Properties of Alkali Activated Lightweight Aggregate Generated from Sidoarjo Volcanic Mud (Lusi), Fly Ash, and Municipal Solid Waste Incineration Bottom Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
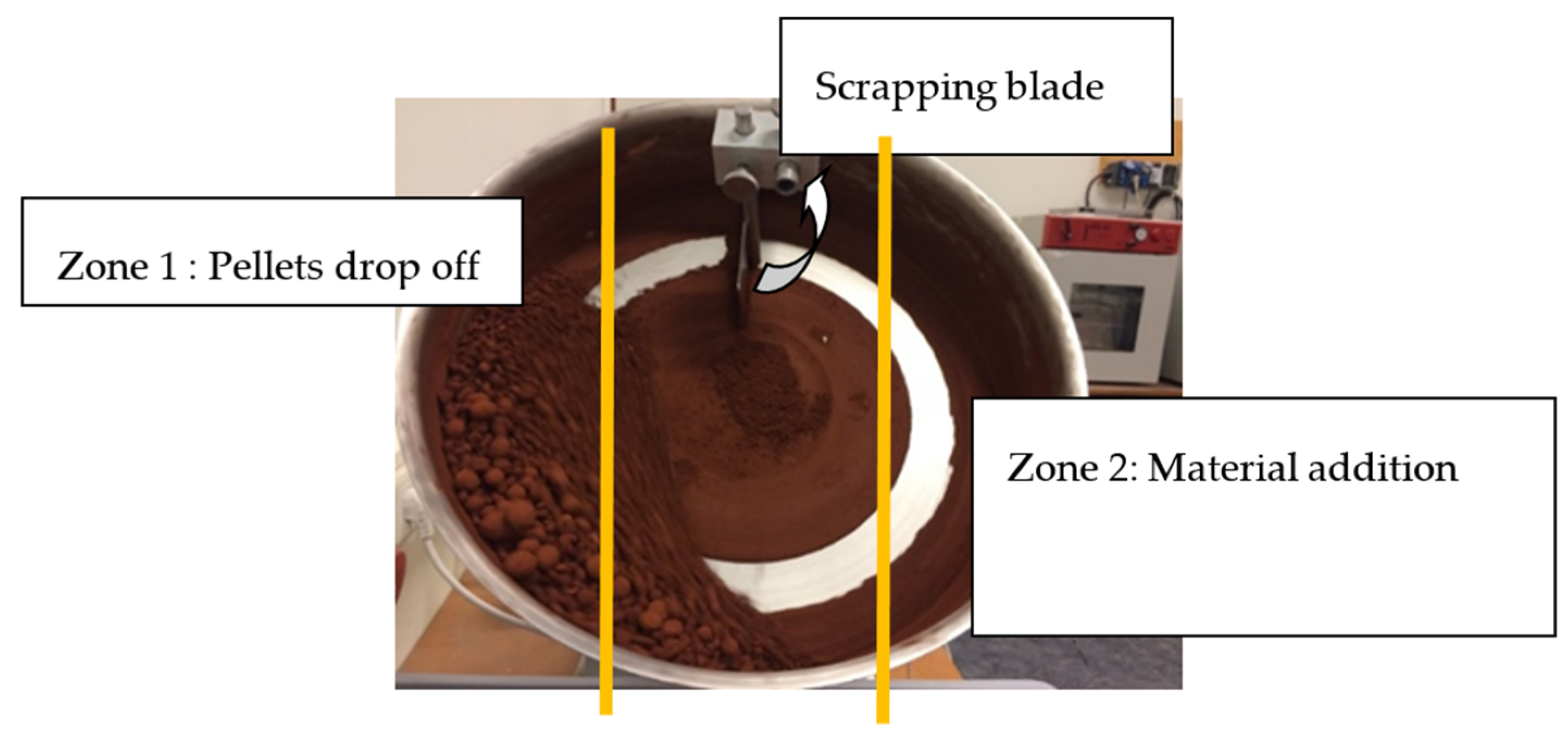
2.2.1. Pelletizing Procedure
2.2.2. Characterization of the Samples
2.2.3. Application of LWA in Mortar
3. Results and Discussion
3.1. Properties of Raw Materials
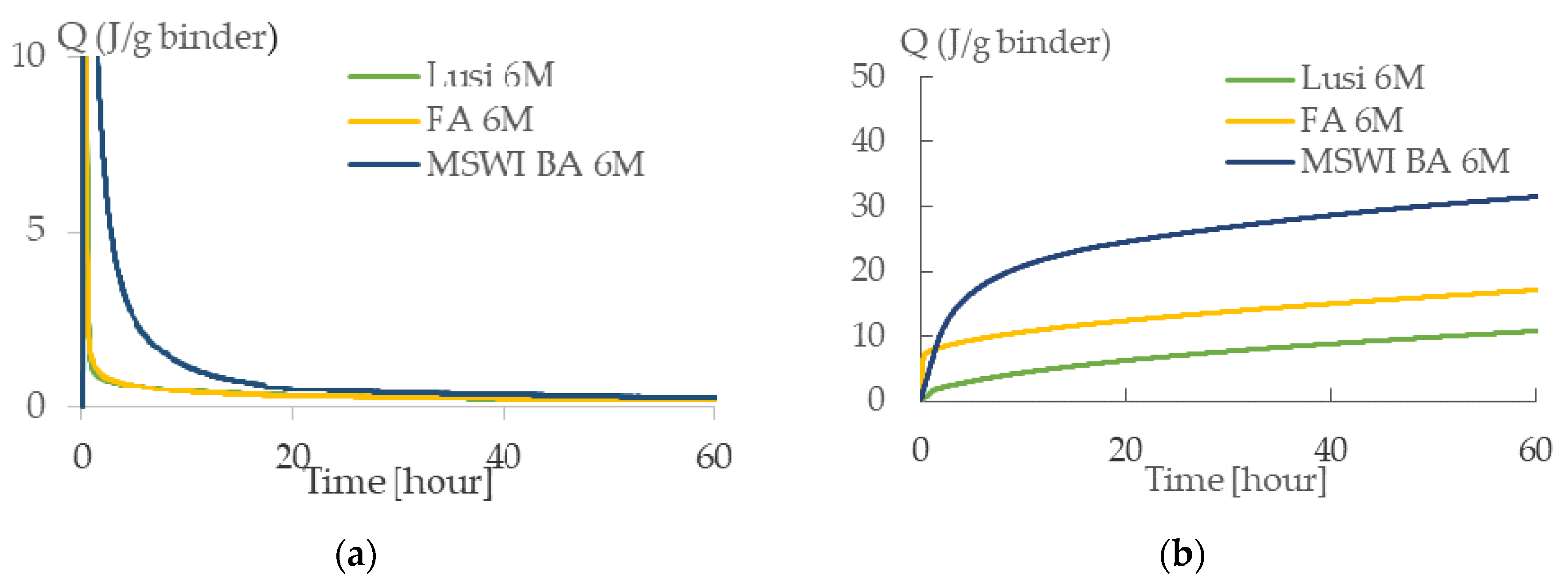
3.2. Heat Evolution
3.3. Density and Water Absorption
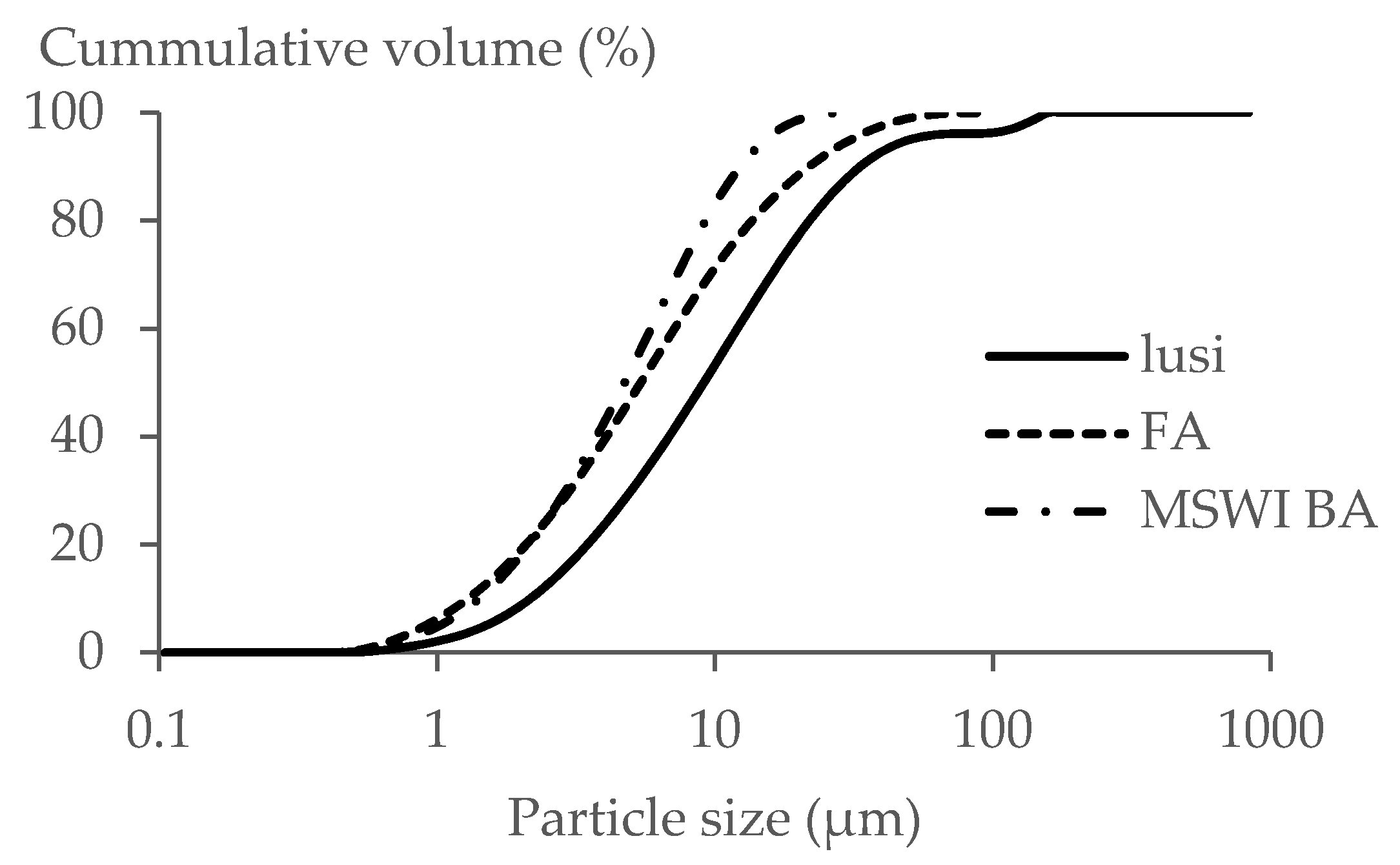
3.4. Particle Size Distribution
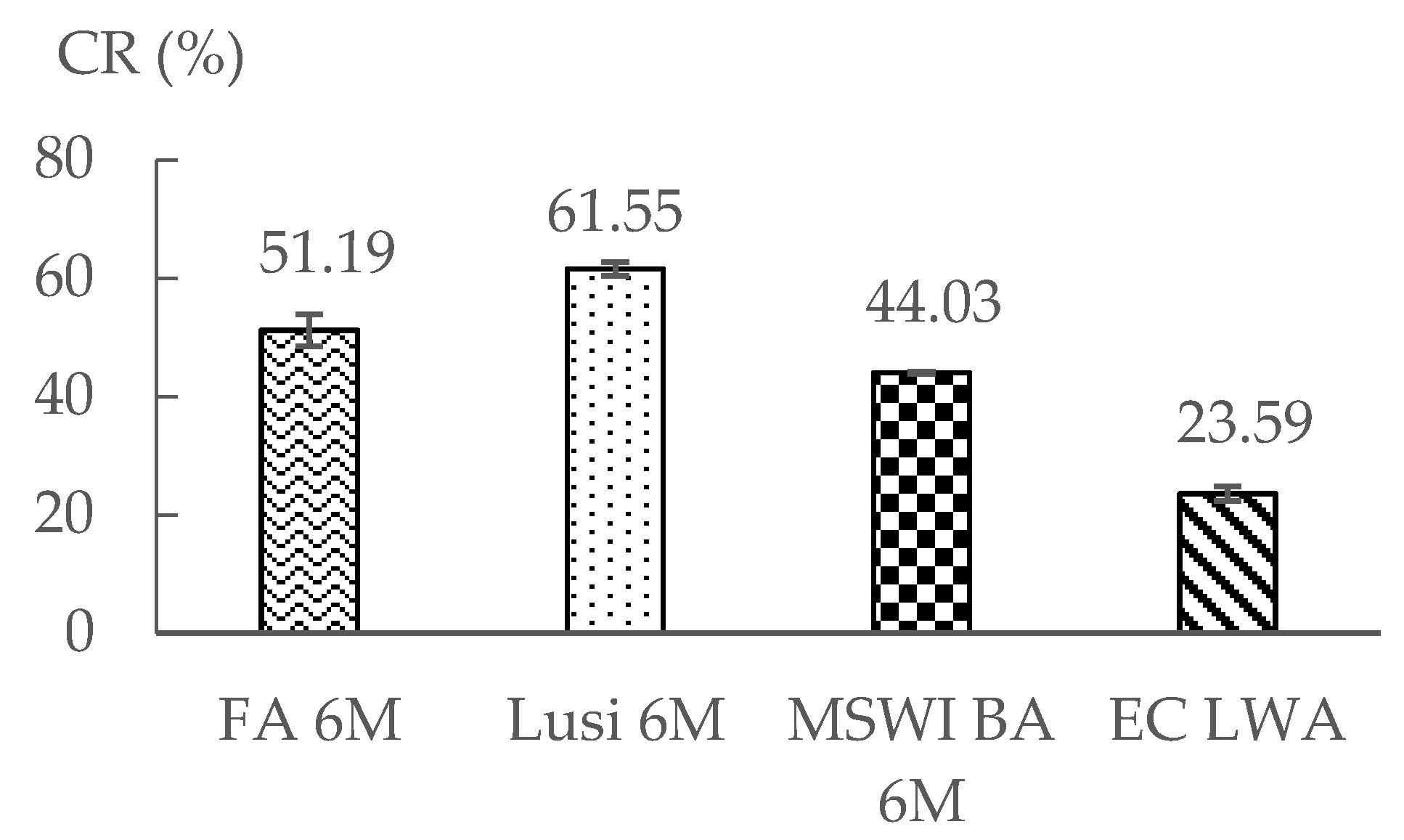
3.5. Crushing Resistance Test
3.6. Porosity
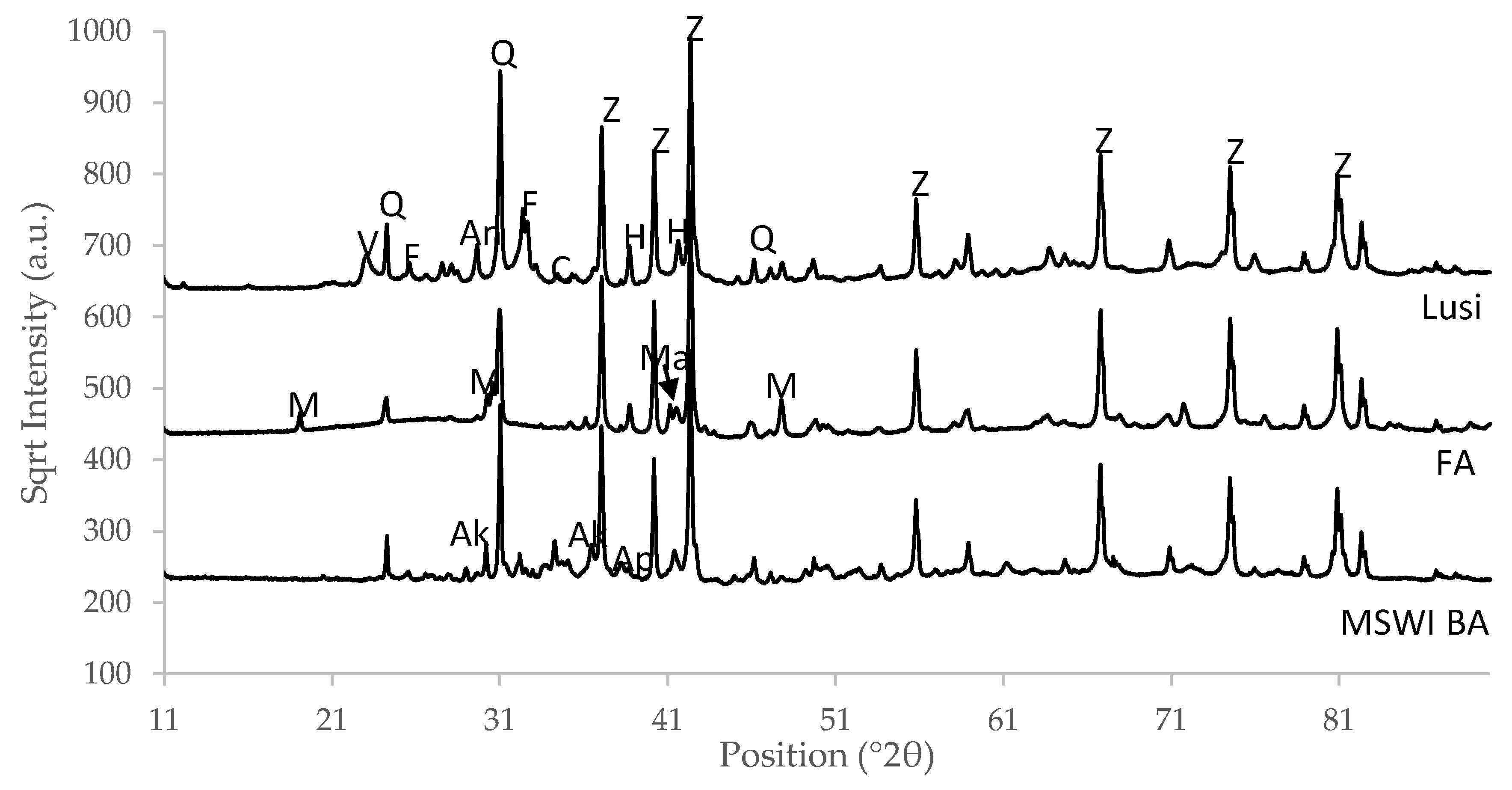
3.7. Mineralogy
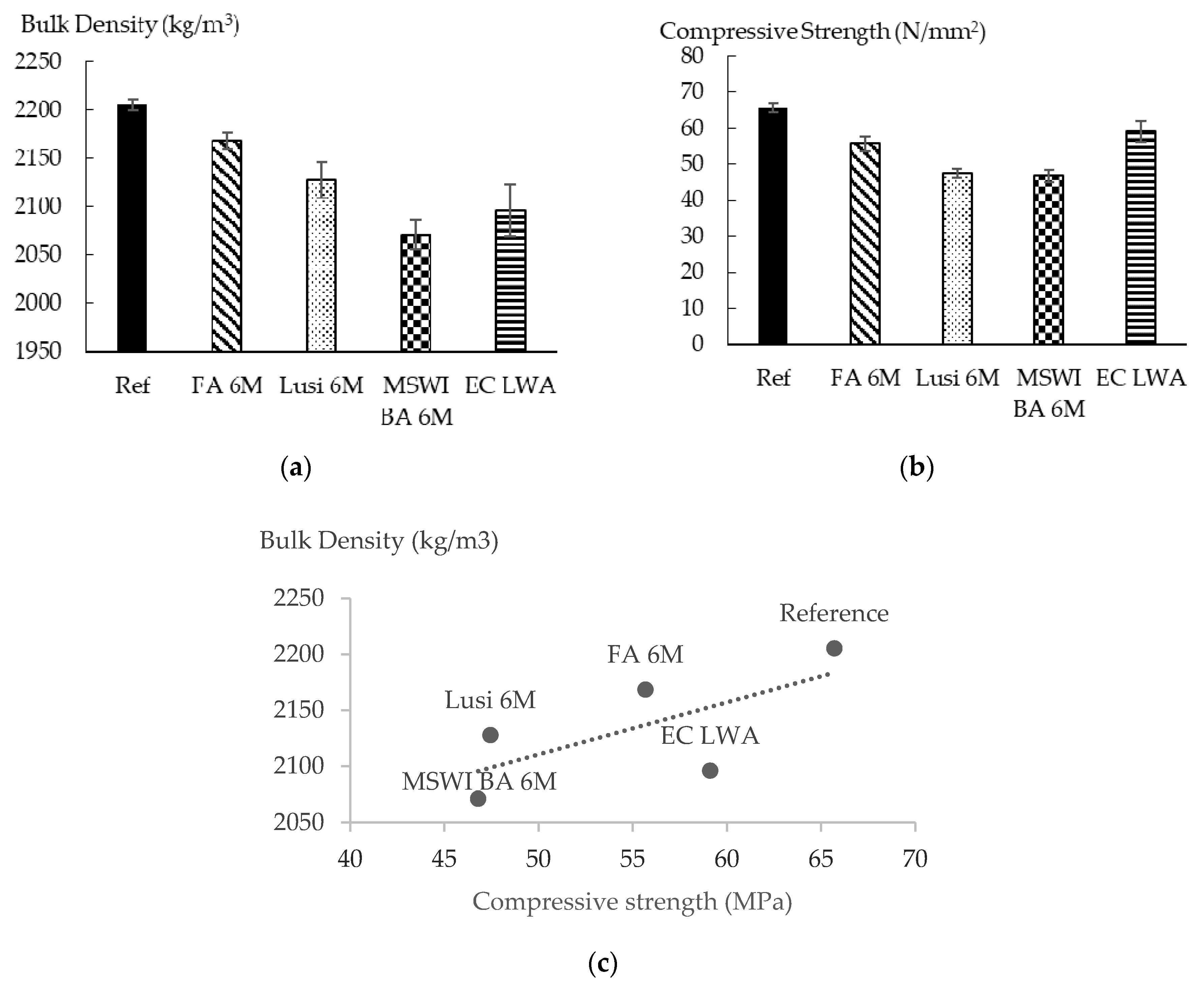
3.8. Volume Density and Compressive Strength of Mortar
