Novel Ternary Heterogeneous Reduction Graphene Oxide (RGO)/BiOCl/TiO2 Nanocomposites for Enhanced Adsorption and Visible-Light Induced Photocatalytic Activity toward Organic Contaminants
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Preparation of RGO/BiOCl/TiO2 Nanocomposites
2.3. Characterization
2.4. Adsorption and Photocatalytic Performance Measurement
2.5. Analysis of Photocatalytic Mechanism
3. Results and Discussions
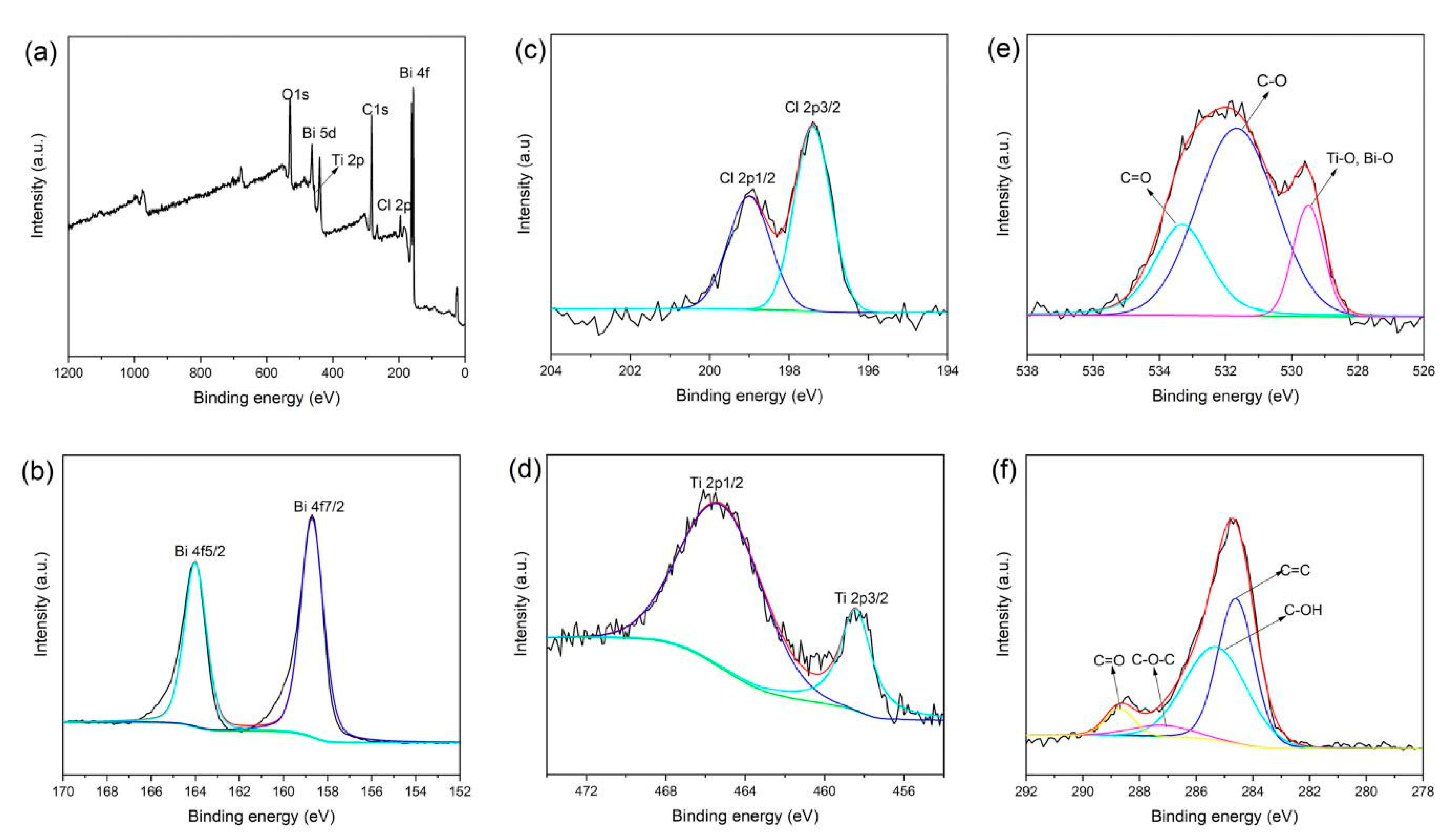
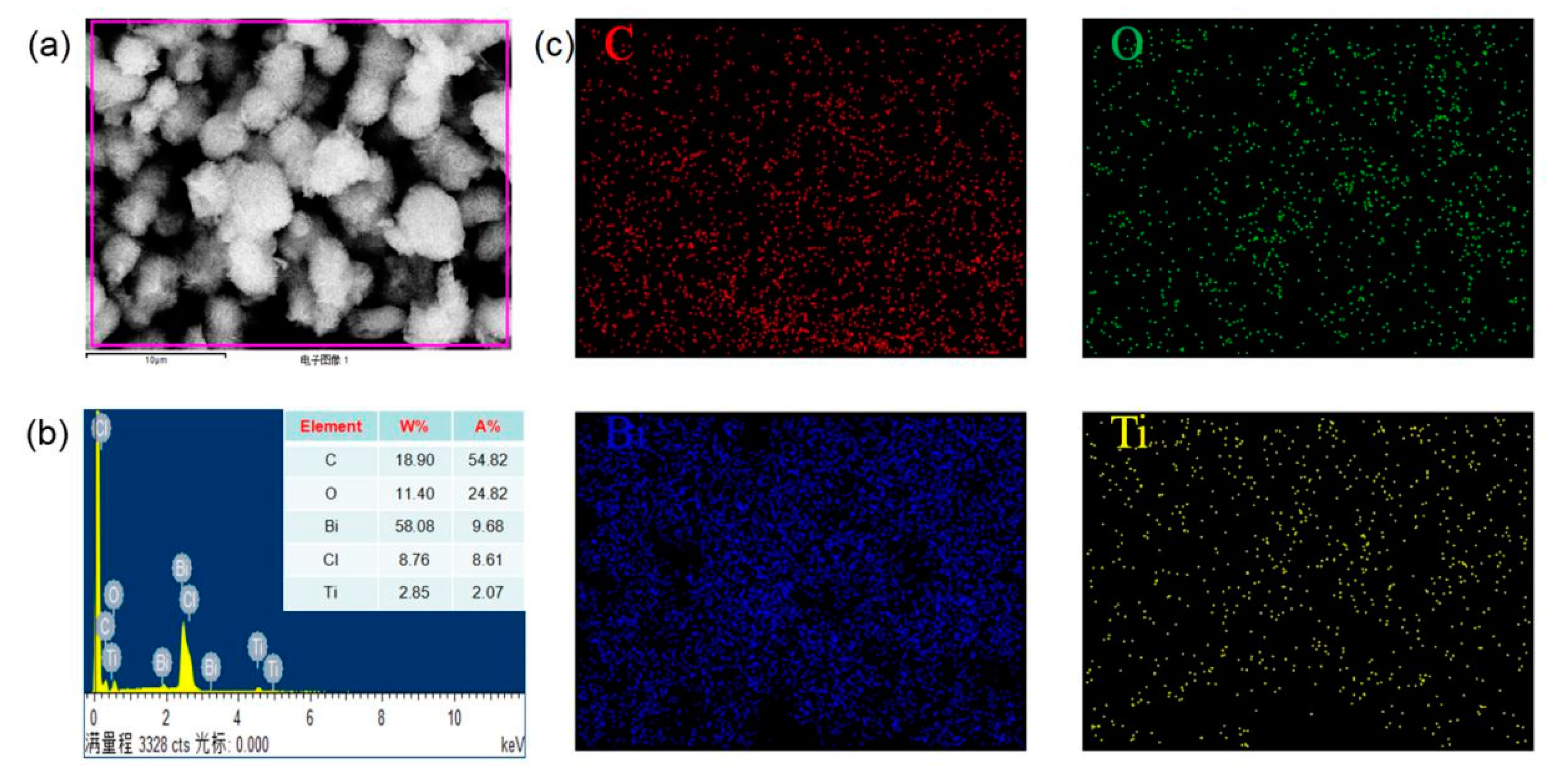
3.1. Chemical Structure of RGO/BiOCl/TiO2 Nanocomposites
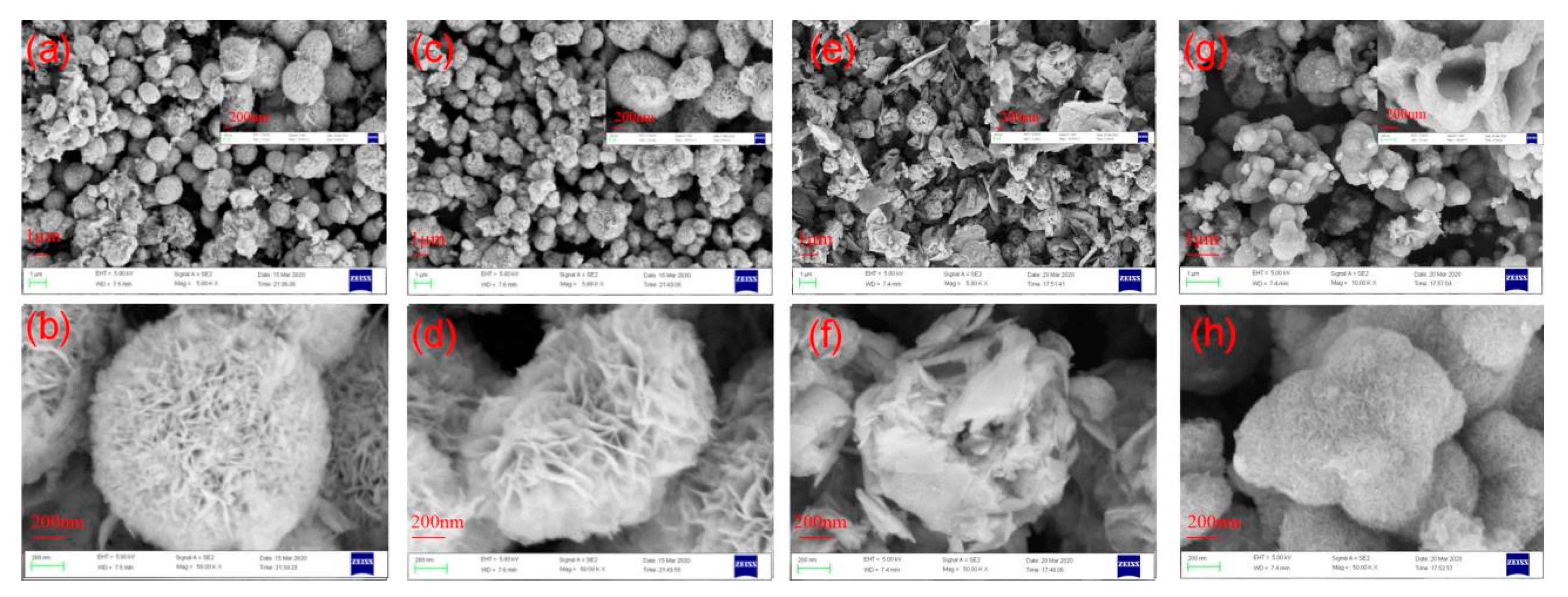
3.2. Morphology of RGO/BiOCl/TiO2 Nanocomposites
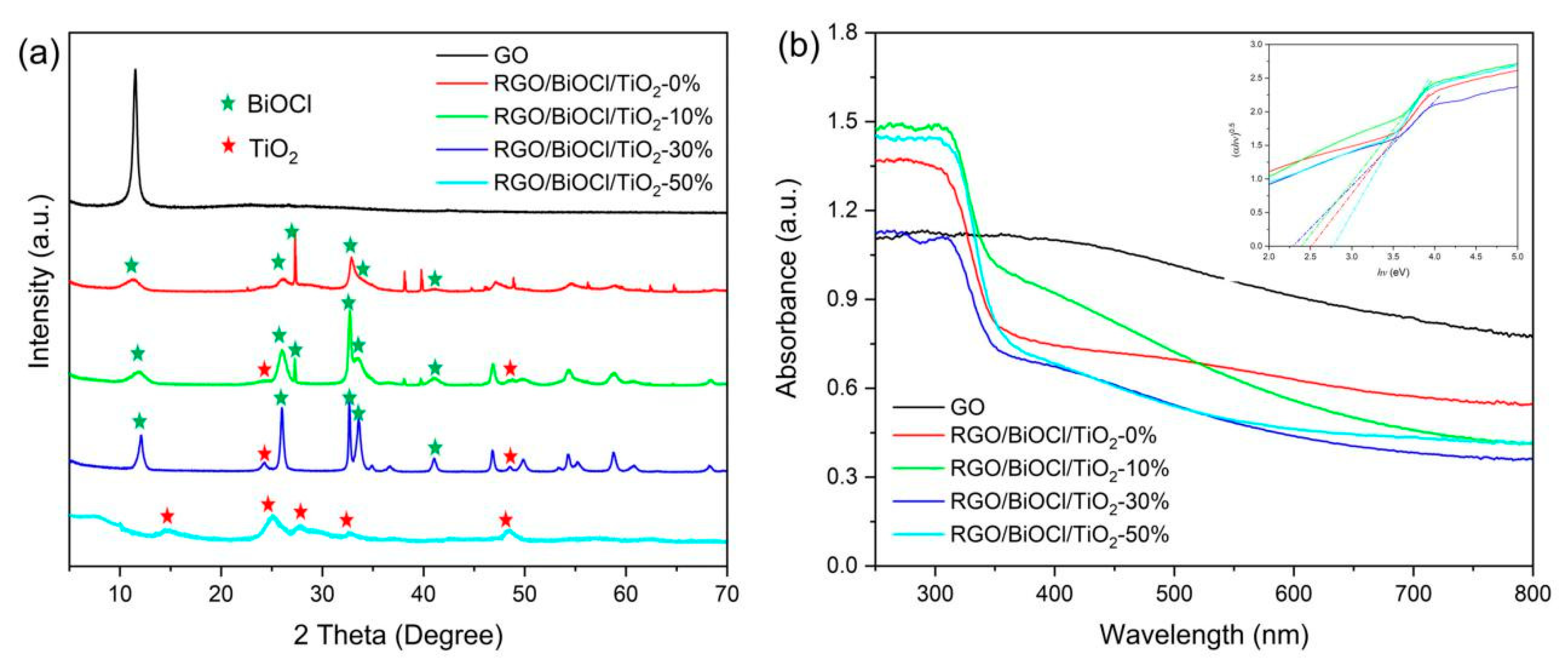
3.3. Phase Structure and Optical Property
3.4. Surface Area and Pore-Size Distribution
3.5. Adsorption Capacity
3.6. Photocatalytic Performance
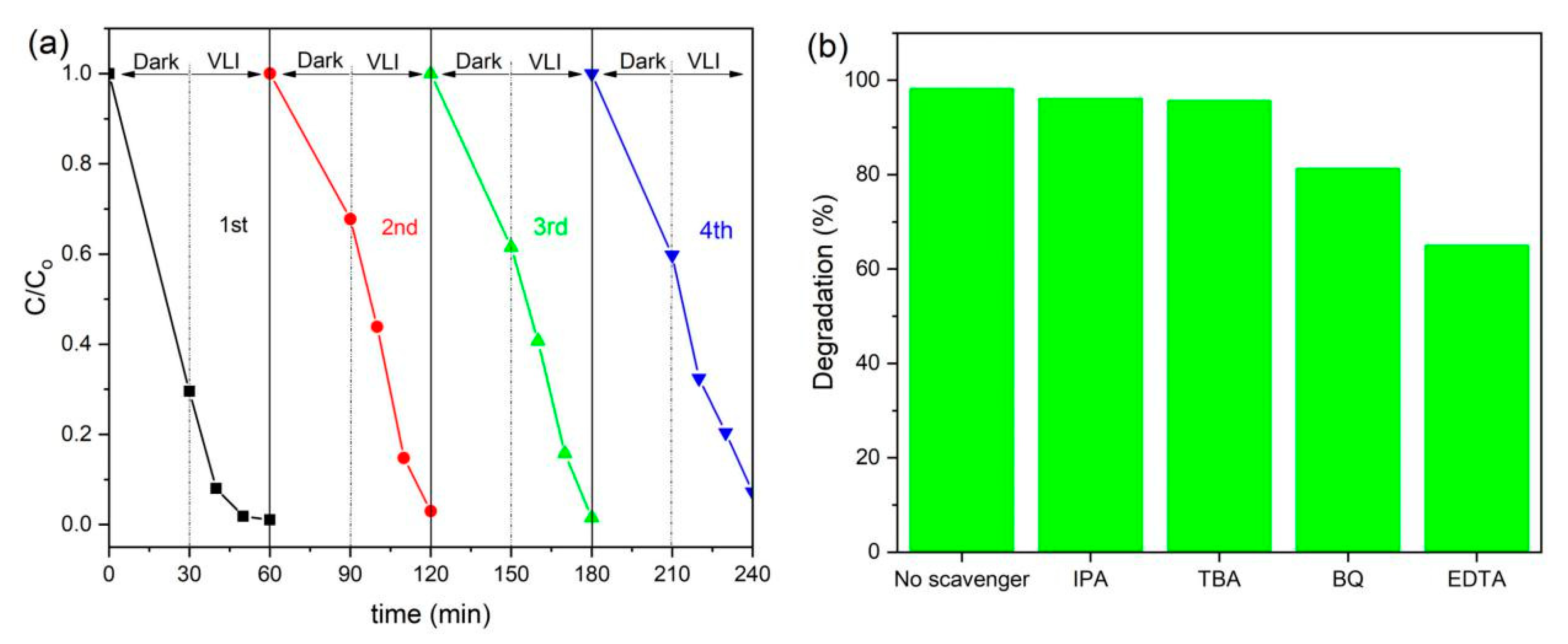
3.7. Reusability Study
3.8. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kong, J.-Z.; Li, A.-D.; Zhai, H.; Li, H.; Yan, Q.-Y.; Ma, J.; Wu, D. Preparation, characterization and photocatalytic properties of ZnTiO3 powders. J. Hazard. Mater. 2009, 171, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.M.; Mohamed, A.R. Roles of titanium dioxide and ion-doped titanium dioxide on photocatalytic degradation of organic pollutants (phenolic compounds and dyes) in aqueous solutions: A review. J. Alloy. Compd. 2011, 509, 1648–1660. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, X.-F.; Chen, X.-T.; Xue, Z.-L. BiOBr hierarchical microspheres: Microwave-assisted solvothermal synthesis, strong adsorption and excellent photocatalytic properties. J. Colloid Interface Sci. 2011, 354, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Salari, D.; Khataee, A. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Chan, S.H.S.; Wu, T.Y.; Juan, J.C.; Teh, C.Y. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process. Process. Intensif. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Lai, C.; Zeng, G.; Huang, D.; Cheng, M.; Wang, J.; Chen, F.; Zhou, C.; Xiong, W. BiOX (X = Cl, Br, I) photocatalytic nanomaterials: Applications for fuels and environmental management. Adv. Colloid Interface Sci. 2018, 254, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Z. New insight into BiOX (X = Cl, Br, and I) hierarchical microspheres in photocatalysis. Mater. Lett. 2018, 225, 152–156. [Google Scholar] [CrossRef]
- Wen, J.; Li, X.; Liu, W.; Fang, Y.; Xie, J.; Xu, Y. Photocatalysis fundamentals and surface modification of TiO2 nanomaterials. Chin. J. Catal. 2015, 36, 2049–2070. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X. Hyderothermal synthesis and photocatalytic activity of zinc oxide hollow spheres. Environ. Sci. Technol. 2008, 42, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, W.; Zhou, L.; Sun, S.; Zhang, L. CTAB-assisted synthesis of monoclinic BiVO4 photocatalyst and its highly efficient degradation of organic dye under visible-light irradiation. J. Hazard. Mater. 2010, 173, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, B.; Lin, H.; Luo, B.; Chen, S. Chemical etching preparation of BiOI/BiOBr heterostructures with enhanced photocatalytic properties for organic dye removal. Chem. Eng. J. 2012, 185, 91–99. [Google Scholar] [CrossRef]
- Sajjad, A.K.L.; Shamaila, S.; Tian, B.; Chen, F.; Zhang, J. One step activation of WOx/TiO2 nanocomposites with enhanced photocatalytic activity. Appl. Catal. B Environ. 2009, 91, 397–405. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. CdS/Graphene Nanocomposite Photocatalysts. Adv. Energy Mater. 2015, 5, 1500010. [Google Scholar] [CrossRef]
- Li, Y.; Ding, Y. Porous AgCl/Ag Nanocomposites with Enhanced Visible Light Photocatalytic Properties. J. Phys. Chem. C 2010, 114, 3175–3179. [Google Scholar] [CrossRef]
