Decontamination of Titanium Surface Using Different Methods: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
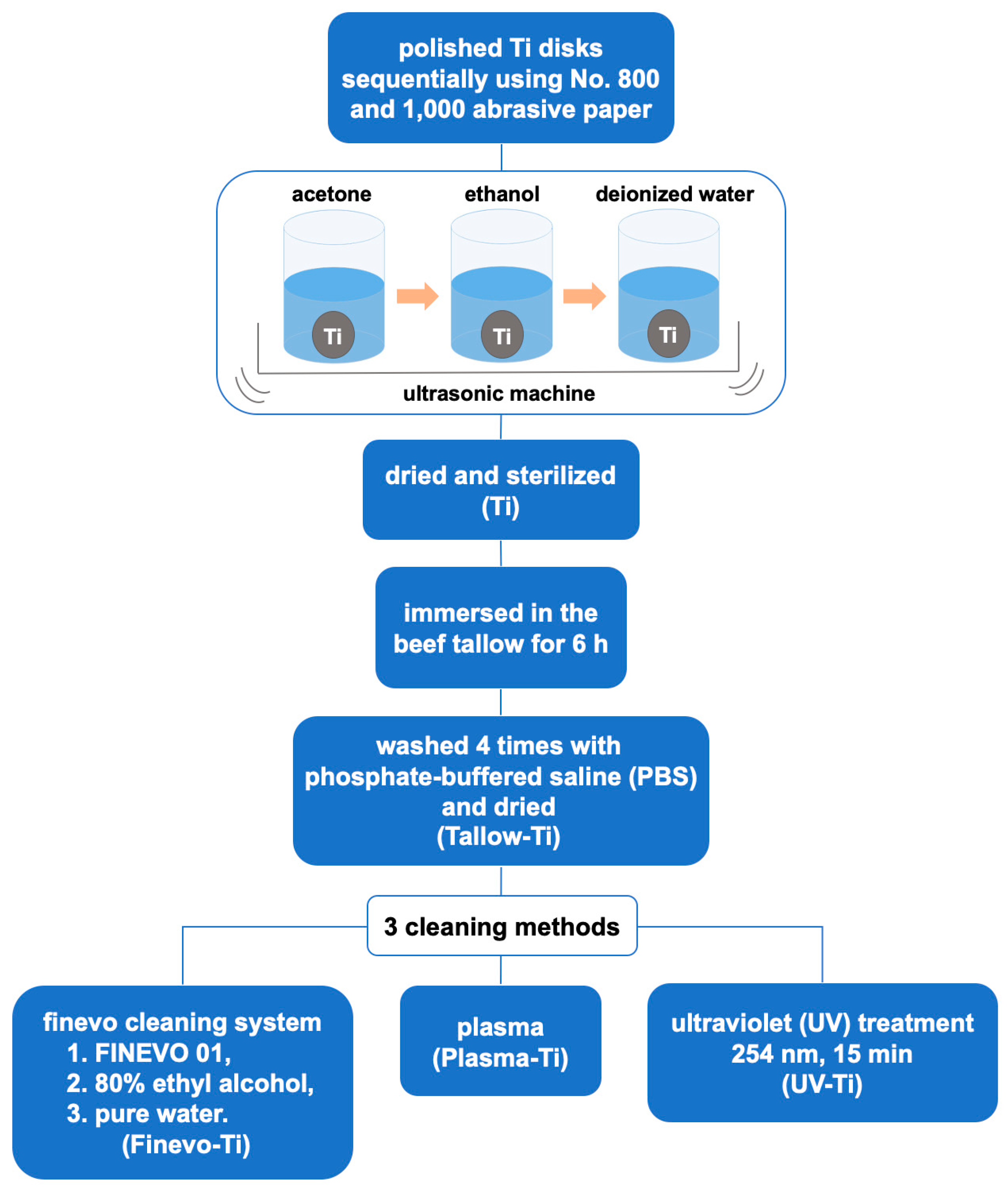
2.1. Sample Preparation
2.2. Surface Characterization
2.3. Cell Culture
2.4. Cell Morphology
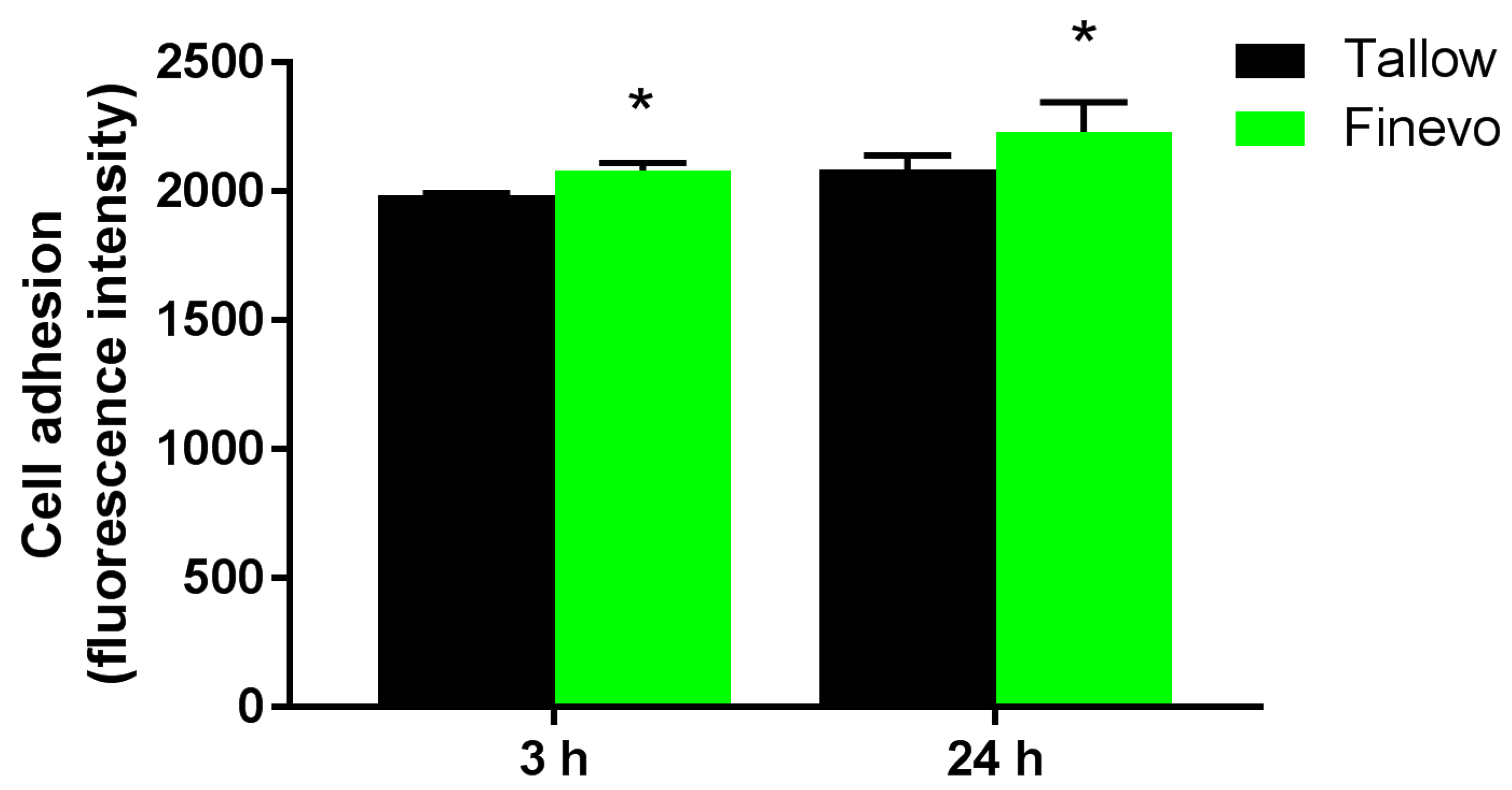
2.5. Cell Adhesion
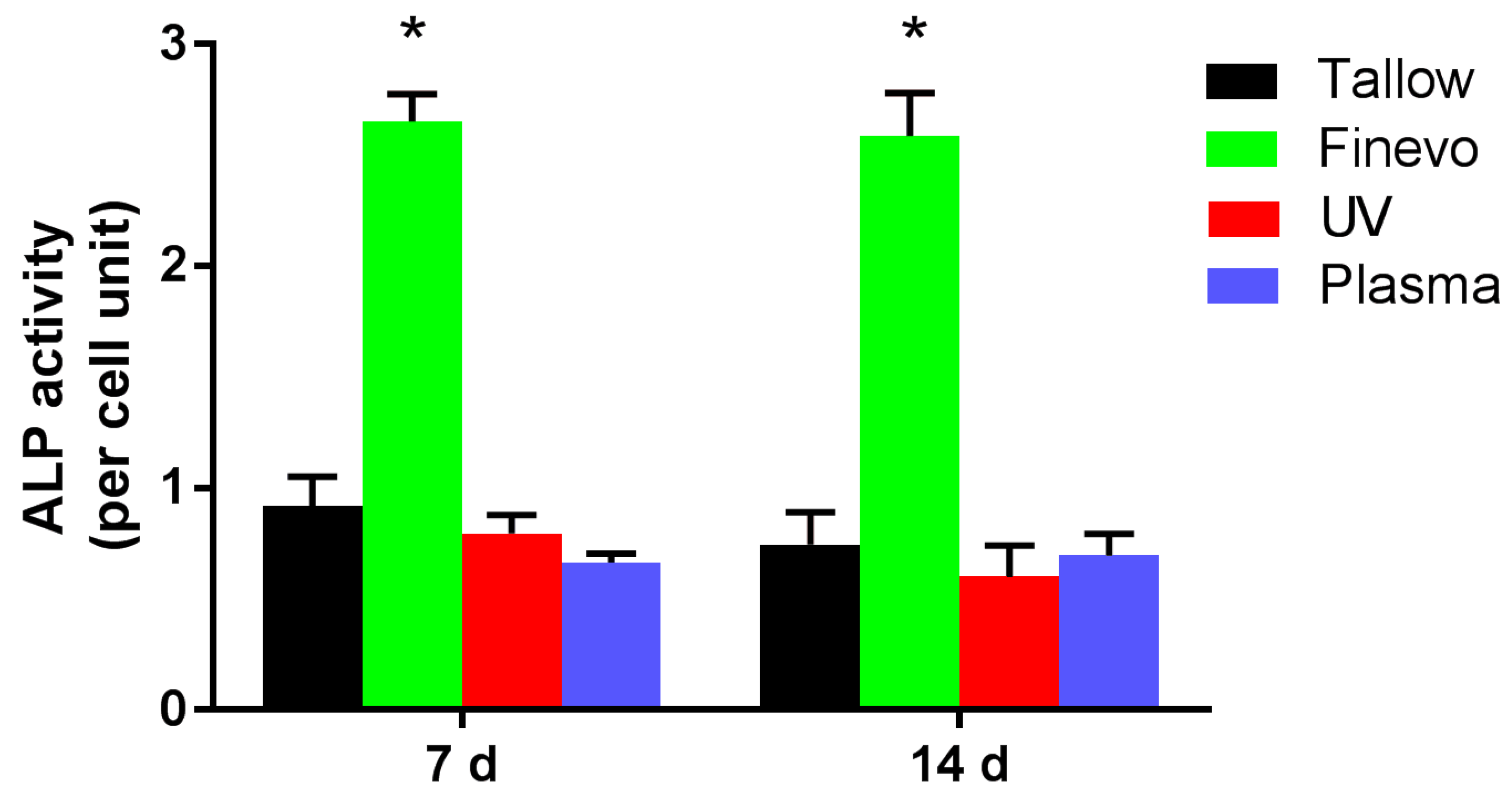
2.6. Alkaline Phosphatase (ALP) Assay
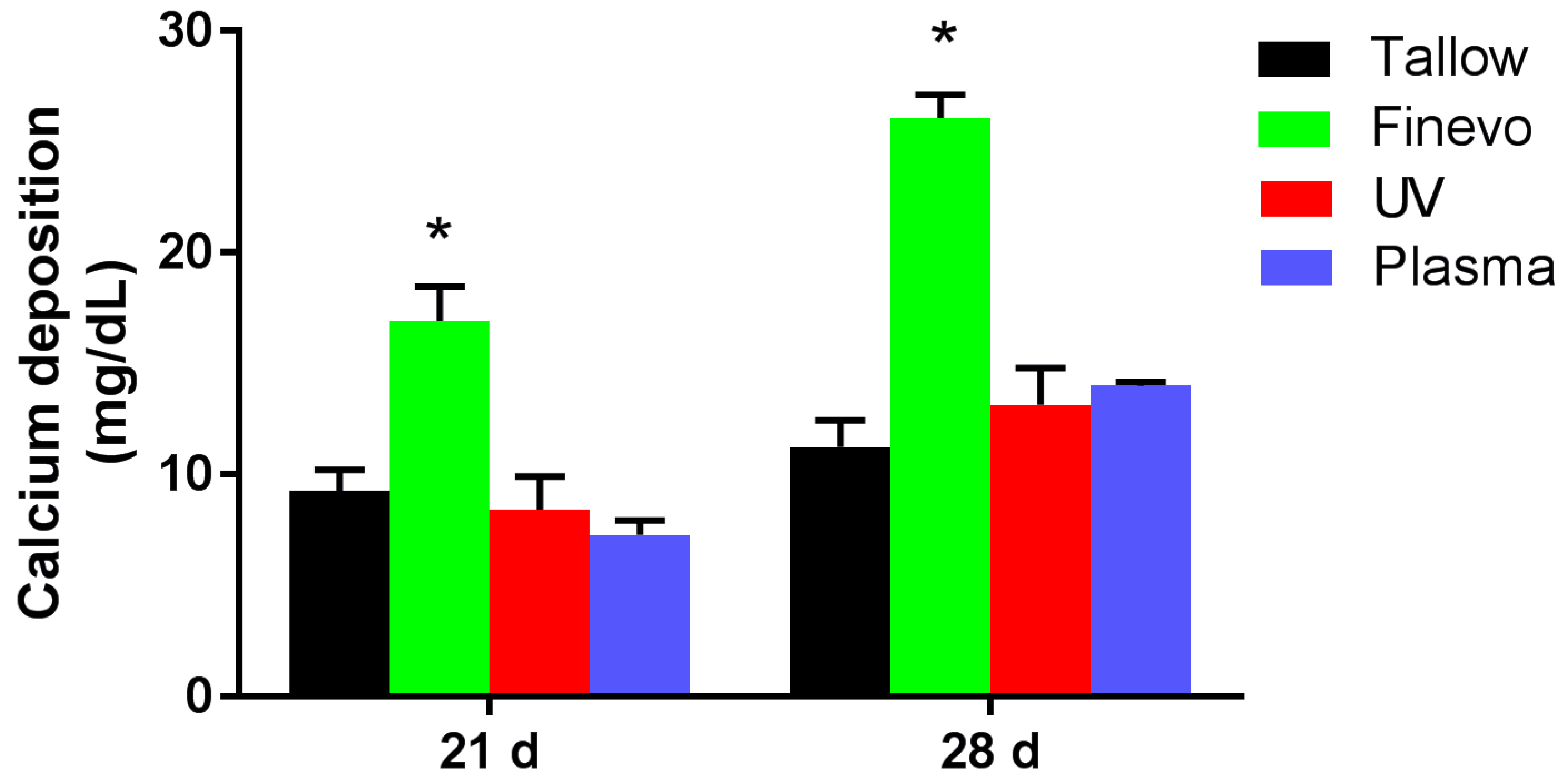
2.7. Quantification of Calcium Deposition in the Extracellular Matrix
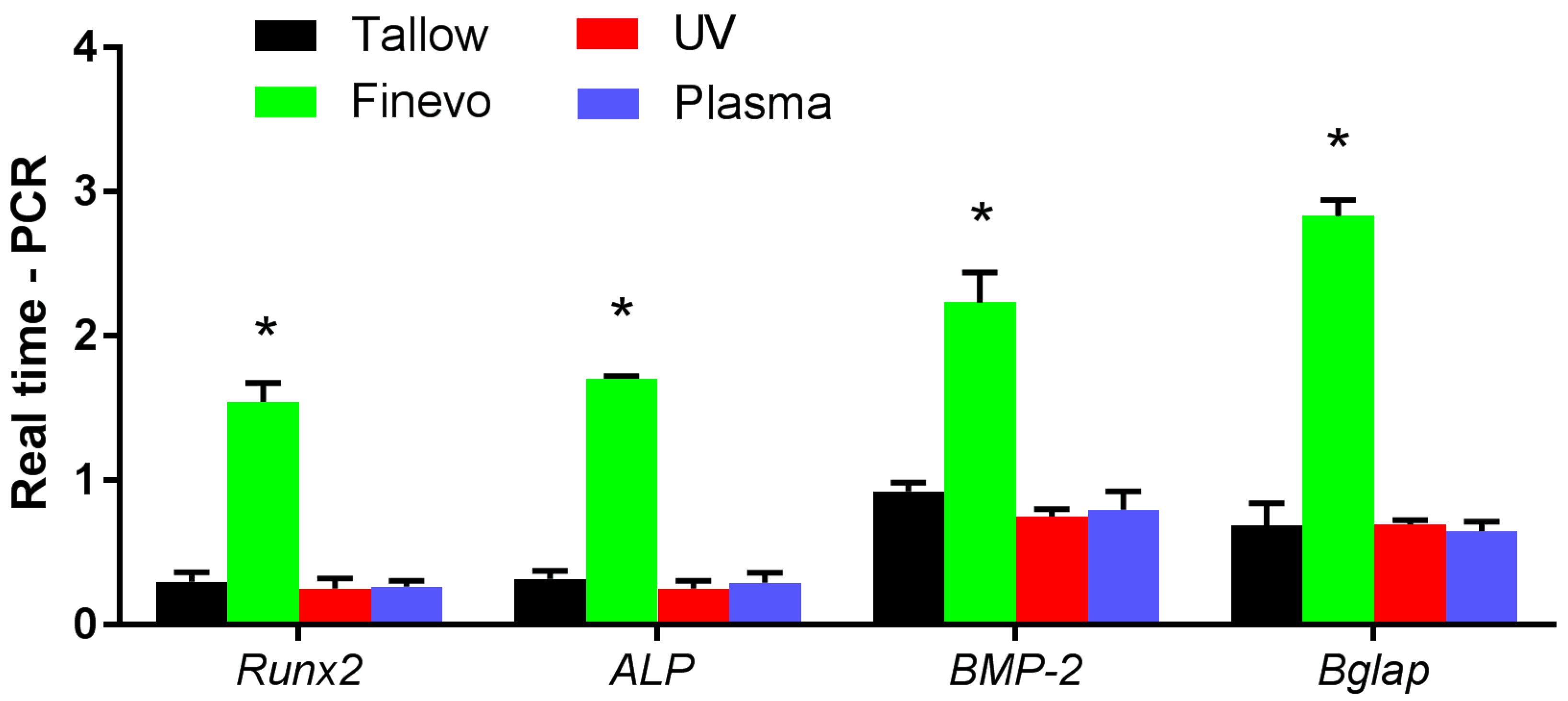
2.8. Analysis of the Expression Levels of Osteogenesis-Related Genes
2.9. Statistical Analysis
3. Results
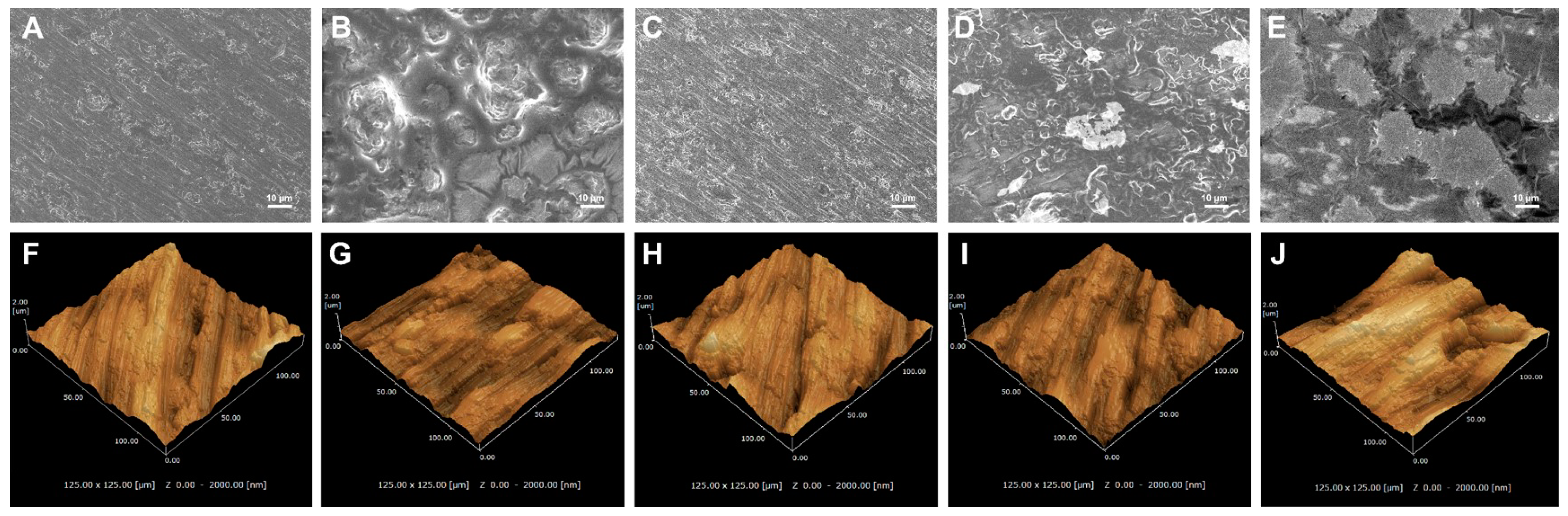
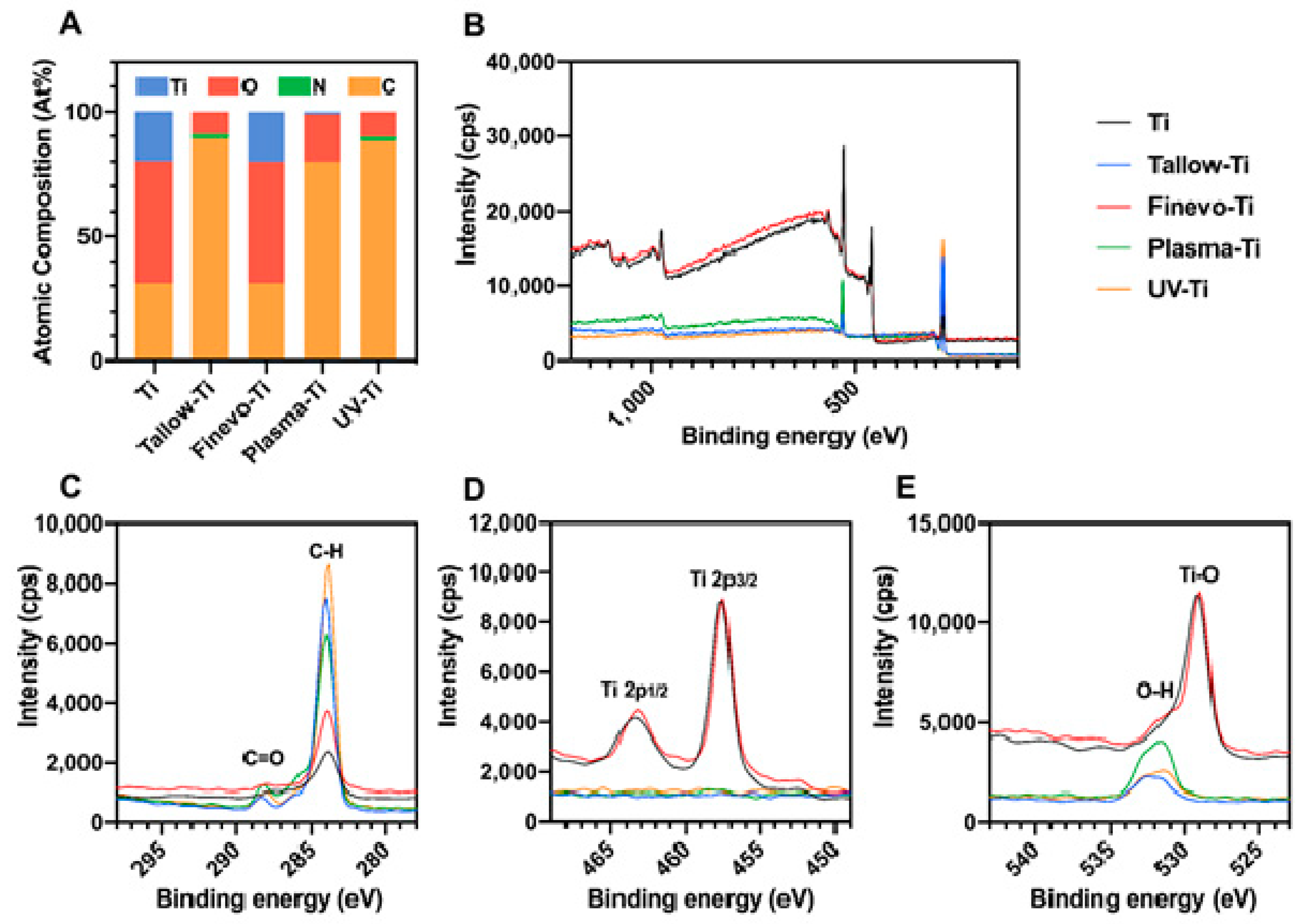
3.1. Surface Characterization
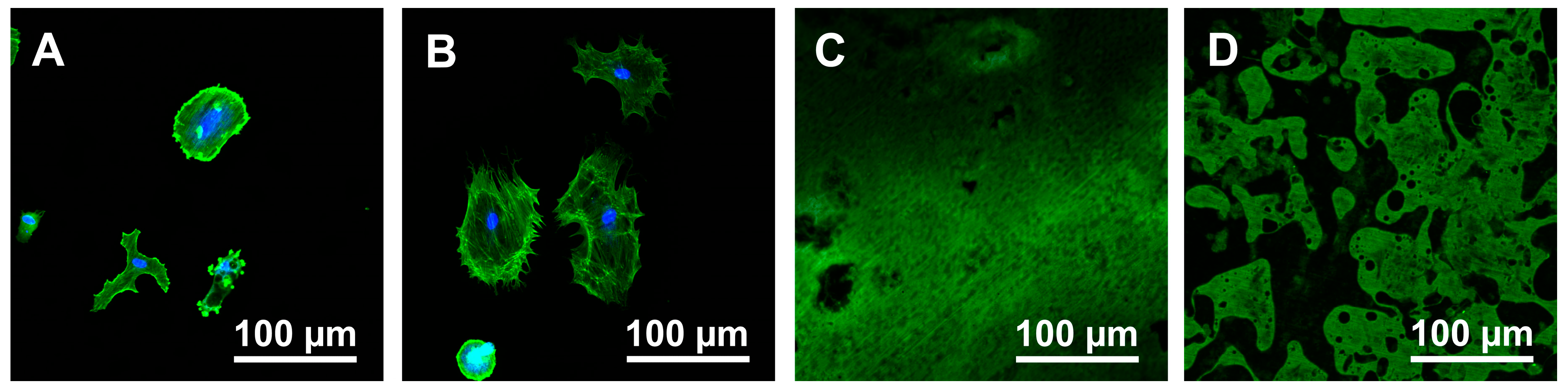
3.2. Cell Morphology
3.3. Cell Adhesion
3.4. Alkaline Phosphatase (ALP) Activity
3.5. Quantification of Calcium Deposition in the Extracellular Matrix
3.6. Analysis of the Expression Levels of Osteogenesis-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, X.; Chen, J.; Scheideler, L.; Reichl, R.; Geis-Gerstorfer, J. Effects of topography and composition of titanium surface oxides on osteoblast responses. Biomaterials 2004, 25, 4087–4103. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; De Soete, M.; Van Steenberghe, D. Infectious risks for oral implants: A review of the literature. Clin. Oral Implant. Res. 2002, 13, 1–19. [Google Scholar] [CrossRef]
