Effect of Optimization of TiO2 Electron Transport Layer on Performance of Perovskite Solar Cells with Rough FTO Substrates
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Device Fabrication
2.2.1. Cleaning FTO Glass
2.2.2. Preparation of Electron Transport Layer
2.2.3. Preparation of Perovskite Absorption Layer
2.2.4. Preparation of Hole Transport Layer
2.2.5. Preparation of Carbon Film Counter Electrode
2.2.6. Solar Cell Package
2.3. Characterization
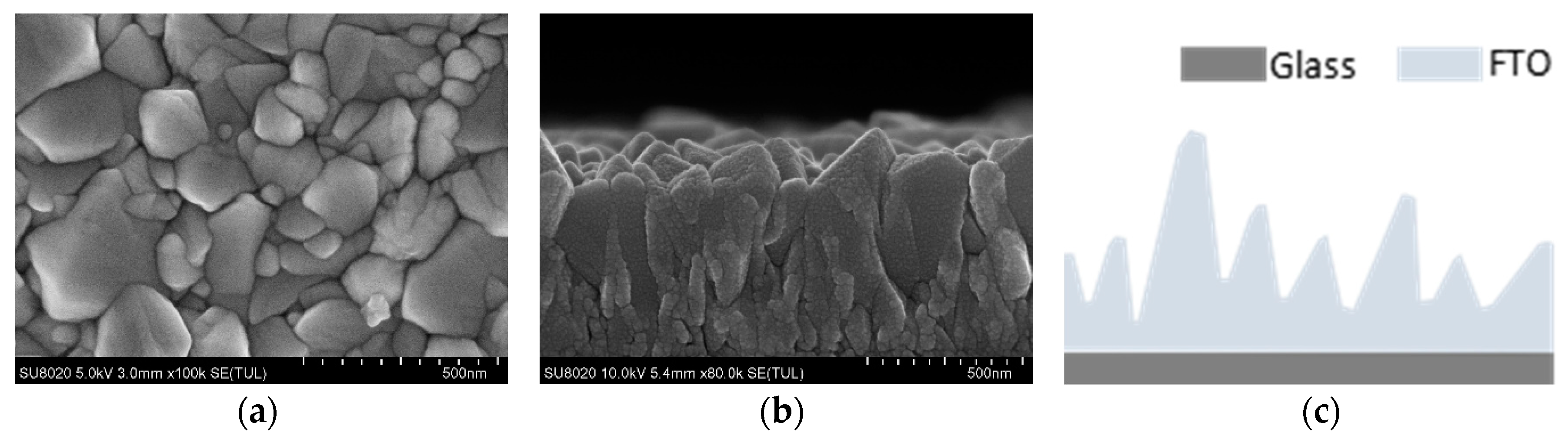
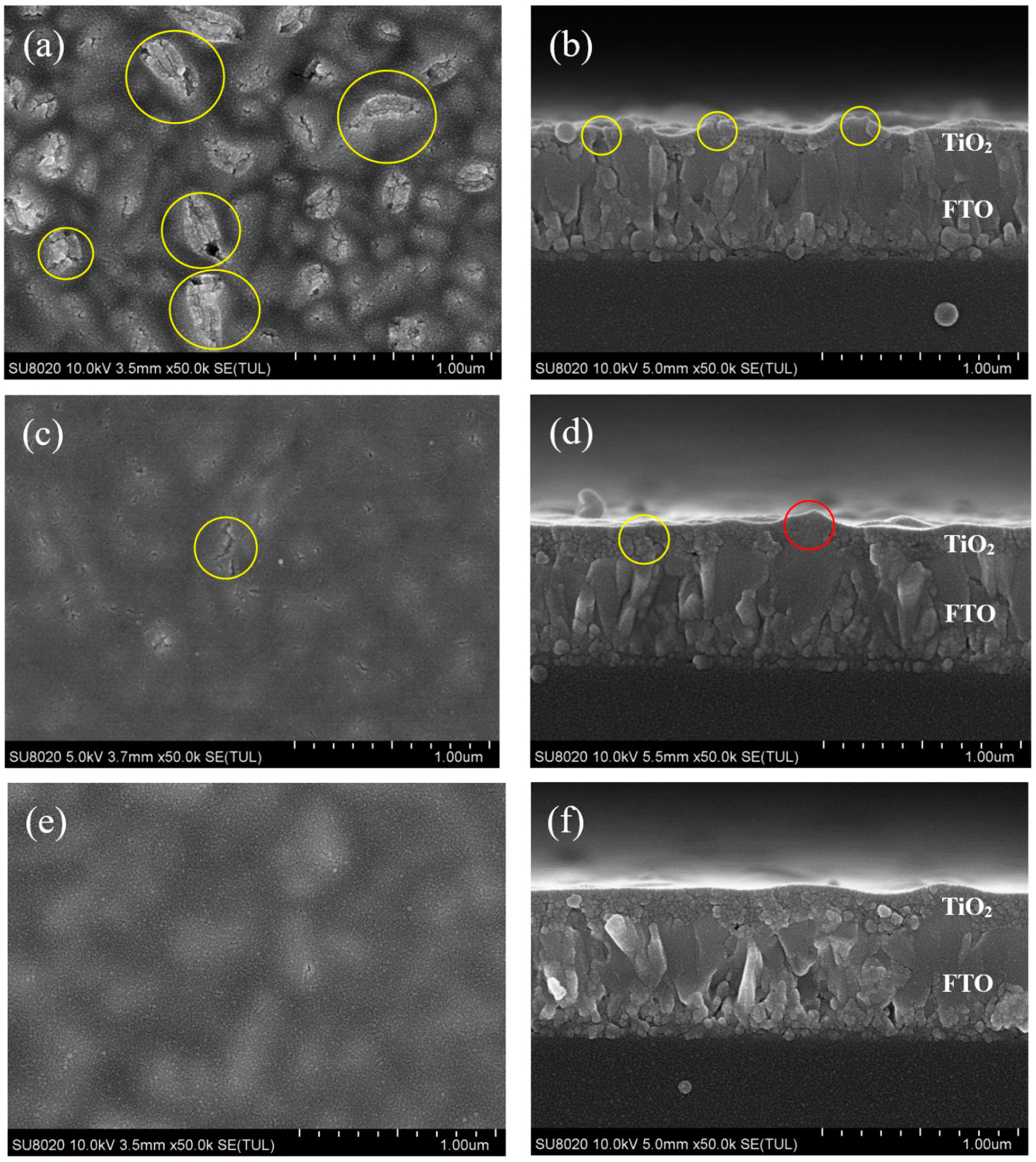
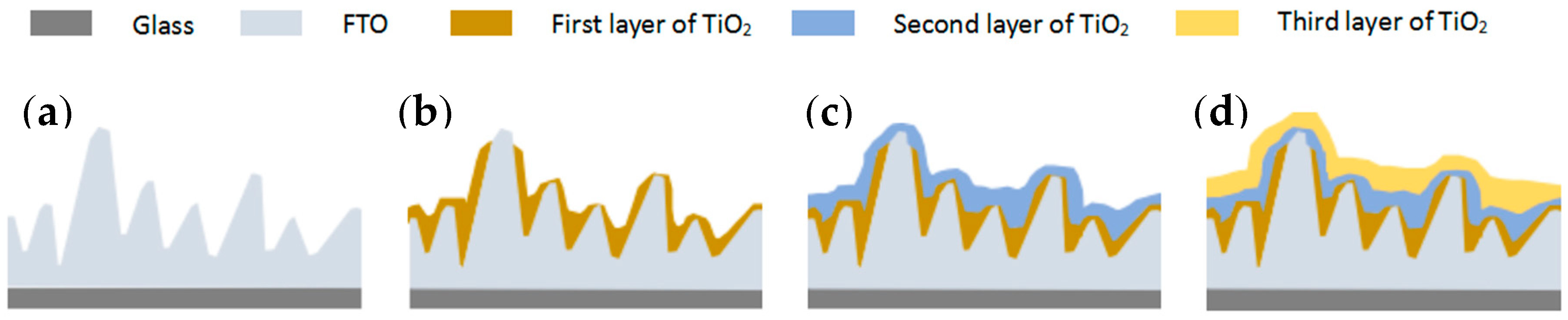
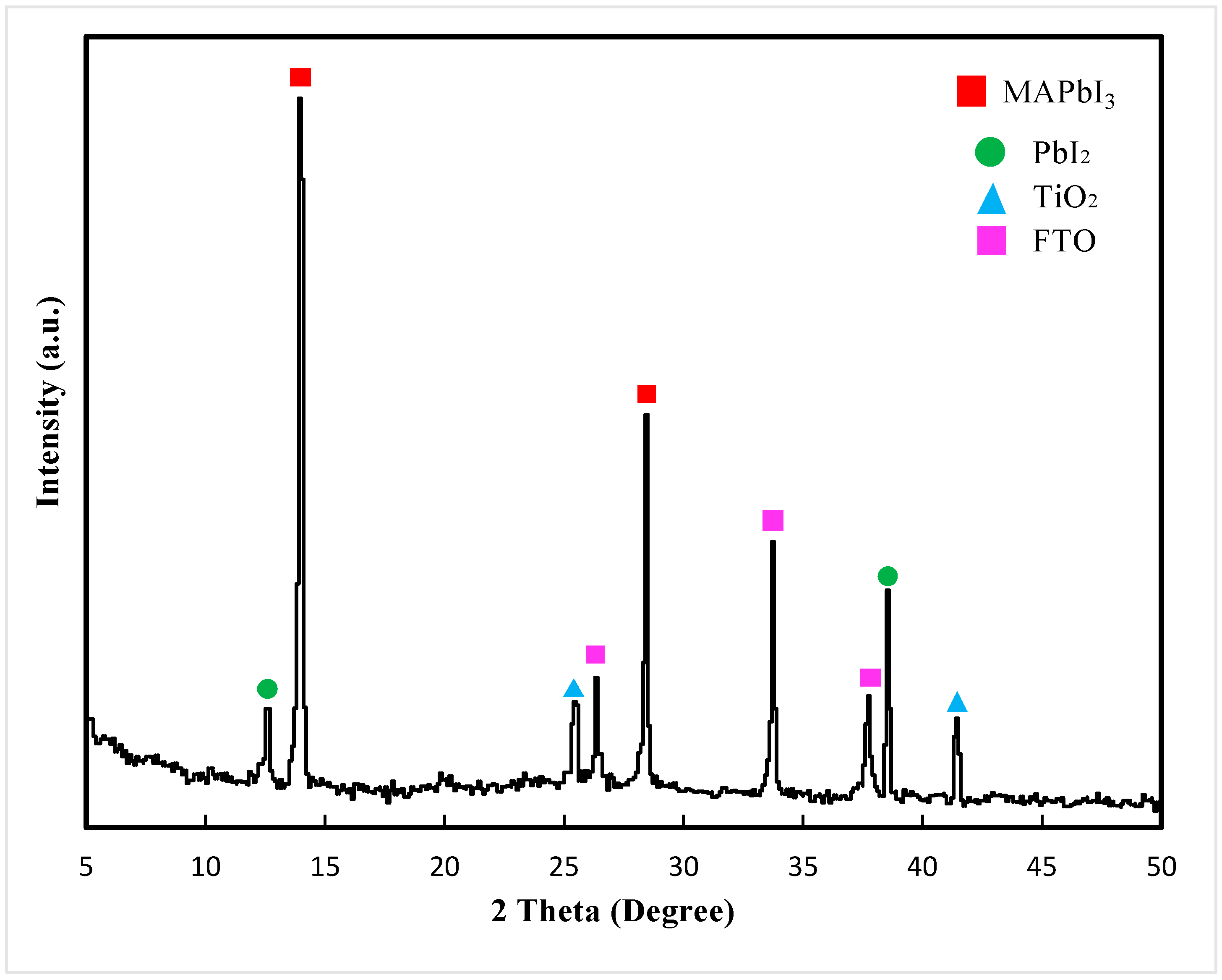
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papež, N.; Skvarenina, L.; Tofel, P.; Sobola, D. Thermal stability of gallium arsenide solar cells. Photonics Prague 2017, 27, 1060313. [Google Scholar]
- Papež, N.; Gajdoš, A.; Dallaev, R.; Sobola, D.; Sedlak, P.; Motúz, R.; Nebojsa, A.; Grmela, L. Performance analysis of GaAs based solar cells under gamma irradiation. Appl. Surf. Sci. 2020, 510, 145329. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The emergence of a new Era for low-cost, high-effificiency solar cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Yang, G.; Tao, H.; Qin, P.; Ke, W.; Fang, G. Recent progress in electron transport layers for efficient perovskite solar cells. J. Mater. Chem. 2016, 4, 3970–3990. