Strontium Retention of Calcium Zirconium Aluminate Cement Paste Studied by NMR, XRD and SEM-EDS
Abstract
1. Introduction
2. Experimental Procedure
2.1. Synthesis and Phase Identification
2.2. Preparation and Treatment of Cement Paste
3. Results and Discussion
3.1. X-ray Diffraction Analysis of Special Cements Hydration
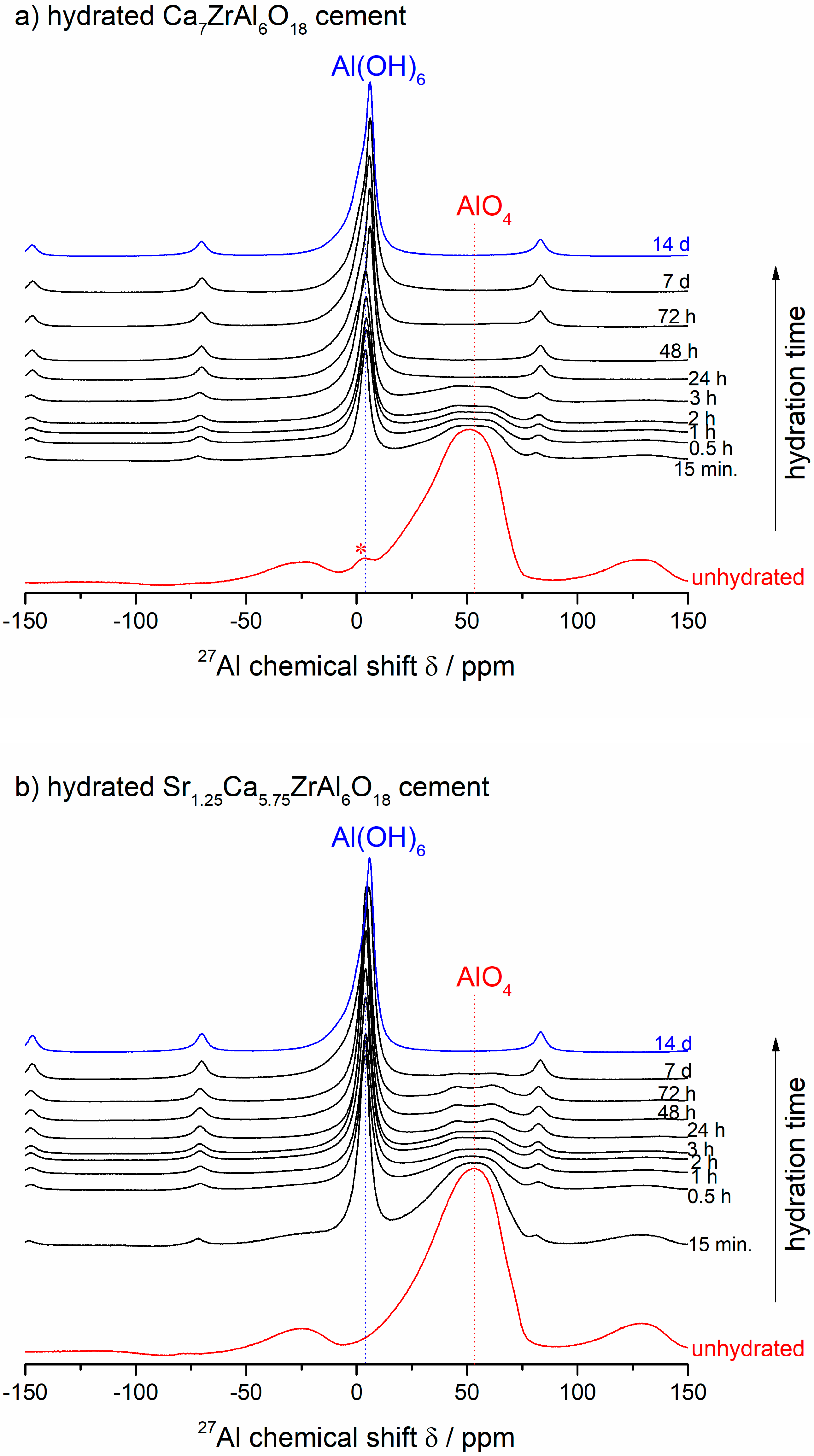
3.2. Ex-Situ 27Al NMR Study of the Hydration Reaction at 50 °C
3.3. Microstructural Studies on the Hydrated Sr1.25Ca5.75ZrAl6O18Cement Paste
4. Conclusions
- (1)
- The Sr-doped cement is developed through structural substitution for Ca ions by Sr ions in the Ca7ZrAl6O18 clinker phase.
