Molten Salt Synthesis of High-Performance, Nanostructured La0.6Sr0.4FeO3−δ Oxygen Electrode of a Reversible Solid Oxide Cell
Abstract
:1. Introduction
2. Materials and Methods
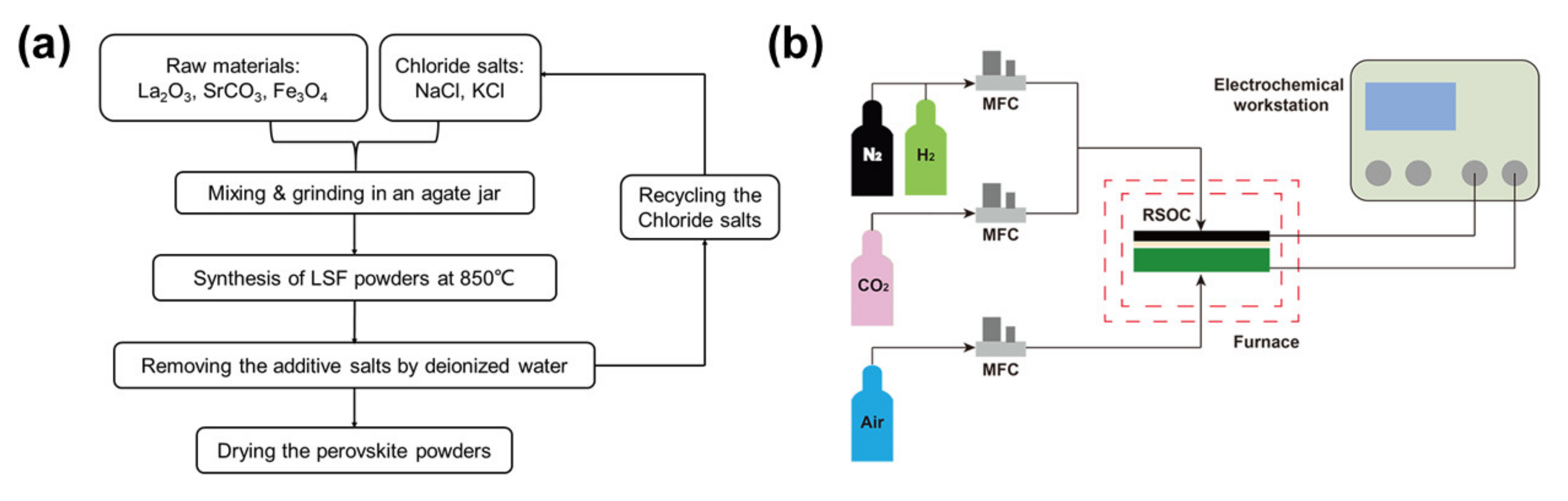
2.1. Synthesis and Characterization of LSF Powders
2.2. Fabrication and Measurement of the Button Cell with Directly Assembled LSF Electrode
3. Results
3.1. Characterization of LSF Powders
3.2. Electrochemical Performance Measurement
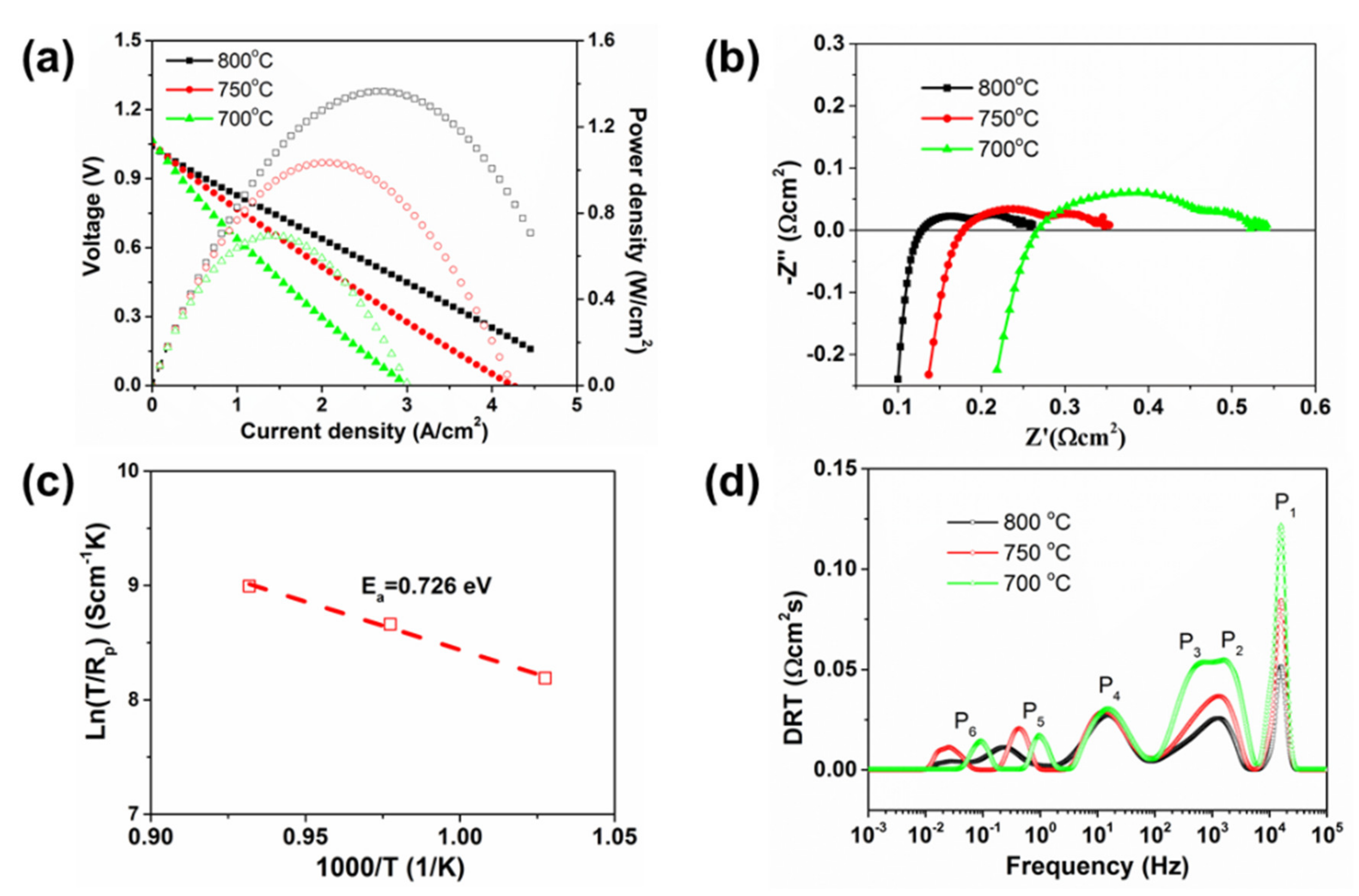
3.2.1. SOFC Performance
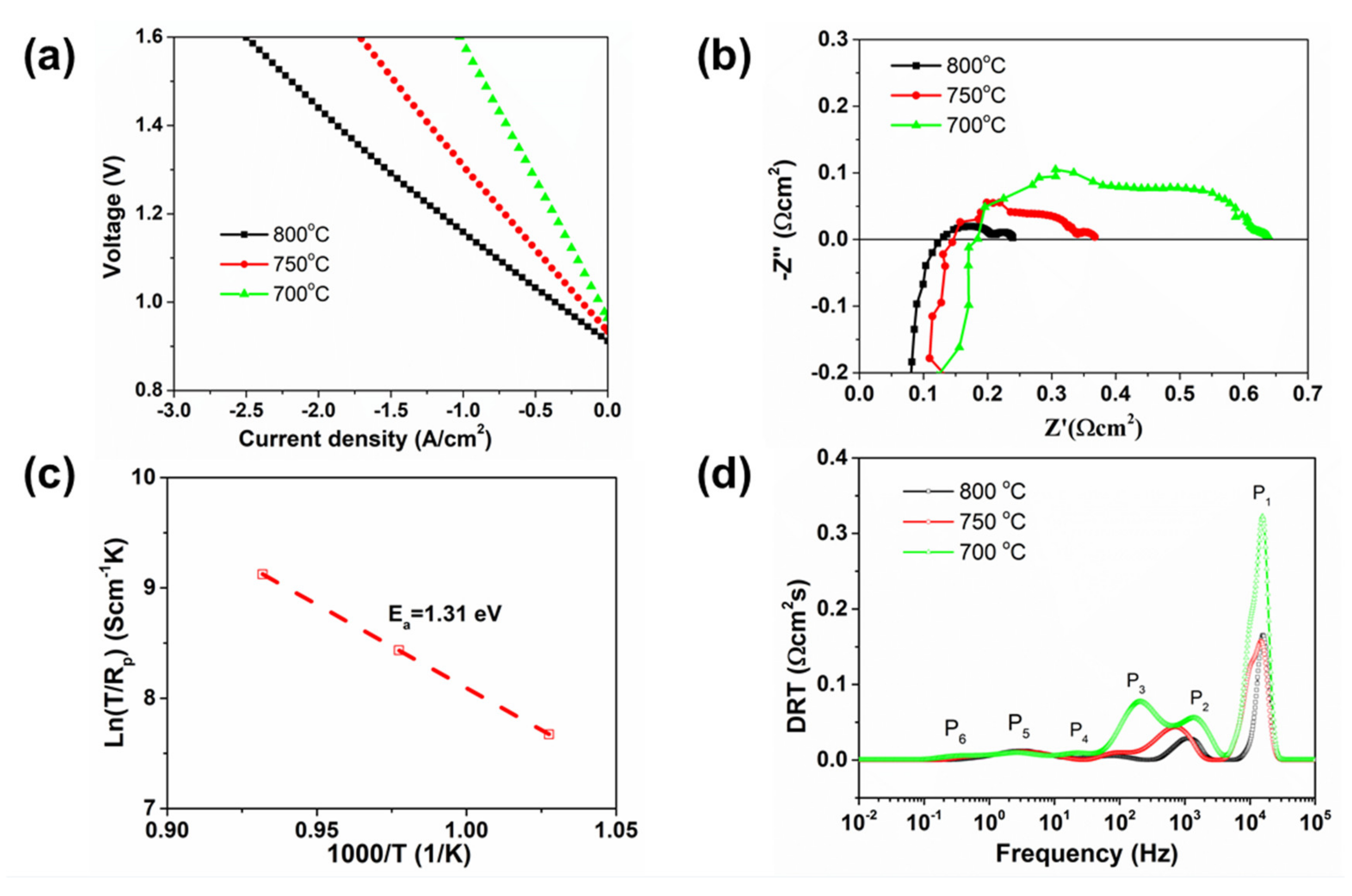
3.2.2. SOEC Performance
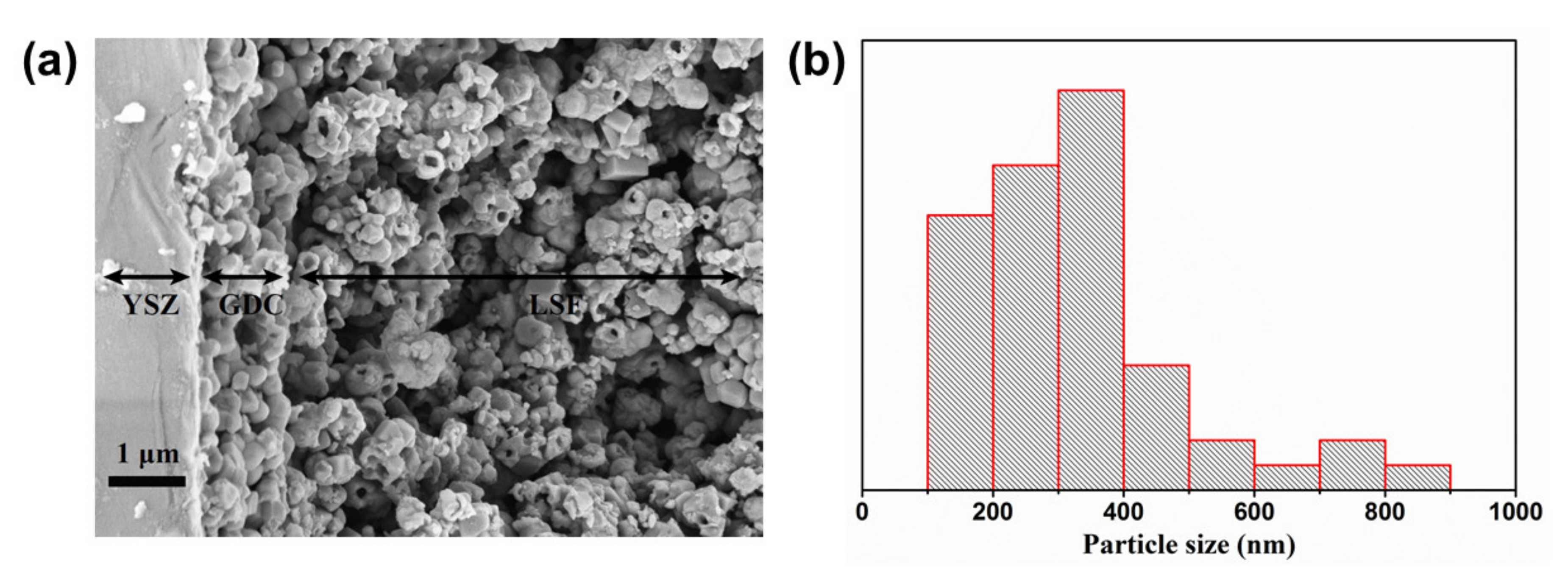
3.3. Microstructure Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jensen, S.H.; Graves, C.; Mogensen, M.; Wendel, C.; Braun, R.; Hughes, G.; Gao, Z.; Barnett, S.A. Large-scale electricity storage utilizing reversible solid oxide cells combined with underground storage of CO2 and CH4. Energy Environ. Sci. 2015, 8, 2471–2479. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic Fuel Cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Hansen, J.B. Solid oxide electrolysis—A key enabling technology for sustainable energy scenarios. Faraday Discuss. 2015, 182, 9–48. [Google Scholar] [CrossRef] [PubMed]
- Risch, M. Perovskite Electrocatalysts for the Oxygen Reduction Reaction in Alkaline Media. Catalysts 2017, 7, 154. [Google Scholar] [CrossRef] [Green Version]
- Simner, S.P.; Bonnett, J.F.; Canfield, N.L.; Meinhardt, K.D.; Shelton, J.P.; Sprenkle, V.L.; Stevenson, J.W. Development of lanthanum ferrite SOFC cathodes. J. Power Sources 2003, 113, 1–10. [Google Scholar] [CrossRef]
