chem. 2019, 290, 648–655. [Google Scholar] [CrossRef]
- Pulgarin, J.A.M.; Molina, A.A.; Garcia, E.J.; Gomez, L.G. A sensitive resonance Rayleigh scattering sensor for dopamine in urine using upconversion nanoparticles. J. Raman Spectrosc. 2019, 1–8. [Google Scholar] [CrossRef]
- Kim, J.; Kumar, R.; Bandodkar, A.J.; Wang, J. Advanced Materials for Printed Wearable Electrochemical Devices: A Review. Adv. Electron. Mater. 2017, 3, 1600260. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Wang, J. Tattoo-Based Wearable Electrochemical Devices: A Review. Electroanalysis 2015, 27, 562–572. [Google Scholar] [CrossRef]
- Nyein, H.Y.Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H.M.; Tai, L.-C.; Ota, H.; Davis, R.W.; et al. A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216–7224. [Google Scholar] [CrossRef]
- Barfidokht, A.; Mishra, R.K.; Seenivasan, R.; Liu, S.; Hubble, L.J.; Wang, J.; Hall, D.A. Wearable electrochemical glove-based sensor for rapid and on-site detection of fentanyl. Sens. Actuator B Chem. 2019, 296, 126422. [Google Scholar] [CrossRef]
- Xuan, X.; Yoon, H.S.; Park, J.Y. A wearable electrochemical glucose sensor based on simple and low-cost fabrication supported micro-patterned reduced graphene oxide nanocomposite electrode on flexible substrate. Biosens. Bioelectron. 2018, 109, 75–82. [Google Scholar] [CrossRef]
- Yang, P.; Pang, J.; Hu, F.; Peng, J.; Jiang, D.; Chu, Z.; Jin, W. An ultrasensitive biosensing flexible chip using a novel silver@Prussian blue core-shell nanocube composite. Sens. Actuator B Chem. 2018, 276, 31–41. [Google Scholar] [CrossRef]
- Tabassum, H.; Zou, R.; Mahmood, A.; Liang, Z.; Wang, Q.; Zhang, H.; Gao, S.; Qu, C.; Guo, W.; Guo, S. A Universal Strategy for Hollow Metal Oxide Nanoparticles Encapsulated into B/N Co-Doped Graphitic Nanotubes as High-Performance Lithium-Ion Battery Anodes. Adv. Mater. 2018, 30, 1705441. [Google Scholar] [CrossRef]
- Lee, T.; Lee, W.; Kim, S.-W.; Kim, J.J.; Kim, B.-S. Flexible Textile Strain Wireless Sensor Functionalized with Hybrid Carbon Nanomaterials Supported ZnO Nanowires with Controlled Aspect Ratio. Adv. Funct. Mater. 2016, 26, 6206–6214. [Google Scholar] [CrossRef]




| Target | System | Nanomaterial | Sensing Probe | Advantage | Detection Limit | Ref. |
|---|---|---|---|---|---|---|
| f-PSA c-PSA | PSA/MGITC@AuNPs/magnetic NPs | AuNPs/magnetic NPs | f-PSA antibody, c-PSA antibody | Effective collection of probe NPs for enhancing the sensitivity | 0.012 ng/mL (f-PSA), 0.15 ng/mL (c-PSA) | [47] |
| HIV p24 | Ab-PtNPs/HIV p24/Nanobody-biotin/polystrept abidin | PtNPs | HIV p24 antibody/PtNPs conjugates | Apparent colorimetric band formation by catalytic reaction derived from porous PtNPs | 0.8 pg/mL | [48] |
| eosinophil cationic protein (ECP) | Hep-Au@Fe3O4 | Au@Fe3O4 | Hep-Au@Fe3O4 | Effective collection of probe NPs for enhancing the sensitivity | 0.3 nM | [50] |
| DNA | DNA/Probe/CDs/AuSPE | CDs | Unmodified oligonucleotides | Synthesis of surface modified CDs immobilized with unmodified oligonucleotides | 0.16 nM (100 times higher than sensor prepared without CDs) | [62] |
| DA, Hg2+, Cu2+, Cd2+ | GQDs/VMSF | GQDs | OH-GQDs, NH2-GQDs | Effective detection of various type of metal ion and small molecule using surface modified GQDs | 120 nM (DA) 0.3 μM (Hg2+) 32 nM (Cu2+) 140 nM (Cd2+) (3 times higher than sensor prepared without GQDs) | [63] |
| Nucleic acid | pDNA/MWCNT/ITO | MWCNT | Amine terminated DNA | Electrochemical signal enhancement | 1 fM (10,000 times higher than sensor prepared without MWCNT) | [64] |
| VP40 | VP40/BSA/MoS2/Au electrode | MoS2 | VP40 antibody | Electrochemical signal enhancement | 2 fM | [74] |
| Carcinoembrynic antigen (CEA) | Anti-CEA/f-Ti3C2/GC | Ti3C2 | Carcino embryonic antibody monoclonal | Synthesis of surface modified Ti3C2 and electrochemical signal enhancement | 0.018 pg/mL | [75] |
| PSA, EphA2 | Highly doped UCNPs | UCNPs | PSA antibody, EphA2 antibody | Luminescence signal enhancement using highly lanthanide ion doped UCNPs | 89 pg/mL (PSA), 400 pg/mL (EphA2) (5 times and 12 times higher than lower doped UCNPs) | [77] |
| DA | GO/Aptamer/SW-UCNPs | GO/UCNPs | DA-specific aptamer | Luminescence signal enhancement due to the sandwich structure of UCNPs | ~1 pM | [88] |
| glucose | GOx/Au/MoS2/Au/PI | Au/MoS2/Au nanofilm | Glucose oxidase | Electrochemical signal enhancement | 10 nM (3 times higher than sensor prepared without MoS2) | [32] |
| H2O2 | AgNCs/PB/PET | AgNCs | Glucose oxidase | Electrochemical signal enhancement | 2 μM (4 times higher than sensor prepared without AgNCs) | [96] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.; Shin, M.; Lee, T.; Choi, J.-W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials 2020, 13, 299. https://doi.org/10.3390/ma13020299
Yoon J, Shin M, Lee T, Choi J-W. Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials. 2020; 13(2):299. https://doi.org/10.3390/ma13020299
Chicago/Turabian StyleYoon, Jinho, Minkyu Shin, Taek Lee, and Jeong-Woo Choi. 2020. "Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review" Materials 13, no. 2: 299. https://doi.org/10.3390/ma13020299
APA StyleYoon, J., Shin, M., Lee, T., & Choi, J.-W. (2020). Highly Sensitive Biosensors Based on Biomolecules and Functional Nanomaterials Depending on the Types of Nanomaterials: A Perspective Review. Materials, 13(2), 299. https://doi.org/10.3390/ma13020299