- Hu, H.; Wang, X.; Liu, F.; Wang, J.; Xu, C. Rapid microwave-assisted synthesis of graphene nanosheets–zinc sulfide nanocomposites: Optical and photocatalytic properties. Synth. Met. 2011, 161, 404–410. [Google Scholar] [CrossRef]
- Duo, F.; Wang, Y.; Fan, C.; Mao, X.; Zhang, X.; Wang, Y.; Liu, J. Low temperature one-step synthesis of rutile TiO2/BiOCl composites with enhanced photocatalytic activity. Mater. Charact. 2015, 99, 8–16. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Q.; Yang, L.; Luo, X.; Wang, A.; Luo, S.; Dionysiou, D.D. Visible-light-responsive graphene-functionalized Bi-bridge Z-scheme black BiOCl/Bi2O3 heterojunction with oxygen vacancy and multiple charge transfer channels for efficient photocatalytic degradation of 2-nitrophenol and industrial wastewater treatment. Appl. Catal. B Environ. 2018, 238, 61–69. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Lv, D.; Zhang, D.; Pu, X.; Kong, D.; Lu, Z.; Shao, X.; Ma, H.; Dou, J. One-pot combustion synthesis of BiVO4/BiOCl composites with enhanced visible-light photocatalytic properties. Sep. Purif. Technol. 2017, 174, 97–103. [Google Scholar] [CrossRef]
- Shamaila, S.; Sajjad, A.K.L.; Chen, F.; Zhang, J. WO3/BiOCl, a novel heterojunction as visible light photocatalyst. J. Colloid Interface Sci. 2011, 356, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Yu, G.; Huang, J.; Li, Z.; Zhu, S.; Yu, P.; Cheng, C.; Deng, S.; Ji, G. Enhancement of photocatalytic activity over NaBiO3/BiOCl composite prepared by an in situ formation strategy. Catal. Today 2010, 153, 193–199. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, D.; Medrano, M.G.M.; Remita, H.; Escobar-Barrios, V. Photocatalytic properties of BiOCl-TiO2 composites for phenol photodegradation. J. Environ. Chem. Eng. 2018, 6, 1601–1612. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Ismail, A.A.; Mohamed, R.M. Enhancement of titania by doping rare earth for photodegradation of organic dye (Direct Blue). J. Hazard. Mater. 2009, 166, 138–143. [Google Scholar] [CrossRef]