- Kim, M.C.; Park, S.J.; Haneda, H.; Tanaka, J.; Shirasaki, S. High temperature electrical conductivity of La1−xSrxFeO3−δ (x > 0.5). Solid State Ionics 1990, 40, 239–243. [Google Scholar]
- Wu, T.; Yu, B.; Zhang, W.; Chen, J.; Zhao, S. Fabrication of a high-performance nano-structured Ln1−xSrxMO3−δ (Ln=La, Sm; M=Mn, Co, Fe) SOC electrode through infiltration. RSC Adv. 2016, 6, 68379–68387. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, Y.; Han, M. Infiltration of La0.6Sr0.4FeO3-δ nanoparticles into YSZ scaffold for solid oxide fuel cell and solid oxide electrolysis cell. J. Alloys Compd. 2017, 723, 620–626. [Google Scholar] [CrossRef]
- Xue, P.; Wu, H.; Lu, Y.; Zhu, X. Recent progress in molten salt synthesis of low-dimensional perovskite oxide nanostructures, structural characterization, properties, and functional applications: A review. J. Mater. Sci. Technol. 2018, 34, 914–930. [Google Scholar] [CrossRef]
- Song, S.; Zhou, J.; Zhang, S.; Zhang, L.; Li, J.; Wang, Y.; Han, L.; Long, Y.; Hu, Z.; Wang, J.-Q. Molten-salt synthesis of porous La0.6Sr0.4Co0.2Fe0.8O2.9 perovskite as an efficient electrocatalyst for oxygen evolution. Nano Res. 2018, 11, 4796–4805. [Google Scholar] [CrossRef]
- Guan, C.; Wang, Y.; Chen, K.; Xiao, G.; Lin, X.; Zhou, J.; Song, S.; Wang, J.-Q.; Zhu, Z.; Zhou, X.-D. Molten salt synthesis of Nb-doped (La, Sr)FeO3 as the oxygen electrode for reversible solid oxide cells. Mater. Lett. 2019, 245, 114–117. [Google Scholar] [CrossRef]
- Hardy, J.S.; Templeton, J.W.; Edwards, D.J.; Lu, Z.; Stevenson, J.W. Lattice expansion of LSCF-6428 cathodes measured by in situ XRD during SOFC operation. J. Power Sources 2012, 198, 76–82. [Google Scholar] [CrossRef]