3.9. Comparison with Commercial LWA
4. Conclusions
- The types of raw materials used affected the water absorption and the density of the resulting LWAs. Fly ash and bottom ash contain more calcium and have a lower LOI than Lusi, so they delivered LWAs with a lower water absorption and denser structure compared to the LWA generated from Lusi.
- The type of raw material had no significant effect on the particle size distribution of the resulting LWA, the fraction size of MSWI BA 6 M and Lusi 6 M LWA was 2/8 mm, while for the FA 6 M LWA, it was 2/10.
- The MIP test results revealed that LWA generated from fly ash and Lusi had two threshold diameters (dth). FA 6 M LWA had a dth of 0.1 µm and 6 µm while Lusi had a dth of 0.06 µm and 6 µm. The largest dth was measured for MSWI BA 6 M, which had a dth of 18 µm. MSWI BA 6 M contained large amounts of macropores. The appearance of micro-cracks on the surface of the MSWI BA 6 M due to the metallic Al reaction could be the reason for the high number of measured macropores in the MSWI BA 6 M sample.
- There was an unexpected result obtained from the crushing resistance test, where Lusi 6 M showed the highest crushing strength, followed by FA 6 M and MSWI BA 6 M. It seems that the crushing resistance test is not suitable in determining the strength of LWA that has a small particle size.
- The compressive strength reduction compared to the equivalent mortar made with expanded clay LWA was in the range between 6% and 21% for mortar with FA 6 M LWA and mortar with MSWI BA 6 M LWA, respectively. LWAs produced in this study are comparable and have even better mechanical properties compared to other lightweight aggregates generated from the geopolymerization of similar waste products.