- Fan, X.; Wan, J.; Liu, E.; Sun, L.; Hu, Y.; Li, H.; Hu, X.; Fan, J. High-efficiency photoelectrocatalytic hydrogen generation enabled by Ag deposited and Ce doped TiO2 nanotube arrays. Ceram. Int. 2015, 41, 5107–5116. [Google Scholar] [CrossRef]
- Zalfani, M.; Hu, Z.Y.; Yu, W.B.; Mahdouani, M.; Bourguiga, R.; Wu, M.; Li, Y.; Tendeloo, G.V.; Djaoued, Y.; Su, B.L. BiVO4/3DOM TiO2 nanocomposites: Effect of BiVO4 as highly efficient visible light sensitizer for highly improved visible light photocatalytic activity in the degradation of dye pollutants. Appl. Catal. B Environ. 2017, 205, 121–132. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Qian, F.; Wang, Y.; Li, M.; Lu, J.; Li, Y.; Bao, M. Constructing a novel ternary composite (C16H33(CH3)3N)4W10O32/g-C3N4/rGO with enhanced visible-light-driven photocatalytic activity for degradation of dyes and phenol. Appl. Catal. B Environ. 2017, 200, 283–296. [Google Scholar] [CrossRef]
- Zhu, Z.; Han, Q.; Yu, D.; Sun, J.; Liu, B. A novel p-n heterojunction of BiVO4/TiO2/GO composite for enhanced visible-light-driven photocatalytic activity. Mater. Lett. 2017, 209, 379–383. [Google Scholar] [CrossRef]
- Johra, F.T.; Jung, W.-G. RGO–TiO2–ZnO composites: Synthesis, characterization, and application to photocatalysis. Appl. Catal. A Gen. 2015, 491, 52–57. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Wang, X.; Yu, H.; Yu, J.; Lei, M.; Wang, Y. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B Environ. 2013, 132, 452–459. [Google Scholar] [CrossRef]