- (2)
- Strontium was used as a retarding agent to block this cement clinker phase hydration at a curing temperature of 50 °C. Hence, the residual unhydrated cement particles in the hardened Sr1.25Ca5.75ZrAl6O18 cement paste were present for much longer than for the undoped Ca7ZrAl6O18 clinker phase sample as it was observed by XRD.
- (3)
- The hydration of both Ca7ZrAl6O18 and Sr1.25Ca5.75ZrAl6O18 cements was also inspected using the 27Al MAS NMR technique. This hydration is accompanied by a change of Al-coordination from tetrahedral to octahedral. This complete conversion from anhydrous 27AlIV to hydrated 27AlVI species was achieved during the first 24 h of hydration at 50 °C for Ca7ZrAl6O18 and during 7 d of hydration at 50 °C for Sr1.25Ca5.75ZrAl6O18.
- (4)
- The hexagonal phases were formed starting in the very first minutes of hydration of these cements. For each cement type tested, these unstable hydrates consist mainly of C4AH19 and probably of C2AH8 as it was observed by XRD.
- (5)
- The formation of a thermodynamically stable phase pure C3AH6 or Ca-rich (Sr,C)3AH6 in the hardened Sr1.25Ca5.75ZrAl6O18 cement paste was preceded by that of a number of less stable phases, i.e.,Sr-rich (Sr,C)3AH6 hydrate and other hexagonal Ca-Al hydrates. The Sr-rich (Sr,C)3AH6 hydrate existing between 0.5 h and 7 d of curing was isostructural with the Ca-rich (Sr,C)3AH6 or pure C3AH6 formed at the age of 7 d.
- (6)
- The transformation of the hexagonal calcium aluminate hydrates and Sr-rich (Sr,C)3AH6 hydrate into the cubic phase Ca-rich (Sr,C)3AH6 or pure C3AH6 was expressed in terms of chemical shift from ca. 4 ppm to ca. 6 ppm in the hardened Sr1.25Ca5.75ZrAl6O18 cement paste at the age of 7 d. The same 27Al NMR chemical shift was detected at the age of 24 h for the reference hardened Ca7ZrAl6O18 cement paste.
Funding
Conflicts of Interest
Nomenclature
| C | CaO |
| A | Al2O3 |
| Z | ZrO2 |
| Sr | SrO |
| H | H2O |
References
- Nowacka, M.; Pacewska, B. Effect of structurally different aluminosilicates on early-age hydration of calcium aluminate cement depending on temperature. Constr. Build. Mater. 2020, 235, 117404. [Google Scholar] [CrossRef]
- Cao, Y.-F.; Tao, Z.; Pan, Z.; Wuhrer, R. Effect of calcium aluminate cement on geopolymer concrete cured at ambient temperature. Constr. Build. Mater. 2018, 191, 242–252. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Lu, C.; Huang, Z. Effects of sodium gluconate on early hydration and mortar performance of Portland cement-calcium aluminate cement-anhydrite binder. Constr. Build. Mater. 2017, 157, 1065–1073. [Google Scholar] [CrossRef]
- Madej, D. Synthesis, formation mechanism and hydraulic activity of novel composite cements belonging to the system CaO-Al2O3-ZrO2. J. Therm. Anal. Calorim. 2017, 130, 1913–1924. [Google Scholar] [CrossRef]
- Litwinek, E.; Madej, D. Structure, microstructure and thermal stability characterizations of C3AH6 synthesized from different precursors through hydration. J. Therm. Anal. Calorim. 2019. [Google Scholar] [CrossRef]
- Madej, D.; Rajska, M.; Kruk, A. Synthesis and hydration behaviour of calcium zirconium aluminate powders by modifying co-precipitation method. Ceram. Int. 2020, 46, 2373–2383. [Google Scholar] [CrossRef]
- Madej, D. A new implementation of electrochemical impedance spectroscopy (EIS) and other methods to monitor the progress of hydration of strontium monoaluminate (SrAl2O4) cement. J. Therm. Anal. 2019, 139, 17–28. [Google Scholar] [CrossRef]