- Despite the fact that the properties of mortar made with EC LWA were slightly better than that for the mortar made with LWAs in this research, the fact that more energy is required during the sintering process of EC LWA should also be a consideration.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Freedonia, World Construction Aggregates—Industry Study with Forecasts for 2015 & 2020, Free. Gr. (2012) 6. Available online: https://www.freedoniagroup.com/industry-study/global-construction-aggregates-3742.htm (accessed on 25 April 2020).
- Abidin, H.Z.; Davies, R.J.; Kusuma, M.A.; Andreas, H.; Deguchi, T. Subsidence and uplift of Sidoarjo (East Java) due to the eruption of the Lusi mud volcano (2006–present). Environ. Geol. 2009, 57, 833–844. [Google Scholar] [CrossRef]
- Triwulan, T.; Ekaputri, J.J. Sidoarjo Mud-Based Lightweight Mortar Using Foaming Agent and Aluminum Powder. In Applied Mechanics and Materials; Trans Tech Publications Ltd.: Baech, Switzerland, 2015. [Google Scholar] [CrossRef]
- Ekaputri, J.J. Lightweight Geopolymer Binder Base on Sidoarjo Mud. In Materials Science Forum; Trans Tech Publications Ltd.: Baech, Switzerland, 2015. [Google Scholar] [CrossRef]
- Hardjito, D.; Wibowo, G.M.; Christianto, D. Pozzolanic Activity Assessment of LUSI (LUmpur SIdoarjo) Mud in Semi High Volume Pozzolanic Mortar. Materials 2012, 5, 1654–1660. [Google Scholar] [CrossRef] [Green Version]
- Cembureau, Activity Report 2017. 2017. Available online: https://cembureau.eu/media/1716/activity-report-2017.pdf (accessed on 26 May 2020).