- Bi, J.; Fang, W.; Li, L.; Li, X.; Liu, M.; Liang, S.; Zhang, Z.; He, Y.; Lin, H.; Wu, L.; et al. Ternary reduced-graphene-oxide/Bi2MoO6/Au nanocomposites with enhanced photocatalytic activity under visible light. J. Alloy. Compd. 2015, 649, 28–34. [Google Scholar] [CrossRef]
- Wang, H.; Peng, D.; Chen, T.; Chang, Y.; Dong, S. A novel photocatalyst AgBr/ZnO/RGO with high visible light photocatalytic activity. Ceram. Int. 2016, 42, 4406–4412. [Google Scholar] [CrossRef]
- Liu, S.; Liu, C.; Wang, W.; Cheng, B.; Yu, J. Unique photocatalytic oxidation reactivity and selectivity of TiO2–graphene nanocomposites. Nanoscale 2012, 4, 3193. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Ho, W.; Lee, S.C. Efficient Visible Light Photocatalytic Removal of NO with BiOBr-Graphene Nanocomposites. J. Phys. Chem. C 2011, 115, 25330–25337. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Li, W.; Tian, Y.; Li, H.; Zhao, C.; Zhang, B.; Zhang, H.; Geng, W.; Zhang, Q. Novel BiOCl/TiO2 hierarchical composites: Synthesis, characterization and application on photocatalysis. Appl. Catal. A: Gen. 2016, 516, 81–89. [Google Scholar] [CrossRef]
- Fang, K.; Shi, L.; Yao, L.; Cui, L. Synthesis of novel magnetically separable Fe3O4/Bi12O17Cl2 photocatalyst with boosted visible-light photocatalytic activity. Mater. Res. Bull. 2020, 129, 110888. [Google Scholar] [CrossRef]
- She, X.; Liu, T.; Wu, N.; Xu, X.; Li, J.; Yang, D.; Frost, R. Spectrum analysis of the reduction degree of two-step reduced graphene oxide (GO) and the polymer/r-GO composites. Mater. Chem. Phys. 2013, 143, 240–246. [Google Scholar] [CrossRef]
- Mallakpour, S.; Abdolmaleki, A.; Khalesi, Z.; Borandeh, S. Surface functionalization of GO, preparation and characterization of PVA/TRIS-GO nanocomposites. Polymer 2015, 81, 140–150. [Google Scholar] [CrossRef]