- Liu, K.; Chen, A.; Shang, X.; Chen, L.; Zheng, L.; Gao, S.; Zhou, Y.; Wang, Q.; Ye, G. The impact of mechanical grinding on calcium aluminate cement hydration at 30 °C. Ceram. Int. 2019, 45, 14121–14125. [Google Scholar] [CrossRef]
- Kerienė, J.; Antonovič, V.; Stonys, R.; Boris, R. The influence of the ageing of calcium aluminate cement on the properties of mortar. Constr. Build. Mater. 2019, 205, 387–397. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Irisawa, K.; Jin, F.; Meguro, Y.; Kinoshita, H. Reduction of water content in calcium aluminate cement with/out phosphate modification for alternative cementation technique. Cem. Concr. Res. 2018, 109, 243–253. [Google Scholar] [CrossRef]
- Madej, D.; Szczerba, J.; Nocuń-Wczelik, W.; Gajerski, R. Hydration of Ca7ZrAl6O18 phase. Ceram. Int. 2012, 38, 3821–3827. [Google Scholar] [CrossRef]
- Madej, D.; Boris, R. Synthesis, characterization and hydration analysis of Ba2+-, Cu2+- or Bi3+-doped CaO-Al2O3-ZrO2-based cements. J. Therm. Anal. Calorim. 2019, 138, 4331–4340. [Google Scholar] [CrossRef]
- Madej, D. Hydration, carbonation and thermal stability of hydrates in Ca7-xSrxZrAl6O18 cement. J. Therm. Anal. Calorim. 2018, 131, 2411–2420. [Google Scholar] [CrossRef]
- Pöllmann, H.; Kaden, R. Mono- (strontium-, calcium-) aluminate based cements. In Calcium Aluminates Proceedings of the International Conference; Building Research Establishment: Watford, UK, 2014; pp. 99–108. [Google Scholar]
- Ukrainczyk, N.; Vrbos, N.; Šipušić, J. Influence of metal chloride salts on calcium aluminate cement hydration. Adv. Cem. Res. 2012, 24, 249–262. [Google Scholar] [CrossRef]
- Duran, A.; Sirera, R.; Nicolas, M.P.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Study of the early hydration of calcium aluminates in the presence of different metallic salts. Cem. Concr. Res. 2016, 81, 1–15. [Google Scholar] [CrossRef]
- Faucon, P.; Charpentier, T.; Bertrandie, D.; Nonat, A.; Virlet, J.; Petit, J.C. Characterization of calcium aluminate hydrates and related hydrates of cement pastes by 27Al MQ-MAS NMR. Inorg. Chem. 1998, 37, 3726–3733. [Google Scholar] [CrossRef]
- Skibsted, J.; Henderson, E.; Jakobsen, H.J. Characterization of calcium aluminate phases in cements by 27Al MAS NMR spectroscopy. Inorg. Chem. 1993, 32, 1013–1027. [Google Scholar] [CrossRef]
- Cong, X.; Kirkpatrick, R.J. Hydration of calcium aluminate cements: A solid-state 27Al NMR study. J. Am. Ceram. Soc. 1991, 76, 409–416. [Google Scholar] [CrossRef]
- Mercury, J.M.R.; Pena, P.; de Aza, A.H.; Turrillas, X.; Sobrados, I.; Sanz, J. Solid-state 27Al and 29Si NMR investigations on Si-substituted hydrogarnets. Acta Mater. 2007, 55, 1183–1191. [Google Scholar] [CrossRef]
- Myers, R.J.; Bernal, S.A.; Gehman, J.D.; Van Deventer, J.S.J.; Provis, J. The role of Al in cross-linking of alkali-activated slag cements. J. Am. Ceram. Soc. 2014, 98, 996–1004. [Google Scholar] [CrossRef]
- Walkley, B.; Provis, J.L. Solid-state nuclear magnetic resonance spectroscopy of cements. Mater. Today Adv. 2019, 1, 100007. [Google Scholar] [CrossRef]
- Müller, D.; Rettel, A.; Gessner, W.; Scheler, G. An application of solid state magic-angle spinning 27Al NMR to the study of cement hydration. J. Magn. Reson. 1984, 57, 152–156. [Google Scholar] [CrossRef]
- Muller, D.; Gessner, W.; Samoson, A.; Lippmaa, E.; Scheler, G. Solid state 27Al NMR studies of polycrystalline aluminates in the system CaO-Al2O3. Polyhedron 1986, 5, 779–785. [Google Scholar] [CrossRef]