- Caillol, N.; Pijolat, M.; Siebert, E. Investigation of chemisorbed oxygen, surface segregation and effect of post-treatments on La0.8Sr0.2MnO3 powder and screen-printed layers for solid oxide fuel cell cathodes. Appl. Surf. Sci. 2007, 253, 4641–4648. [Google Scholar] [CrossRef]
- Kindermann, L.; Das, D.; Nickel, H.; Hilpert, K. Chemical compatibility of the LaFeO3 base perovskites (La0.6Sr0.4)(z)Fe(0.8)M(0.2)O(3-delta) (z=1, 0.9; M=Cr, Mn, Co, Ni) with yttria stabilized zirconia. Solid State Ionics 1996, 89, 215–220. [Google Scholar] [CrossRef]
- Ardigò, M.R.; Perron, A.; Combemale, L.; Heintz, O.; Caboche, G.; Chevalier, S. Interface reactivity study between La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF) cathode material and metallic interconnect for fuel cell. J. Power Sources 2011, 196, 2037–2045. [Google Scholar] [CrossRef]
- Jiang, S.P. Thermally and Electrochemically Induced Electrode/Electrolyte Interfaces in Solid Oxide Fuel Cells: An AFM and EIS Study. J. Electrochem. Soc. 2015, 162, F1119–F1128. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Ai, N.; Chen, K.; Cheng, Y.; He, S.; Saunders, M.; Dodd, A.; Suvorova, A.; Jiang, S.P. In situ assembled La0.8Sr0.2MnO3 cathodes on a Y2O3–ZrO2 electrolyte of solid oxide fuel cells-interface and electrochemical activity. RSC Adv. 2016, 6, 99211–99219. [Google Scholar] [CrossRef]
- Chen, K.; Li, N.; Ai, N.; Cheng, Y.; Rickard, W.D.A.; Jiang, S.P. Polarization-Induced Interface and Sr Segregation of in Situ Assembled La0.6Sr0.4Co0.2Fe0.8O3−δ Electrodes on Y2O3-ZrO2 Electrolyte of Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2016, 8, 31729–31737. [Google Scholar] [CrossRef]