- Ye, L.; Li, Z. Rapid microwave-assisted synthesis of reduced graphene oxide (RGO)/ZnIn2S4 microspheres as superior noble-metal-free photocatalyst for hydrogen evolutions under visible light. Appl. Catal. B Environ. 2014, 160/161, 552–557. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, C.; Lin, H.; Xu, B.; Chen, S. Surface modification of m-BiVO4 with wide band-gap semiconductor BiOCl to largely improve the visible light induced photocatalytic activity. Appl. Surf. Sci. 2013, 284, 263–269. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Z.; Hu, X.; Song, A.; Zheng, S. Synthesis of BiOCl/TiO2–zeolite composite with enhanced visible light photoactivity. J. Taiwan Inst. Chem. Eng. 2017, 81, 435–444. [Google Scholar] [CrossRef]
- Shen, X.; Lin, X.; Yousefi, N.; Jia, J.; Kim, J.-K. Wrinkling in graphene sheets and graphene oxide papers. Carbon 2014, 66, 84–92. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Graphene oxide (GO) enhanced polyamide (PA) thin-film nanocomposite (TFN) membrane for water purification. Desalination 2016, 379, 93–101. [Google Scholar] [CrossRef]
- Wu, S.; Jia, Q.; Dai, W. Synthesis of RGO/TiO2 hybrid as a high performance photocatalyst. Ceram. Int. 2017, 43, 1530–1535. [Google Scholar] [CrossRef]
- Cheng, X.; Shang, Y.; Cui, Y.; Shi, R.; Zhu, Y.; Yang, P. Enhanced photoelectrochemical and photocatalytic properties of anatase-TiO2(B) nanobelts decorated with CdS nanoparticles. Solid State Sci. 2020, 99, 106075. [Google Scholar] [CrossRef]
- Wang, Q.; Li, P.; Zhang, Z.; Jiang, C.; Zuojiao, K.; Liu, J.; Wang, Y. Kinetics and mechanism insights into the photodegradation of tetracycline hydrochloride and ofloxacin mixed antibiotics with the flower-like BiOCl/TiO2 heterojunction. J. Photochem. Photobiol. A: Chem. 2019, 378, 114–124. [Google Scholar] [CrossRef]
- Mady, A.H.; Baynosa, M.L.; Tuma, D.; Shim, J.-J. Facile microwave-assisted green synthesis of Ag-ZnFe2O4@rGO nanocomposites for efficient removal of organic dyes under UV- and visible-light irradiation. Appl. Catal. B: Environ. 2017, 203, 416–427. [Google Scholar] [CrossRef]