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A. Perovskite solar cells: The birth of a new era in photovoltaics. ACS Energy Lett. 2017, 2, 822–830. [Google Scholar] [CrossRef]
- Green, M.A.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Yoshita, M.; Ho-Baillie, A.W.Y. Solar cell efficiency tables (Version 55). Prog. Photovolt. 2020, 28, 3–15. [Google Scholar] [CrossRef]
- Eperon, C.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological control for high performance, solution-processed planar heterojunction perovskite solar cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Liu, D.; Kelly, T.L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photonics 2014, 8, 133–138. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.B.; Duan, H.S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J.; et al. Low-temperature solution-processed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Sun, B.; Liu, X.Y.; Han, J.H.; Ye, H.B.; Tu, Y.X.; Chen, C.; Shi, T.L.; Lang, Z.R.; Liao, G.L. 15% effificient carbon based planar-heterojunction perovskite solar cells using TiO2/SnO2 bilayer as electron transport layer. J. Mater. Chem. A 2018, 6, 7409–7419. [Google Scholar] [CrossRef]
- Ren, Z.Q.; Wang, N.; Zhu, M.H.; Li, X.; Qi, J.Y. A NH4F interface passivation strategy to produce air-processed high-performance planar perovskite solar cells. Electrochim. Acta 2018, 282, 653–661. [Google Scholar] [CrossRef]