- Ai, N.; He, S.; Li, N.; Zhang, Q.; Rickard, W.D.A.; Chen, K.; Zhang, T.; Jiang, S.P. Suppressed Sr segregation and performance of directly assembled La0.6Sr0.4Co0.2Fe0.8O3−δ oxygen electrode on Y2O3-ZrO2 electrolyte of solid oxide electrolysis cells. J. Power Sources 2018, 384, 125–135. [Google Scholar] [CrossRef]
- Graat, P.C.J.; Somers, M.A.J. Simultaneous determination of composition and thickness of thin iron-oxide films from XPS Fe 2p spectra. Appl. Surf. Sci. 1996, 100, 36–40. [Google Scholar] [CrossRef]
- Seyama, H.; Soma, M. Bonding-state characterization of the constituent elements of silicate minerals by X-ray photoelectron spectroscopy. J. Chem. Soc. Faraday Trans. 1985, 81, 485–495. [Google Scholar] [CrossRef]
- Allen, G.C.; Curtis, M.T.; Hooper, A.J. X-Ray photoelectron spectroscopy of iron-oxygen systems. J. Chem. Soc. Dalton Trans. 1974, 14, 1525–1530. [Google Scholar] [CrossRef]
- Langevoort, J.C.; Sutherland, I.; Hanekamp, L.J.; Gellings, P.J. On the oxide formation on stainless steels AISI 304 and incoloy 800H investigated with XPS. Appl. Surf. Sci. 1987, 28, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wei, B.; Cao, Z.; Yue, X.; Zhang, Y.; Lü, Z. Niobium doped lanthanum strontium ferrite as a redox-stable and sulfur-tolerant anode for solid oxide fuel cells. ChemSusChem 2018, 11, 254–263. [Google Scholar] [CrossRef]
- Nitadori, T.; Misono, M. Catalytic properties of La1−xA′xFeO3(A′=Sr, Ce) and La1−xCexCoO3. J. Catal. 1985, 93, 459–466. [Google Scholar] [CrossRef]