- Romeo, E.; Ghisolfi, M.C.D. Peri-implant diseases. A systematic review of the literature. Minerva Stomatol. 2004, 53, 215–230. [Google Scholar]
- Esposito, M.; Hirsch, J.; Lekholm, U.; Thomsen, P. Differential diagnosis and treatment strategies for biologic complications and failing oral implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 1999, 14, 473–490. [Google Scholar]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17, 68–81. [Google Scholar] [CrossRef]
- Traini, T.; Danza, M.; Zollino, I.; Altavilla, R.; Lucchese, A.; Sollazzo, V.; Trapella, G.; Brunelli, G.; Carinci, F. Histomorphometric evaluation of an immediately loaded implant retrieved from human mandible after 2 years. Int. J. Immunopathol. Pharmacol. 2011, 24, 31–36. [Google Scholar] [CrossRef]
- Albrektsson, T.; Isidor, F. Consensus report of session IV. In Proceedings of the First European Workshop on Periodontology; Lang, N.P., Karring, T., Eds.; Quintessence: London, UK, 1994; pp. 365–369. [Google Scholar]
- Mombelli, A.; Lang, N.P. The diagnosis and treatment of peri-implantitis. Periodontol 2000, 17, 63–76. [Google Scholar] [CrossRef]
- Branemark, P.I.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lundkvist, S.; Rockler, B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 2006, 4, 17–20. [Google Scholar]