- Li, X.; Yang, J.Y.; Jiang, Q.H.; Lai, H.; Li, S.P.; Xin, J.W.; Chu, W.J.; Hou, J.D. Low-temperature solution-processed ZnSe electron transport layer for effificient planar perovskite solar cells with negligible hysteresis and improved photostability. ACS Nano 2018, 12, 5605–5614. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.J.; Yang, G.; Qin, M.C.; Zheng, X.L.; Lei, H.W.; Chen, C.; Chen, Z.L.; Guo, Y.X.; Han, H.W.; Zhao, X.Z.; et al. MgO nanoparticle modifified anode for highly effificient SnO2-based planar perovskite solar cells. Adv. Sci. 2017, 4, 1700031. [Google Scholar] [CrossRef]
- Yu, X.; Zou, X.; Cheng, J.; Chen, D.; Yao, Y.; Chang, C.; Liu, B.; Wang, J.; Zhou, Z.; Li, G. Investigation on Low-temperature Annealing Process of Solution-processed TiO2 Electron Transport Layer for Flexible Perovskite Solar Cell. Materials 2020, 13, 1031. [Google Scholar] [CrossRef]
- Nwankwo, U.; Ngqoloda, S.; Nkele, A.C.; Arendse, C.J.; Ozoemena, K.I.; Ekwealor, A.B.C.; Jose, R.; Maaza, M.; Ezema, F.I. Effects of alkali and transition metal-doped TiO2 hole blocking layers on the perovskite solar cells obtained by a two-step sequential deposition method in air and under vacuum. RSC Adv. 2020, 10, 13139. [Google Scholar] [CrossRef]