- LagunaBercero, M.; Hanifi, A.; Monzon, H.; Cunningham, J.; Etsell, T.H.; Sarkar, P. High performance of microtubular solid oxide fuel cells using Nd2NiO4+δ-based composite cathodes. J. Mater. Chem. A 2014, 2, 9764–9770. [Google Scholar] [CrossRef] [Green Version]
- Simner, S.P.; Bonnett, J.F.; Canfield, N.L.; Meinhardt, K.D.; Sprenkle, V.L.; Stevenson, J.W. Optimized Lanthanum Ferrite-Based Cathodes for Anode-Supported SOFCs. Electrochem. Solid State Lett. 2002, 5, A173–A175. [Google Scholar] [CrossRef]
- Yu, T.; Mao, X.; Ma, G. Performance of cobalt-free perovskite La0.6Sr0.4Fe1−xNbxO3−δ cathode materials for proton-conducting IT-SOFC. J. Alloys Compd. 2014, 608, 30–34. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, X.; Xiao, G.; Zhao, F.; Chen, F. A Novel Electrode Material for Symmetrical SOFCs. Adv. Mater. 2010, 22, 5478–5482. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; He, T. Cobalt-free cathode material SrFe0.9Nb0.1O3−δ for intermediate-temperature solid oxide fuel cells. Electrochem. Commun. 2010, 12, 285–287. [Google Scholar] [CrossRef]
- Ling, Y.; Zhao, L.; Lin, B.; Dong, Y.; Zhang, X.; Meng, G.; Liu, X. Investigation of cobalt-free cathode material Sm0.5Sr0.5Fe0.8Cu0.2O3−δ for intermediate temperature solid oxide fuel cell. Int. J. Hydrogen Energy 2010, 35, 6905–6910. [Google Scholar] [CrossRef]
- Jiang, S.; Liang, F.; Zhou, W.; Shao, Z. Hierarchical porous cobalt-free perovskite electrode for highly efficient oxygen reduction. J. Mater. Chem. 2012, 22, 16214–16218. [Google Scholar] [CrossRef]