- Yoshihara, C.; Ueno, T.; Chen, P.; Tsutsumi, Y.; Hanawa, T.; Wakabayashi, N. Inverse response of osteoblasts and fibroblasts to growth on carbon-deposited titanium surfaces. J. Biomed. Mater. Res.Part B Appl. Biomater. 2018, 106, 1869–1877. [Google Scholar] [CrossRef]
- Hayashi, R.; Ueno, T.; Migita, S.; Tsutsumi, Y.; Doi, H.; Ogawa, T.; Hanawa, T.; Wakabayashi, N. Hydrocarbon deposition attenuates osteoblast activity on titanium. J. Dent. Res. 2014, 93, 698–703. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Massaro, C.; Rotolo, P.; De Riccardis, F.; Milella, E.; Napoli, A.; Wieland, M.; Textor, M.; Spencer, N.D.; Brunette, D. Comparative investigation of the surface properties of commercial titanium dental implants. Part I: Chemical composition. J. Mater. Sci. Mater. Med. 2002, 13, 535–548. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakamoto, K.; Martra, G.; Coluccia, S.; Anpo, M. Mechanism of photoinduced superhydrophilicity on the TiO2 photocatalyst surface. J. Phys. Chem. B 2005, 109, 15422–15428. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Takeuchi, M.; Martra, G.; Coluccia, S.; Anpo, M. Verification of the photoadsorption of H2O molecules on TiO2 semiconductor surfaces by vibrational absorption spectroscopy. J. Phys. Chem. C 2007, 111, 9811–9817. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Iwasa, F.; Yamada, M.; Ueno, T.; Ogawa, T. The effect of UV-photofunctionalization on the time-related bioactivity of titanium and chromium-cobalt alloys. Biomaterials 2009, 30, 4268–4276. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma-A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar]
- Whittaker, A.G.; Graham, E.M.; Baxter, R.L.; Jones, A.C.; Richardson, P.R.; Meek, G.; Campbell, G.A.; Aitken, A.; Baxter, H.C. Plasma cleaning of dental instruments. J. Hosp. Infect. 2004, 56, 37–41. [Google Scholar] [CrossRef]
- Fricke, K.; Koban, I.; Tresp, H.; Jablonowski, L.; Schröder, K.; Kramer, A.; Weltmann, K.D.; Von Woedtke, T.; Kocher, T. Atmospheric pressure plasma: A high-performance tool for the efficient removal of biofilms. PLoS ONE 2012, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bentley, E.M. The value of ultrasonic cleaners in dental practice. Br. Dent. J. 1994, 177, 53–56. [Google Scholar] [CrossRef]
- Gehrke, P.; Tabellion, A.; Fischer, C. Microscopical and chemical surface characterization of CAD/CAM zircona abutments after different cleaning procedures. A qualitative analysis. J. Adv. Prosthodont. 2015, 7, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, P.; Smeets, R.; Gosau, M.; Friedrich, R.E.; Madani, E.; Duddeck, D.; Fischer, C.; Tebbel, F.; Sader, R.; Hartjen, P. The influence of an ultrasonic cleaning protocol for CAD/CAM abutment