- Supasai, T.; Henjongchom, N.; Tang, I.M.; Deng, F.; Rujisamphan, N. Compact nanostructured TiO2 deposited by aerosol spray pyrolysis for the hole-blocking layer in a CH3NH3PbI3 perovskite solar cell. Sol. Energy 2016, 136, 515–524. [Google Scholar] [CrossRef]
- Hu, H.; Dong, B.; Hu, H.; Chen, F.; Kong, M.; Zhang, Q.; Luo, T.; Zhao, L.; Guo, Z.; Li, J.; et al. Atomic layer deposition of TiO2 for high-efficiency hole-blocking layer in hole-conductor-free perovskite solar cells processed in ambient air. ACS Appl. Mater. Inter. 2016, 8, 17999–18007. [Google Scholar] [CrossRef]
- Ke, W.J.; Fang, G.J.; Wang, J.; Qin, P.L.; Tao, H.; Lei, H.W.; Liu, Q.; Dai, X.; Zhao, X.Z. Perovskite Solar Cell with an Efficient TiO2 Compact Film. ACS Appl. Mater. Inter. 2014, 6, 15959–15965. [Google Scholar] [CrossRef]
- Darvishzadeh, P.; Redzwan, G.; Ahmadi, R.; Gorji, N.E. Modeling the degradation/recovery of short-circuit current density in perovskite and thin film photovoltaics. Org. Electron. 2017, 43, 247–252. [Google Scholar] [CrossRef]
- Xiao, M.D.; Huang, F.Z.; Huang, W.C.; Dkhissi, Y.; Zhu, Y.; Etheridge, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.B.; Spiccia, L. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. 2014, 126, 10056–10061. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.Y.; Jang, I.H.; Kang, S.M.; Choi, M.; Park, N.G. Highly reproducible perovskite solar cells with average efficiency of 18.3% and best efficiency of 19.7% fabricated via lewis base adduct of lead (II) iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Zhao, D.; Cimaroli, A.J.; Grice, C.R.; Qin, P.; Liu, Q.; Xiong, L.; Yan, Y.; Fang, G. Effects of annealing temperature of tin oxide electron selective layers on the performance of perovskite solar cells. J. Mater. Chem. A 2015, 3, 24163. [Google Scholar] [CrossRef]
- Dao, V.D.; Larina, L.L.; Choi, H.S. Minimizing energy losses in perovskite solar cells using plasma-treated transparent conducting layers. Thin Solid Films 2015, 593, 10–16. [Google Scholar] [CrossRef]
- Song, S.; Moon, B.J.; Hörantner, M.T.; Lim, J.; Kang, G.; Park, M.; Kim, J.Y.; Snaith, H.J.; Park, T. Interfacial Electron Accumulation for Efficient Homo-Junction Perovskite Solar Cells. Nano Energy 2016, 28, 269. [Google Scholar] [CrossRef]
- Baek, S.; Han, J.W.; Vidyasagar, D.; Cho, H.; HA, H.H.; Kim, D.H.; Heo, Y.W.; Lee, S. Room-Temperature-Processed Amorphous Sn-In-O Electron Transport Layer for Perovskite Solar Cells. Materials 2020, 13, 32. [Google Scholar] [CrossRef]
- Zuo, L.J.; Gu, Z.W.; Ye, T.; Fu, W.F.; Wu, G.; Li, H.Y.; Chen, H.Z. Enhanced Photovoltaic Performance of CH3NH3PbI3 Perovskite Solar Cells through Interfacial Engineering Using Self-Assembling Monolayer. J. Am. Chem. Soc. 2015, 137, 2674–2679. [Google Scholar] [CrossRef]