- Sun, X.; Bonaccorso, A.D.; Graves, C.; Ebbesen, S.D.; Jensen, S.H.; Hagen, A.; Holtappels, P.; Hendriksen, P.V.; Mogensen, M.B. Performance Characterization of Solid Oxide Cells Under High Pressure. Fuel Cells 2015, 15, 697–702. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Song, Y.; Zhang, X.; Guan, F.; Lv, H.; Liu, Q.; Miao, S.; Wang, G.; Bao, X. Enhancing CO2 electrolysis performance with vanadium-doped perovskite cathode in solid oxide electrolysis cell. Nano Energy 2018, 50, 43–51. [Google Scholar] [CrossRef]
- Zhou, N.; Yin, Y.-M.; Li, J.; Xu, L.; Ma, Z.-F. A robust high-performance cobalt-free oxygen electrode La0.5Sr0.5Fe0.8Cu0.15Nb0.05O3−δ for reversible solid oxide electrochemical cell. J. Power Sources 2017, 340, 373–379. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, C.; Dong, X.; Chen, F. Perovskite Sr2Fe1.5Mo0.5O6−δ as electrode materials for symmetrical solid oxide electrolysis cells. Int. J. Hydrogen Energy 2010, 35, 10039–10044. [Google Scholar] [CrossRef]
- Chauveau, F.; Mougin, J.; Mauvy, F.; Bassat, J.-M.; Grenier, J.-C. Development and operation of alternative oxygen electrode materials for hydrogen production by high temperature steam electrolysis. Int. J. Hydrogen Energy 2011, 36, 7785–7790. [Google Scholar] [CrossRef]
- Yang, S.; Wen, Y.; Zhang, J.; Lu, Y.; Ye, X.; Wen, Z. Electrochemical performance and stability of cobalt-free Ln1.2Sr0.8NiO4(Ln=La and Pr) air electrodes for proton-conducting reversible solid oxide cells. Electrochim. Acta 2018, 267, 269–277. [Google Scholar] [CrossRef]