surfaces on cell viability and inflammatory response in vitro. Vivo 2019, 33, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Carinci, F.; Brunelli, G.; Monguzzi, R. Everstick® and Ribbond® fiber reinforced composites: Scanning Electron Microscope (SEM) comparative analysis. Eur. J. Inflamm. 2011, 9, 73–79. [Google Scholar]
- Zhang, H.; Komasa, S.; Mashimo, C.; Sekino, T.; Okazaki, J. Effect of ultraviolet treatment on bacterial attachment and osteogenic activity to alkali-treated titanium with nanonetwork structures. Int. J. Nanomed. 2017, 12, 4633–4646. [Google Scholar] [CrossRef]
- Xing, H.; Komasa, S.; Taguchi, Y.; Sekino, T.; Okazaki, J. Osteogenic activity of titanium surfaces with nanonetwork structures. Int. J. Nanomed. 2014, 9, 1741–1755. [Google Scholar] [CrossRef]
- Lausmaa, J.; Kasemo, B.; Mattsson, H. Surface spectroscopic characterization of titanium implant materials. Appl. Surf. Sci. 1990, 44, 133–146. [Google Scholar] [CrossRef]
- Karlsson, U.; Gotfredsen, K.; Olsson, C. A 2-year report on maxillary and mandibular fixed partial dentures supported by Astra Tech dental implants: A comparison of 2 implants with different surface textures. Clin. Oral Implant. Res. 1998, 9, 235–242. [Google Scholar] [CrossRef]