- Heidrich, C.; Feuerborn, H.; Weir, A. Coal Combustion Products: A global perspective. In Proceedings of the World of Coal Ash Conference, Lexington, MA, USA, 22–25 April 2013. [Google Scholar]
- Joseph, A.M.; Snellings, R.; van den Heede, P.; Matthys, S. The Use of Municipal Solid Waste Incineration Ash in Various Building Materials: A Belgian Point of View. Materials 2018, 11, 141. [Google Scholar] [CrossRef] [Green Version]
- Oehmig, W.N.; Roessler, J.G.; Blaisi, N.I.; Townsend, T.G. ScienceDirect Contemporary practices and findings essential to the development of effective MSWI ash reuse policy in the United States. Environ. Sci. Policy 2015, 51, 304–312. [Google Scholar] [CrossRef]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator ( MSWI ) bottom ash in high calcium fl y ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Tang, P.; Florea, M.V.A.; Brouwers, H.J.H. Employing cold bonded pelletization to produce lightweight aggregates from incineration fine bottom ash. J. Clean. Prod. 2017, 165, 1371–1384. [Google Scholar] [CrossRef]
- Gesoǧlu, M.; Özturan, T.; Güneyisi, E. Effects of fly ash properties on characteristics of cold-bonded fly ash lightweight aggregates. Constr. Build. Mater. 2007, 21, 1869–1878. [Google Scholar] [CrossRef]
- Gomathi, P.; Sivakumar, A. Accelerated curing effects on the mechanical performance of cold bonded and sintered fly ash aggregate concrete. Constr. Build. Mater. 2015, 77, 276–287. [Google Scholar] [CrossRef]
- Tang, P.; Brouwers, H.J.H. Integral recycling of municipal solid waste incineration (MSWI) bottom ash fines (0–2 mm) and industrial powder wastes by cold-bonding pelletization. Waste Manag. 2017, 62, 125–138. [Google Scholar] [CrossRef]
- Görhan, G.; Kürklü, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Part B Eng. 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Shivaprasad, K.N.; Das, B.B. Determination of optimized geopolymerization factors on the properties of pelletized fly ash aggregates. Constr. Build. Mater. 2018, 163, 428–437. [Google Scholar] [CrossRef]
- Xu, H.; van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef] [Green Version]
- Heah, C.Y.; Kamarudin, H.; al Bakri, A.M.M.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Liew, Y.M. Study on solids-to-liquid and alkaline activator ratios on kaolin-based geopolymers. Constr. Build. Mater. 2012, 35, 912–922. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Hardjito, D. Studies on Fly Ash-Based Geopolymer Concrete. Curtin Univ. Technol. 2005, 94. [Google Scholar] [CrossRef]
- Salman, M.; Cizer, Ö.; Pontikes, Y.; Vandewalle, L.; Blanpain, B. Effect of curing temperatures on the alkali activation of crystalline continuous casting stainless steel slag. Constr. Build. Mater. 2014, 71, 308–316. [Google Scholar] [CrossRef]
- Thomas, J.; Harilal, B. Properties of cold bonded quarry dust coarse aggregates and its use in concrete. Cem. Concr. Compos. 2015, 62, 67–75. [Google Scholar] [CrossRef]
- González-corrochano, B.; Alonso-azcárate, J.; Rodríguez, L.; Pérez, A.; Fernández, M.; José, J.; Ramos, T.; Dolores, M.; Muro, C. Valorization of washing aggregate sludge and sewage sludge for lightweight aggregates production. Constr. Build. Mater. 2016, 116, 252–262. [Google Scholar] [CrossRef]
- Hamidi, R.M.; Man, Z.; Azizli, K.A. Concentration of NaOH and the Effect on the Properties of Fly Ash Based Geopolymer. Procedia Eng. 2016, 148, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Baykal, G.; Döven, A.G. Utilization of fly ash by pelletization process; theory, application areas and research results. Conserv. Recycl. 2000, 30, 59–77. [Google Scholar] [CrossRef]
- Snoeck, D.; Velasco, L.F.; Mignon, A.; van Vlierberghe, S.; Dubruel, P.; Lodewyckx, P.; de Belie, N. The in fluence of different drying techniques on the water sorption properties of cement-based materials. Cem. Concr. Res. 2014, 64, 54–62. [Google Scholar] [CrossRef]