- Lai, W.C.; Lin, K.W.; Guo, T.F.; Chen, P.; Wang, Y.T. Conversion efficiency improvement of inverted ch3nh3pbi3 perovskite solar cells with room temperature sputtered zno by adding the c60. Appl. Phys. Lett. 2015, 107, 253301. [Google Scholar] [CrossRef]
- Ren, X.D.; Yang, D.; Yang, Z.; Feng, J.S.; Zhu, X.J.; Niu, J.Z.; Liu, Y.C.; Zhao, W.G.; Liu, S.F. Solution-Processed Nb:SnO2 Electron Transport Layer for Efficient Planar Perovskite Solar Cells. ACS Appl. Mater. Inter. 2017, 9, 2421–2429. [Google Scholar] [CrossRef]
- Ren, H.Y.; Zou, X.P.; Cheng, J.; Ling, T.; Bai, X.; Chen, D. Facile Solution Spin-Coating SnO2 Thin Film Covering Cracks of TiO2 Hole Blocking Layer for Perovskite Solar Cells. Coatings 2018, 8, 314. [Google Scholar] [CrossRef]
- Malviya, K.D.; Dotan, H.; Yoon, K.R.; Kim, I.D.; Rothschild, A. Rigorous substrate cleaning process for reproducible thin film hematite (alpha-Fe2O3) photoanodes. J. Mater. Res. 2016, 31, 1565–1573. [Google Scholar] [CrossRef]
- Im, J.H.; Jang, I.H.; Pellet, N.; Gratzel, M.; Park, N.G. Growth of CH3NH3PbI3 Cuboids with Controlled Size for High-Efficiency Perovskite Solar Cells. Nat. Nanotechnol. 2014, 9, 927–932. [Google Scholar] [CrossRef]