- Cassinelli, C.; Morra, M.; Bruzzone, G.; Carpi, A.; Di Santi, G.; Giardino, R.; Fini, M. Surface Chemistry effects of topographic modification of titanium dental implant surfaces: 2. In Vitro Experiments. Int. J. Oral Maxillofac. Implant. 2003, 18, 46–52. [Google Scholar]
- Sul, Y.T.; Byon, E.; Wennerberg, A. Surface characteristics of electrochemically oxidized implants and acid-etched implants: Surface chemistry, morphology, pore configurations, oxide thickness, crystal structure, and roughness. Int. J. Oral Maxillofac. Implant. 2008, 23, 631–640. [Google Scholar]
- Kikuchi, L.; Park, J.Y.; Victor, C.; Davies, J.E. Platelet interactions with calcium-phosphate-coated surfaces. Biomaterials 2005, 26, 5285–5295. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Olivares-Navarrete, R.; Baier, R.E.; Meyer, A.E.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta Biomater. 2012, 8, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.; Cassinelli, C. Evaluation of surface contamination of titanium dental implants by LV-SEM: Comparison with XPS measurements. Surf. Interface Anal. 1997, 25, 983–988. [Google Scholar] [CrossRef]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Mata, A.; Su, X.; Fleischman, A.J.; Roy, S.; Banks, B.A.; Miller, S.K.; Midura, R.J. Osteoblast attachment to a textured surface in the absence of exogenous adhesion proteins. IEEE Trans. Nanobiosci. 2003, 2, 287–293. [Google Scholar] [CrossRef]