- Lin, W.; Yu, X.; Zhu, Y.; Zhang, Y. Graphene Oxide/BiOCl Nanocomposite Films as Efficient Visible Light Photocatalysts. Front. Chem. 2018, 6, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samsudin, M.F.R.; Jayabalan, P.J.; Ong, W.J.; Ng, Y.H.; Sufian, S. Photocatalytic degradation of real industrial poultry wastewater vis platinum decorated BiVO4/g-C3N4 photocatalyst under solar light. J. Photoch. Photobio. A Chem. 2019, 378, 46–56. [Google Scholar] [CrossRef]
- Sun, X.-F.; Liu, B.; Jing, Z.; Wang, H. Preparation and adsorption property of xylan/poly(acrylic acid) magnetic nanocomposite hydrogel adsorbent. Carbohydr. Polym. 2015, 118, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Wu, T.; Lin, Y.-W. Preparation and characterization of bismuth oxychloride/reduced graphene oxide for photocatalytic degradation of rhodamine B under white-light light-emitting-diode and sunlight irradiation. J. Photochem. Photobiol. A Chem. 2019, 371, 355–364. [Google Scholar] [CrossRef]
- Hou, G.; Li, Y.; An, W.; Gao, S.; Zhang, W.; Cui, W. Fabrication and photocatalytic activity of magnetic core@shell ZnFe2O4@Ag3PO4 heterojunction. Mater. Sci. Semicond. Process. 2017, 63, 261–268. [Google Scholar] [CrossRef]

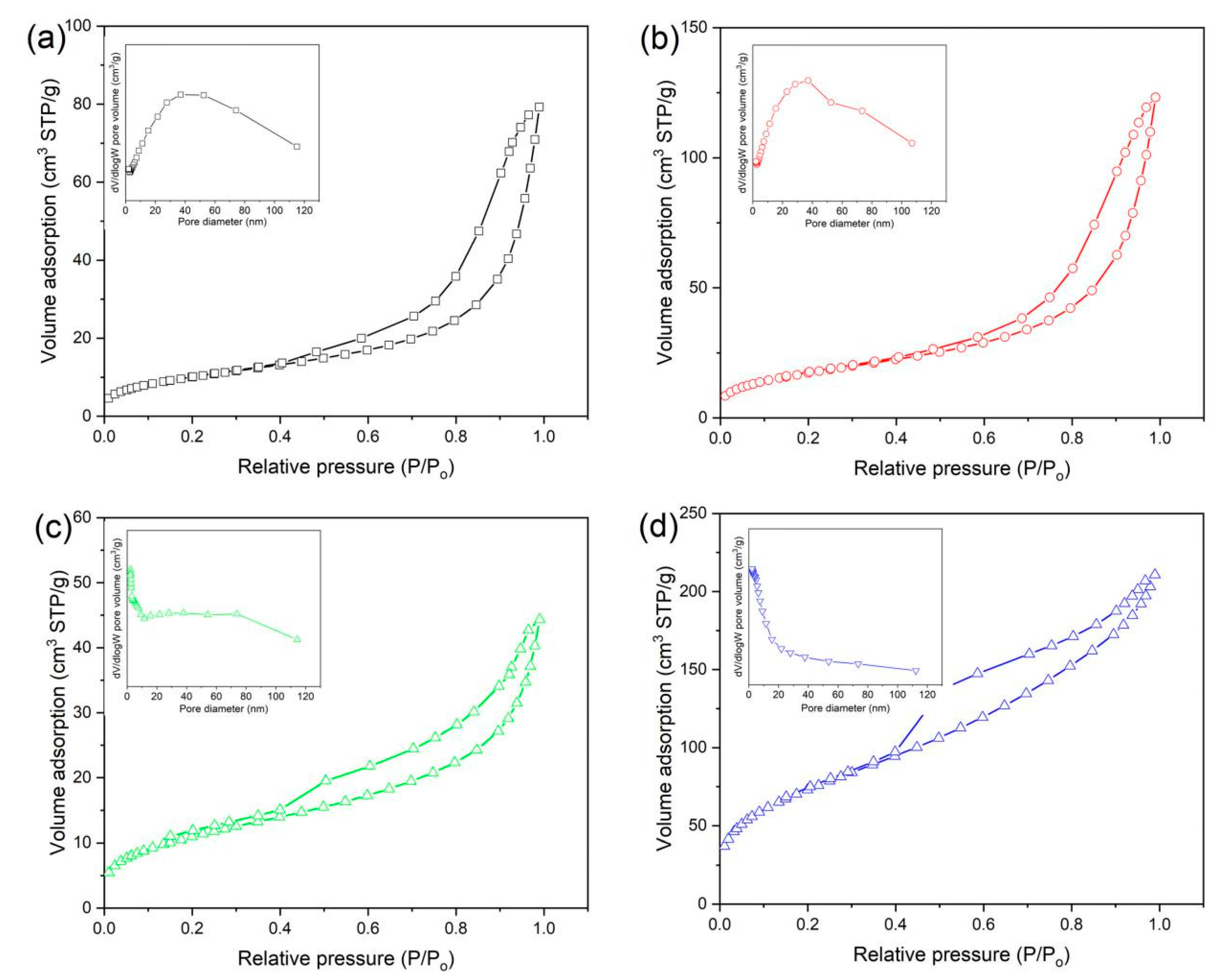
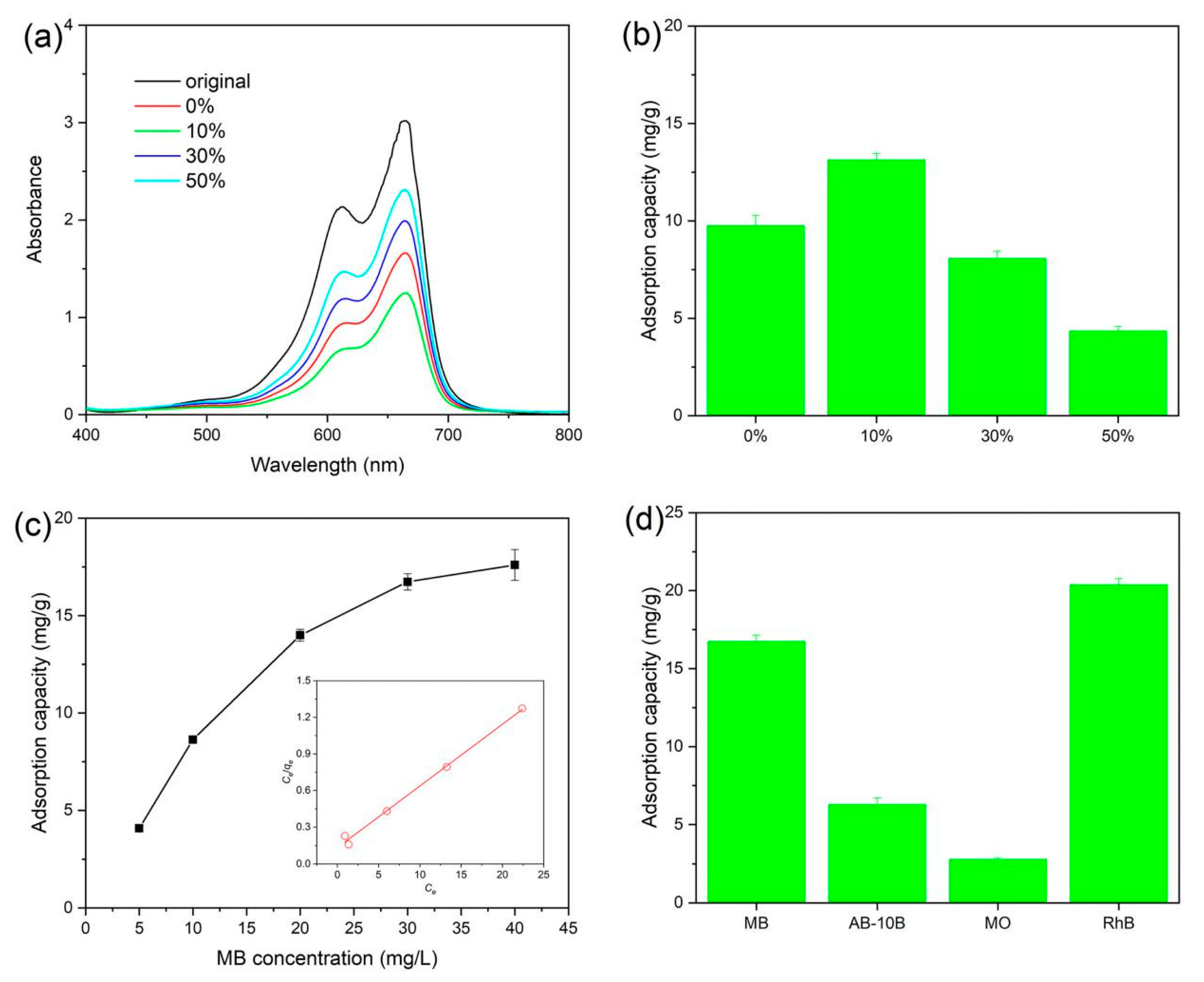
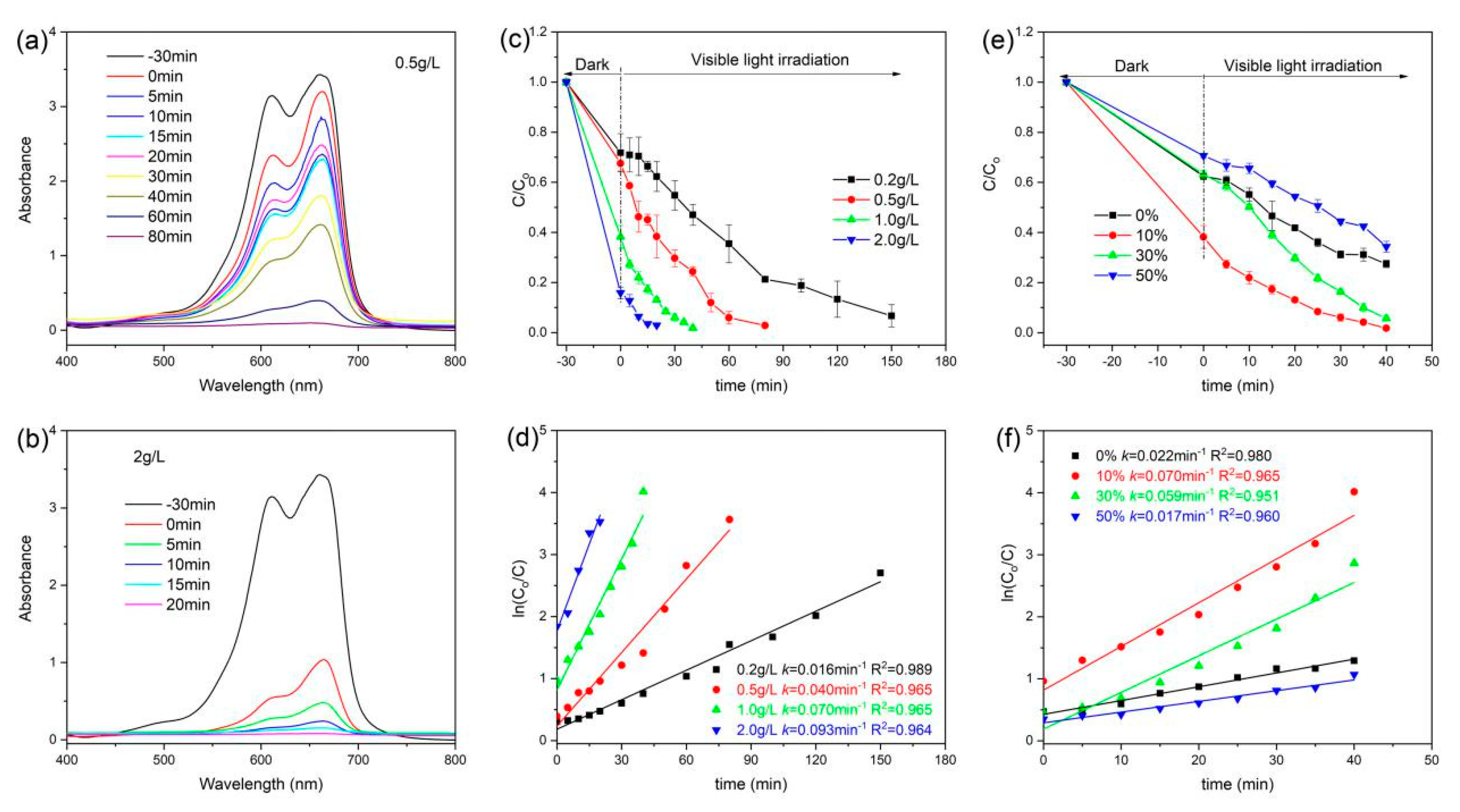
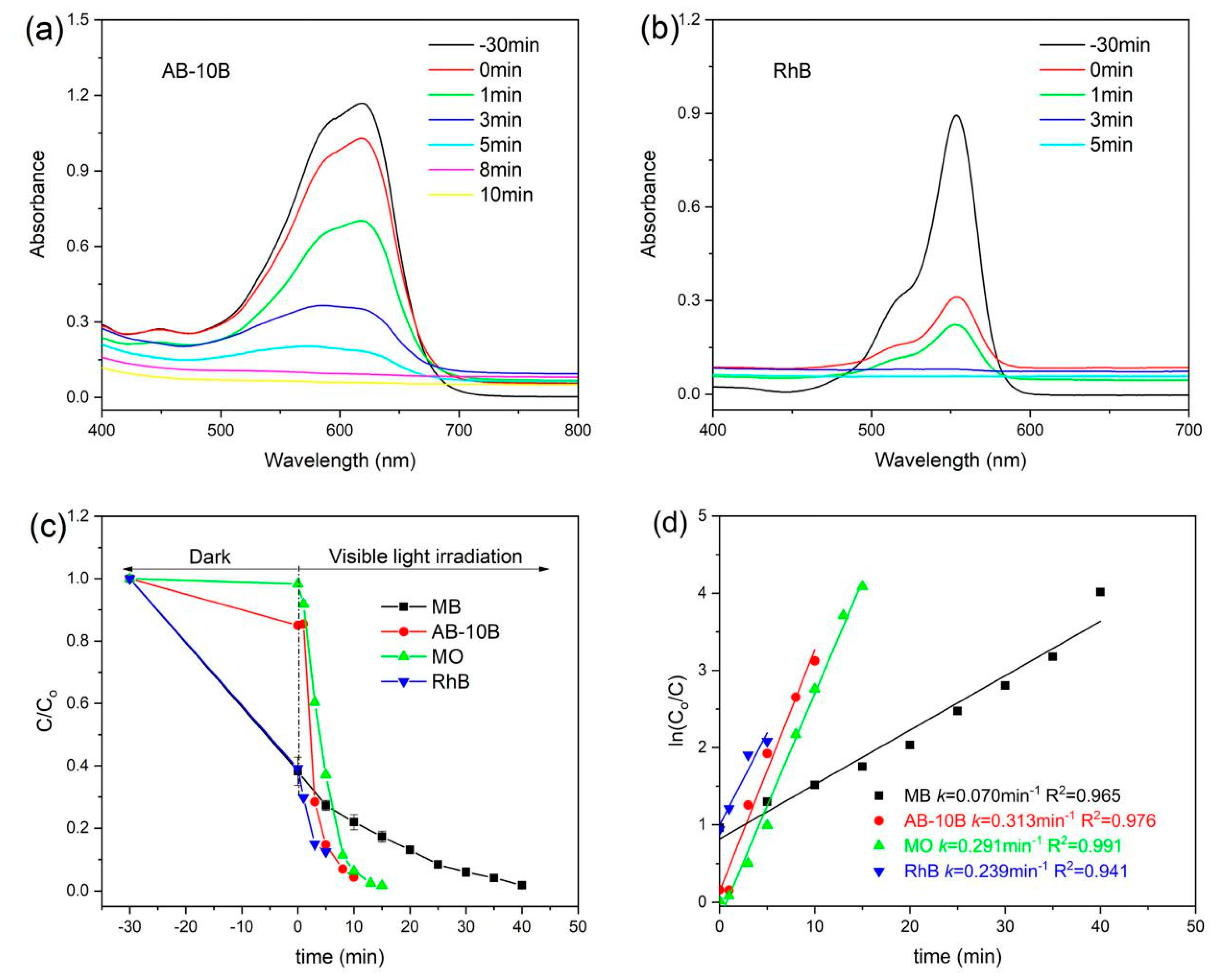

| Nanocomposites | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Band Gap (eV) |
|---|---|---|---|---|
| RGO/BiOCl/TiO2-0% | 37.58 | 0.121 | 13.05 | 2.51 |
| RGO/BiOCl/TiO2-10% | 64.21 | 0.188 | 12.52 | 2.39 |
| RGO/BiOCl/TiO2-30% | 40.44 | 0.069 | 7.60 | 2.30 |
| RGO/BiOCl/TiO2-50% | 270.59 | 0.331 | 5.27 | 2.77 |