- Zhang, N.; Guo, Y.; Yin, X.; He, M.; Zou, X. Spongy carbon film deposited on a separated substrate as counter electrode for perovskite-based solar cell. Mater. Lett. 2016, 182, 248–252. [Google Scholar] [CrossRef]
- Mohammadpour, F.; Altomare, M.; So, S.; Lee, K.; Schmuki, P. High-temperature annealing of TiO2 nanotube membranes for efficient dye-sensitized solar cells. Semicond. Sci. Technol. 2016, 31, 014010. [Google Scholar] [CrossRef]
- Cao, D.H.; Stoumpos, C.C.; Malliakas, C.D.; Katz, M.J.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G. Remnant PbI2, an unforeseen necessity in high-efficiency hybrid perovskite-based solar cells? APL Mater. 2014, 2, 091101. [Google Scholar] [CrossRef]

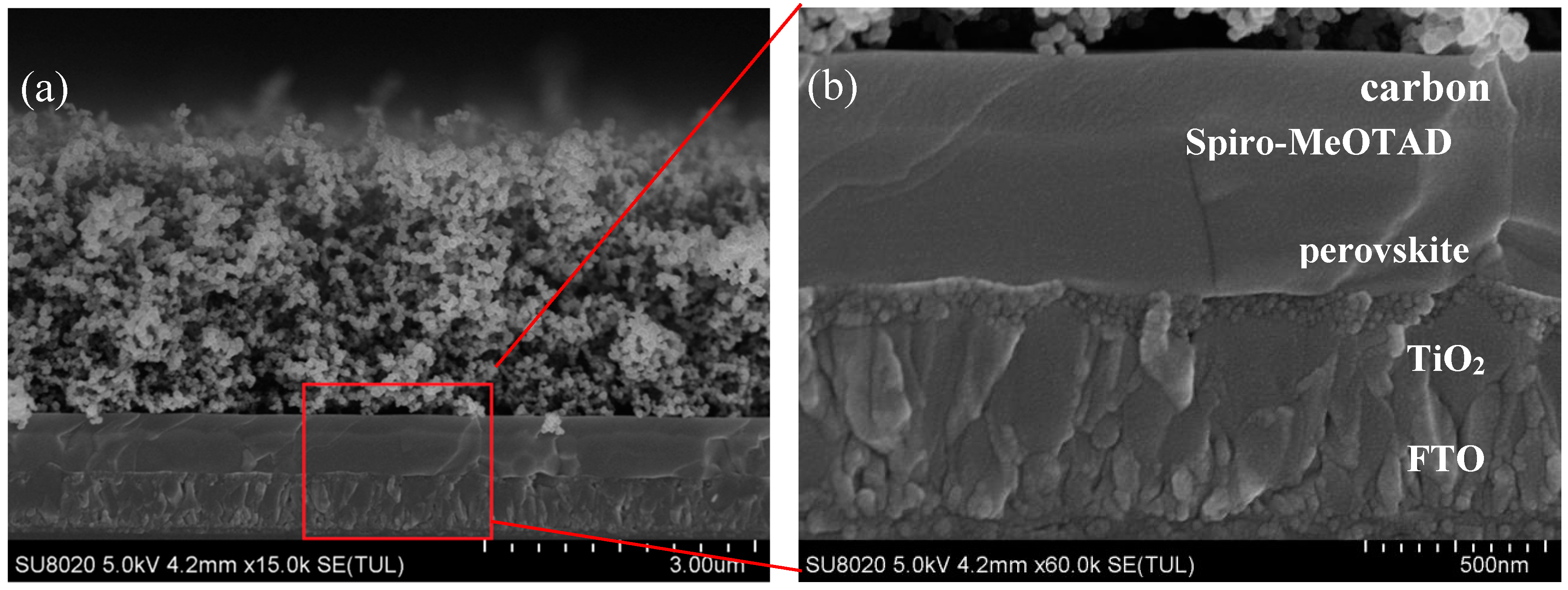
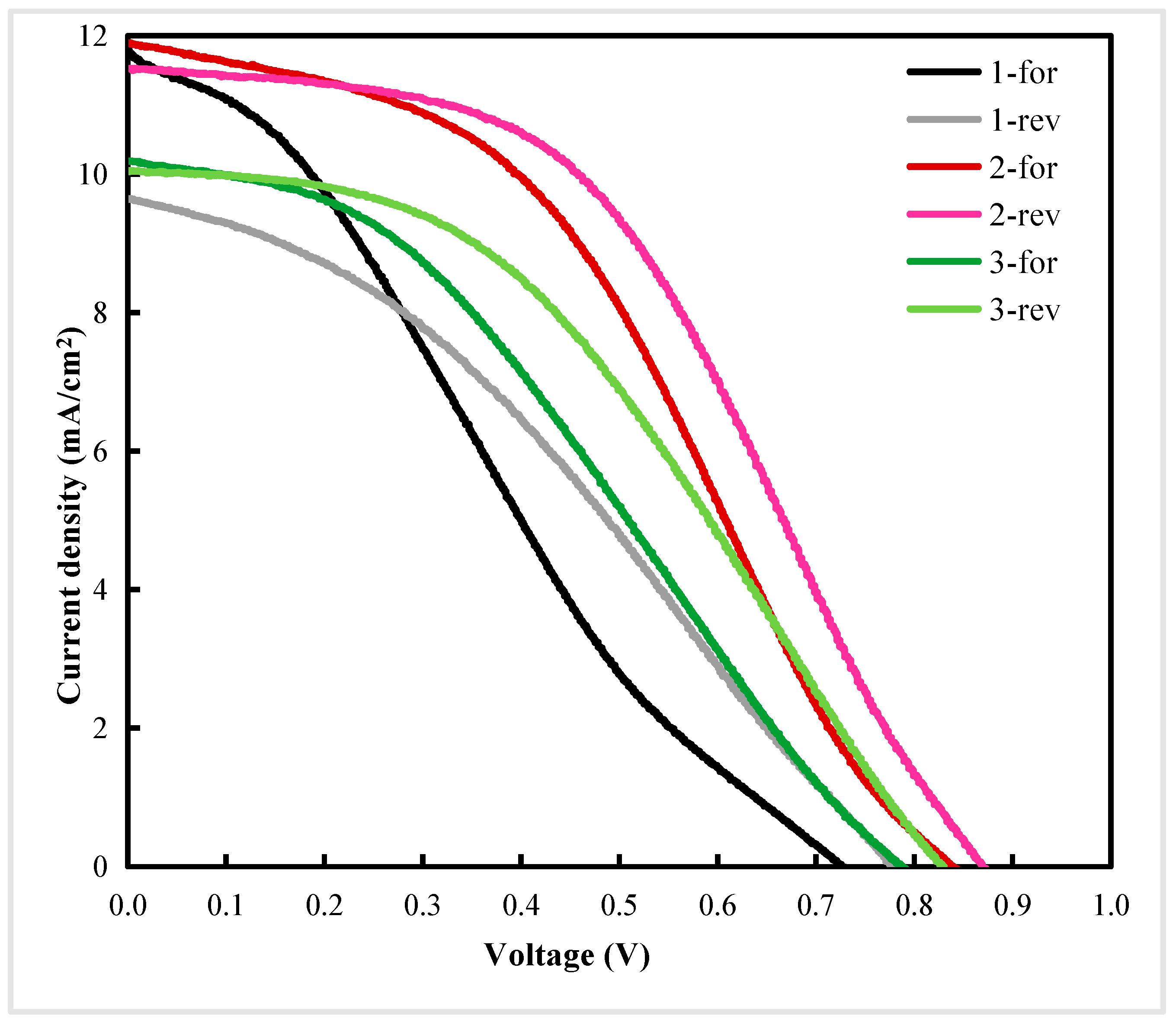
| Sample | PCE a (%) | Voc b (V) | Jsc c (mA/cm2) | FF d | Rs e (Ωcm2) | Rsh f (Ωcm2) | Average Thickness g (nm) | Standard Error of Thickness (nm) | RMS h (nm) | Standard Error of Roughness (nm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-for | 2.26 | 0.73 | 11.81 | 0.26 | 39.88 | 159.59 | 101 | 1.64 | 32 | 1.72 |
| 1-rev | 2.59 | 0.78 | 9.65 | 0.34 | 53.15 | 268.20 | ||||
| 2-for | 4.14 | 0.84 | 11.90 | 0.42 | 33.15 | 322.82 | 150 | 1.77 | 19 | 1.20 |
| 2-rev | 4.68 | 0.87 | 11.53 | 0.47 | 32.80 | 1026.60 | ||||
| 3-for | 2.87 | 0.79 | 10.19 | 0.36 | 48.42 | 378.79 | 189 | 2.45 | 16 | 0.97 |