- Hughes, C.E.; Walkley, B.; Gardner, L.; Walling, S.A.; Bernal, S.A.; Iuga, D.; Provis, J.; Harris, K.D.M. Exploiting in-situ solid-state NMR spectroscopy to probe the early stages of hydration of calcium aluminate cement. Solid State Nucl. Magn. Reson. 2019, 99, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Garces, P.; Alcocel, E.; Chinchon, S.; Andreu, C.; Alcaide, J. Alcaide, Effect of curing temperature in some hydration characteristics of calcium aluminate cement compared with those of portland cement. Cem. Concr. Res. 1997, 27, 1343–1355. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, G.; Gu, W.; Ding, D.; Chen, L.; Zhu, L. Conversion of calcium aluminate cement hydrates at 60 °C with and without water. J. Am. Ceram. Soc. 2018, 101, 2712–2717. [Google Scholar] [CrossRef]
- Luz, A.P.; Pandolfelli, V.C. Halting the calcium aluminate cement hydration process. Ceram. Int. 2011, 37, 3789–3793. [Google Scholar] [CrossRef]
- Fukuda, K.; Iwata, T.; Nishiyuki, K. Crystal structure, structural disorder, and hydration behavior of calcium zirconium aluminate, Ca7ZrAl6O18. Chem. Mater. 2007, 19, 3726–3731. [Google Scholar] [CrossRef]
- Das, S.K.; Kumar, S.K.; Das, P.K. Crystal morphology of calcium aluminates hydrated for 14 days. J. Mater. Sci. Lett. 1997, 16, 735–736. [Google Scholar] [CrossRef]
- Kumar, S.; Das, S.K.; Daspoddar, P.K. Microstructure of calcium aluminates and their mixes after 3 days of hydration. Trans. Indian Ceram. Soc. 1999, 58, 115–117. [Google Scholar] [CrossRef]
- Antonovič, V.; Keriené, J.; Boris, R.; Aleknevičius, M. The effect of temperature on the formation of the hydrated calcium aluminate cement structure. Procedia Eng. 2013, 57, 99–106. [Google Scholar] [CrossRef]
- López, A.H.; Calvo, J.L.G.; Olmo, J.G.; Petit, S.; Alonso, M.C. Microstructural evolution of calcium aluminate cements hydration with silica fume and fly ash additions by scanning electron microscopy, and mid and near-infrared spectroscopy. J. Am. Ceram. Soc. 2008, 91, 1258–1265. [Google Scholar] [CrossRef]
- Rashid, S.; Barnes, P.; Bensted, J.; Turrillas, X. Conversion of calcium aluminate cement hydrates re-examined with synchrotron energy-dispersive diffraction. J. Mater. Sci. Lett. 1994, 13, 1232–1234. [Google Scholar] [CrossRef]









| Sample Designation | Cement/Sample | Hydration Time | Water-to-Cement Ratio (w/c), Temperature |
|---|---|---|---|
| A | Sr1.25Ca5.75ZrAl6O18(as a solid solution) | 15 min, 0.5 h, 1 h, 2 h, 3 h, 24 h, 48 h, 72 h, 7 d and 14 d | w/c = 1.0, T = 50 °C |
| B | SrAl2O4(as a reference undoped compound) | 7 d | w/c = 0.5, T = 50 °C |
| C | Ca7ZrAl6O18(as a reference undoped compound) | 15 min, 0.5 h, 1 h, 2 h, 3 h, 24 h, 48 h, 72 h, 7 d and 14 d | w/c = 1.0, T = 50 °C |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madej, D. Strontium Retention of Calcium Zirconium Aluminate Cement Paste Studied by NMR, XRD and SEM-EDS. Materials 2020, 13, 2366. https://doi.org/10.3390/ma13102366
Madej D. Strontium Retention of Calcium Zirconium Aluminate Cement Paste Studied by NMR, XRD and SEM-EDS. Materials. 2020; 13(10):2366. https://doi.org/10.3390/ma13102366
Chicago/Turabian StyleMadej, Dominika. 2020. "Strontium Retention of Calcium Zirconium Aluminate Cement Paste Studied by NMR, XRD and SEM-EDS" Materials 13, no. 10: 2366. https://doi.org/10.3390/ma13102366
APA StyleMadej, D. (2020). Strontium Retention of Calcium Zirconium Aluminate Cement Paste Studied by NMR, XRD and SEM-EDS. Materials, 13(10), 2366. https://doi.org/10.3390/ma13102366