- Washburn, E.W. The Dynamics of Capillary Flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Santos, R.M.; Mertens, G.; Salman, M.; Cizer, Ö.; van Gerven, T. Comparative study of ageing, heat treatment and accelerated carbonation for stabilization of municipal solid waste incineration bottom ash in view of reducing regulated heavy metal / metalloid leaching. J. Environ. Manag. 2013, 128, 807–821. [Google Scholar] [CrossRef] [Green Version]
- Nath, S.K.; Kumar, S. Role of alkali concentration on reaction kinetics of fl y ash geopolymerization. J. Non-Cryst. Solids 2019, 505, 241–251. [Google Scholar] [CrossRef]
- Kang, S.; Jeong, Y.; Ook, M.; Moon, J. Pozzolanic reaction on alkali-activated Class F fly ash for ambient condition curable structural materials. Constr. Build. Mater. 2019, 218, 235–244. [Google Scholar] [CrossRef]
- Chithiraputhiran, S.; Neithalath, N. Isothermal reaction kinetics and temperature dependence of alkali activation of slag, fly ash and their blends. Constr. Build. Mater. 2013, 45, 233–242. [Google Scholar] [CrossRef]
- Geetha, S.; Ramamurthy, K. Properties of geopolymerised low-calcium bottom ash aggregate cured at ambient temperature. Cem. Concr. Compos. 2013, 43, 20–30. [Google Scholar] [CrossRef]
- Yliniemi; Paiva; Ferreira; Tiainen; Illikainen. Development and incorporation of lightweight waste-based geopolymer aggregates in mortar and concrete. Constr. Build. Mater. 2017, 131, 784–792. [Google Scholar] [CrossRef]
- Gesoǧlu, M.; Güneyisi, E.; Alzeebaree, R.; Mermerdaş, K. Effect of silica fume and steel fiber on the mechanical properties of the concretes produced with cold bonded fly ash aggregates. Constr. Build. Mater. 2013, 40, 982–990. [Google Scholar] [CrossRef]
- Vandeputte, N. Sustainable High Quality Recycling of Bottom Ashes from Waste-To-Energy for Use in Cement-Bound Materials. Master’s Thesis, Ghent University, Ghent, Belgium, 2018. [Google Scholar]
- Caprai, V.; Schollbach, K.; Brouwers, H.J.H. Influence of hydrothermal treatment on the mechanical and environmental performances of mortars including MSWI bottom ash. Waste Manag. 2018, 78, 639–648. [Google Scholar] [CrossRef]
- Sun, Z.; Vollpracht, A. Isothermal calorimetry and in-situ XRD study of the NaOH activated fly ash, metakaolin and slag. Cem. Concr. Res. 2018, 103, 110–122. [Google Scholar] [CrossRef]
- van den Heede, P.; Ringoot, N.; Beirnaert, A.; van Brecht, A.; van den Brande, E.; de Schutter, G.; de Belie, N. Sustainable high quality recycling of aggregates from waste-to-energy, treated in awet bottom ash processing installation, for use in concrete products. Materials 2016, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everett, D.H. International union of pure and applied chemistry division of physical chemistry manual of symbols and terminology for physicochemical quantities and units appendix II, Part I. Pure Appl. Chem. 1972, 34, 577–638. [Google Scholar] [CrossRef]
- Tziviloglou, E. Biogenic Self-Healing Mortar. Ph.D. Thesis, TU Delft, Delft, The Netherlands, 2018. [Google Scholar]
- Korat, L.; Ducman, V.; Legat, A.; Mirtic, B. Characterisation of the pore-forming process in lightweight aggregate based on silica sludge by means of X-ray micro-tomography ( micro-CT ) and mercury intrusion porosimetry (MIP). Ceram. Int. 2013, 39, 6997–7005. [Google Scholar] [CrossRef]
- Singh, G.V.P.B.; Subramaniam, K.V.L. Quantitative XRD study of amorphous phase in alkali activated low calcium siliceous fly ash. Constr. Build. Mater. 2016, 124, 139–147. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.S.; Zhan, B.J.; Sharma, U.; Poon, C.S. Compressive strength and microstructural properties of dry-mixed geopolymer pastes synthesized from GGBS and sewage sludge ash. Constr. Build. Mater. 2018, 182, 597–607. [Google Scholar] [CrossRef]
- Kong, L.; Hou, L.; Du, Y. Chemical reactivity of lightweight aggregate in cement paste. Constr. Build. Mater. 2014, 64, 22–27. [Google Scholar] [CrossRef]
- Eva, T.Ĥ. Effect of firing temperature on the structure of the aggregate from sintered ashes. Procedia Eng. 2016, 151, 345–351. [Google Scholar] [CrossRef]