| 3-rev | 3.51 | 0.83 | 10.05 | 0.42 | 45.60 | 1637.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zou, X.; Zhu, J.; Cheng, J.; Chen, D.; Bai, X.; Yao, Y.; Chang, C.; Yu, X.; Liu, B.; et al. Effect of Optimization of TiO2 Electron Transport Layer on Performance of Perovskite Solar Cells with Rough FTO Substrates. Materials 2020, 13, 2272. https://doi.org/10.3390/ma13102272
Wang J, Zou X, Zhu J, Cheng J, Chen D, Bai X, Yao Y, Chang C, Yu X, Liu B, et al. Effect of Optimization of TiO2 Electron Transport Layer on Performance of Perovskite Solar Cells with Rough FTO Substrates. Materials. 2020; 13(10):2272. https://doi.org/10.3390/ma13102272
Chicago/Turabian StyleWang, Junqi, Xiaoping Zou, Jialin Zhu, Jin Cheng, Dan Chen, Xiao Bai, Yujun Yao, Chuangchuang Chang, Xing Yu, Baoyu Liu, and et al. 2020. "Effect of Optimization of TiO2 Electron Transport Layer on Performance of Perovskite Solar Cells with Rough FTO Substrates" Materials 13, no. 10: 2272. https://doi.org/10.3390/ma13102272
APA StyleWang, J., Zou, X., Zhu, J., Cheng, J., Chen, D., Bai, X., Yao, Y., Chang, C., Yu, X., Liu, B., Zhou, Z., & Li, G. (2020). Effect of Optimization of TiO2 Electron Transport Layer on Performance of Perovskite Solar Cells with Rough FTO Substrates. Materials, 13(10), 2272. https://doi.org/10.3390/ma13102272