- Stoots, C.M.; O’Brien, J.E.; Herring, J.S.; Hartvigsen, J.J. Syngas production via high-temperature coelectrolysis of steam and carbon dioxide. J. Fuel. Cell Sci. Technol. 2009, 6, 011014. [Google Scholar] [CrossRef]
- Vignesh, K.; Perumal, A.E.; Velmurugan, P. Optimization of resistance spot welding process parameters and microstructural examination for dissimilar welding of AISI 316L austenitic stainless steel and 2205 duplex stainless steel. Int. J. Adv. Manuf. Technol. 2017, 93, 455–465. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, X.; Chen, Z.; Guan, C.; Chen, K.; Song, S.; Xiao, G.; Pang, Y.; Wang, J.-Q. Molten Salt Synthesis of High-Performance, Nanostructured La0.6Sr0.4FeO3−δ Oxygen Electrode of a Reversible Solid Oxide Cell. Materials 2020, 13, 2267. https://doi.org/10.3390/ma13102267
Zuo X, Chen Z, Guan C, Chen K, Song S, Xiao G, Pang Y, Wang J-Q. Molten Salt Synthesis of High-Performance, Nanostructured La0.6Sr0.4FeO3−δ Oxygen Electrode of a Reversible Solid Oxide Cell. Materials. 2020; 13(10):2267. https://doi.org/10.3390/ma13102267
Chicago/Turabian StyleZuo, Xiaodong, Zhiyi Chen, Chengzhi Guan, Kongfa Chen, Sanzhao Song, Guoping Xiao, Yuepeng Pang, and Jian-Qiang Wang. 2020. "Molten Salt Synthesis of High-Performance, Nanostructured La0.6Sr0.4FeO3−δ Oxygen Electrode of a Reversible Solid Oxide Cell" Materials 13, no. 10: 2267. https://doi.org/10.3390/ma13102267
APA StyleZuo, X., Chen, Z., Guan, C., Chen, K., Song, S., Xiao, G., Pang, Y., & Wang, J.-Q. (2020). Molten Salt Synthesis of High-Performance, Nanostructured La0.6Sr0.4FeO3−δ Oxygen Electrode of a Reversible Solid Oxide Cell. Materials, 13(10), 2267. https://doi.org/10.3390/ma13102267