| Sample Codes | Liquid/Solid |
|---|---|
| FA 6M | 0.25 ± 0.01 |
| Lusi 6M | 0.51 ± 0.01 |
| MSWI BA 6M | 0.26 ± 0.01 |
| Type | Sand 2/4 (g) | LWAOD1 2/4 (g) | Absorbed Water (g) | Sand 0/2 (g) | Cement (g) | Water (g) |
|---|---|---|---|---|---|---|
| Reference | 220 | 0 | 0 | 1130 | 450 | 225 |
| Lusi 6M | 0 | 116 | 37 | 1196 | 450 | 225 |
| FA 6M | 0 | 122 | 32 | 1196 | 450 | 225 |
| MSWI BA 6M | 0 | 132 | 23 | 1195 | 450 | 225 |
| EC LWA | 0 | 82 | 17 | 1251 | 450 | 225 |
| Composition (%) | MSWI BA | FA | Lusi |
|---|---|---|---|
| Cl | 0.37 | — | 0.15 |
| CaO | 16.80 | 3.79 | 2.27 |
| SiO2 | 48.40 | 57.40 | 52.90 |
| Al2O3 | 10.10 | 26.17 | 22.20 |
| Fe2O3 | 7.69 | 5.99 | 7.64 |
| K2O | 1.13 | 1.88 | 1.59 |
| Na2O | 6.43 | — | — |
| MgO | 2.98 | 1.43 | 2.52 |
| CuO | 0.25 | 0.02 | 0.01 |
| ZnO | 0.47 | 0.02 | 0.01 |
| SO3 | 2.06 | 0.98 | 1.83 |
| P2O5 | 1.91 | 0.88 | — |
| TiO2 | 1.27 | 1.13 | 0.81 |
| LOI | 0.15 | 0.32 | 8.08 |
| Specific Density (g/cm3) | 2.61 | 1.99 | 2.75 |
| Mineral | MSWI BA | FA | Lusi |
|---|---|---|---|
| Akermanite | 2.1 | — | — |
| Quartz | 5.3 | 4.6 | 8.0 |
| Mullite | 3.8 | 11.1 | — |
| Calcite | 2.3 | — | 0.4 |
| Anhydrite | — | 0.3 | 1.0 |
| Magnetite | 0.6 | 3.0 | — |
| Wuestite | 0.4 | — | — |
| Sodalite | — | 0.4 | — |
| Iron | 0.4 | — | — |
| Cristobalite | 0.1 | — | — |
| MgAlSiO | 0.7 | — | — |
| Feldspar | 2.8 | — | 16.3 |
| Hematite | 1.0 | — | 4.1 |
| Vermiculite | — | — | 1.7 |
| Anatase | — | — | 0.6 |
| Corundum | 1.9 | — | — |
| Apatite | 2.2 | — | — |
| (Na,K)Cl | 1.4 | — | — |
| Periclase | 1.4 | — | — |
| Perovskite | — | 0.3 | — |
| Dolomite | 0.9 | — | — |
| Susannite | 0.1 | — | — |
| Amorphous | 72.8 | 80.4 | 67.9 |
| Test | Lusi 6 M | FA 6 M | MSWI BA 6 M | EC LWA |
|---|---|---|---|---|
| Apparent particle density ρa (kg/m3) | 2.60 ± 0.09 | 2.23 ± 0.01 | 2.61 ± 0.03 | 1.25 ± 0.01 |
| Oven Dried Density ρrd (kg/m3) | 1.40 ± 0.02 | 1.47 ± 0.01 | 1.59 ± 0.01 | 0.99 ± 0.01 |
| SSD particle density ρssd (kg/m3) | 1.86 ± 0.03 | 1.81 ± 0.00 | 1.98 ± 0.01 | 1.19 ± 0.01 |
| Water absorption (%) | 32.8 ± 0.28 | 23.69 ± 0.63 | 24.80 ± 0.56 | 21.14 ± 0.30 |
| Porosity (%) | 45.98 ± 0.96 | 34.12 ± 0.75 | 39.32 ± 0.78 | 20.85 ± 0.09 |
| Mineral | MSWI BA 6 M | FA 6 M | Lusi 6 M |
|---|---|---|---|
| akermanite | 1.8 | — | — |
| quartz | 3.9 | 4.0 | 5.7 |
| mullite | — | 6.9 | — |
| calcite | 5.0 | 0.7 | 0.4 |
| magnetite | 2.4 | — | — |
| wuestite | 0.3 | — | — |
| AlFe3 | — | 0.2 | — |
| silimanite | — | 1.0 | — |
| gupeiite | — | 0.1 | — |
| gaylusite | — | 1.7 | — |
| sodalite | — | 0.2 | — |
| magnesioferrite | — | 2.2 | — |
| iron | 0.3 | — | — |
| cristobalite | 0.2 | — | — |
| MgAlSiO | 0.1 | — | — |
| feldspar | 1.8 | — | 10.8 |
| hematite | 0.3 | 1.2 | 2.6 |
| vermiculite | — | — | 1.0 |
| ulvoespinel | — | — | 1.3 |
| anatase | — | — | 0.5 |
| corundum | 0.9 | — | — |
| apatite | 0.3 | — | — |
| (Na,K)Cl | 0.7 | — | — |
| tobermorite 11 a | 3.7 | — | — |
| perovskite | 1.9 | — | — |
| ankerite | 0.9 | — | — |
| amorphous | 75.3 | 82.0 | 77.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Risdanareni, P.; Villagran, Y.; Schollbach, K.; Wang, J.; De Belie, N. Properties of Alkali Activated Lightweight Aggregate Generated from Sidoarjo Volcanic Mud (Lusi), Fly Ash, and Municipal Solid Waste Incineration Bottom Ash. Materials 2020, 13, 2528. https://doi.org/10.3390/ma13112528
Risdanareni P, Villagran Y, Schollbach K, Wang J, De Belie N. Properties of Alkali Activated Lightweight Aggregate Generated from Sidoarjo Volcanic Mud (Lusi), Fly Ash, and Municipal Solid Waste Incineration Bottom Ash. Materials. 2020; 13(11):2528. https://doi.org/10.3390/ma13112528
Chicago/Turabian StyleRisdanareni, Puput, Yury Villagran, Katrin Schollbach, Jianyun Wang, and Nele De Belie. 2020. "Properties of Alkali Activated Lightweight Aggregate Generated from Sidoarjo Volcanic Mud (Lusi), Fly Ash, and Municipal Solid Waste Incineration Bottom Ash" Materials 13, no. 11: 2528. https://doi.org/10.3390/ma13112528