| No. | Catalyst | Irradiation Type | Dyes | Removal Ratio (%) | Time (min) | Dye (mg/L) | Catalyst (g/L) | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1 | WOx/TiO2 | visible light | MO | 83.0 | 300 | 40 | 1.0 | [14] |
| 2 | WO3/BiOCl | visible light | RhB | 100 | 180 | 30 | 0.5 | [22] |
| 3 | BiVO4/TiO2/GO | visible light | RB-19 | 95.87 | 90 | 0.05 | 0.6 | [29] |
| 4 | BiOCl/TiO2-zeolite | visible light | RhB | ~100 | 90 | 10 | 1.0 | [43] |
| 5 | BiOCl/TiO2 | visible light | RhB | ~100 | 35 | 20 | 1.0 | [37] |
| 6 | RGO/BiOCl/TiO2 | visible light | MB | 98.2 | 40 | 30 | 1.0 | In this study |
| 7 | RGO/BiOCl/TiO2 | visible light | AB-10B | 96.0 | 10 | 30 | 1.0 | In this study |
| 8 | RGO/BiOCl/TiO2 | visible light | MO | 98.3 | 15 | 30 | 1.0 | In this study |
| 9 | RGO/BiOCl/TiO2 | visible light | RhB | 90.5 | 5 | 30 | 1.0 | In this study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, Z.; Dai, X.; Xian, X.; Zhang, Q.; Zhong, H.; Li, Y. Novel Ternary Heterogeneous Reduction Graphene Oxide (RGO)/BiOCl/TiO2 Nanocomposites for Enhanced Adsorption and Visible-Light Induced Photocatalytic Activity toward Organic Contaminants. Materials 2020, 13, 2529. https://doi.org/10.3390/ma13112529
Jing Z, Dai X, Xian X, Zhang Q, Zhong H, Li Y. Novel Ternary Heterogeneous Reduction Graphene Oxide (RGO)/BiOCl/TiO2 Nanocomposites for Enhanced Adsorption and Visible-Light Induced Photocatalytic Activity toward Organic Contaminants. Materials. 2020; 13(11):2529. https://doi.org/10.3390/ma13112529
Chicago/Turabian StyleJing, Zhanxin, Xiangyi Dai, Xueying Xian, Qiongshan Zhang, Huojiao Zhong, and Yong Li. 2020. "Novel Ternary Heterogeneous Reduction Graphene Oxide (RGO)/BiOCl/TiO2 Nanocomposites for Enhanced Adsorption and Visible-Light Induced Photocatalytic Activity toward Organic Contaminants" Materials 13, no. 11: 2529. https://doi.org/10.3390/ma13112529