- Benya, P.D.; Shaffer, J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982, 30, 215–224. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ohsaki, Y.; Goto, T.; Nakasima, A.; Iijima, T. Effects of static magnetic fields on bone formation in rat osteoblast cultures. J. Dent. Res. 2003, 82, 962–966. [Google Scholar] [CrossRef]
- Birmingham, E.; Niebur, G.L.; Mchugh, P.E.; Shaw, G.; Barry, F.P.; McNamara, L.M. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cells Mater. 2012, 23, 13–27. [Google Scholar] [CrossRef]
- Aubin, J.E. Regulation of Osteoblast Formation and Function. Rev. Endocr. Metab. Disord. 2001, 2, 81–94. [Google Scholar] [CrossRef]
- Masaki, C.; Schneider, G.B.; Zaharias, R.; Seabold, D.; Stanford, C. Effects of implant surface microtopography on osteoblast gene expression. Clin. Oral Implant. Res. 2005, 16, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Desai, H.V.; Voruganti, I.S.; Jayasuriya, C.; Chen, Q.; Darling, E.M. Live-cell, temporal gene expression analysis of osteogenic differentiation in adipose-derived stem cells. Tissue Eng. Part A 2013, 19, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Katagiri, T.; Ikeda, T.; Wozney, J.M.; Rosen, V.; Wang, E.A.; Kahn, A.J.; Suda, T.; Yoshiki, S. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J. Cell Biol. 1991, 113, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wan, L.; Zhang, X.; Rong, M.; Guo, Z.; Zhou, L. Effects of hydrocarbons contamination on initial responses of osteoblast-like cells on acid-etched titanium surface. Rare Met. Mater. Eng. 2013, 42, 1558–1562. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, S.; Li, M.; Komasa, S.; Agariguchi, A.; Yang, Y.; Zeng, Y.; Takao, S.; Zhang, H.; Tashiro, Y.; Kusumoto, T.; et al. Decontamination of Titanium Surface Using Different Methods: An In Vitro Study. Materials 2020, 13, 2287. https://doi.org/10.3390/ma13102287
Yan S, Li M, Komasa S, Agariguchi A, Yang Y, Zeng Y, Takao S, Zhang H, Tashiro Y, Kusumoto T, et al. Decontamination of Titanium Surface Using Different Methods: An In Vitro Study. Materials. 2020; 13(10):2287. https://doi.org/10.3390/ma13102287
Chicago/Turabian StyleYan, Sifan, Min Li, Satoshi Komasa, Akinori Agariguchi, Yuanyuan Yang, Yuhao Zeng, Seiji Takao, Honghao Zhang, Yuichiro Tashiro, Tetsuji Kusumoto, and et al. 2020. "Decontamination of Titanium Surface Using Different Methods: An In Vitro Study" Materials 13, no. 10: 2287. https://doi.org/10.3390/ma13102287
APA StyleYan, S., Li, M., Komasa, S., Agariguchi, A., Yang, Y., Zeng, Y., Takao, S., Zhang, H., Tashiro, Y., Kusumoto, T., Kobayashi, Y., Chen, L., Kashiwagi, K., Matsumoto, N., Okazaki, J., & Kawazoe, T. (2020). Decontamination of Titanium Surface Using Different Methods: An In Vitro Study. Materials, 13(10), 2287. https://doi.org/10.3390/ma13102287




