Investigating the Properties of Cetyltrimethylammonium Bromide/Hydroxylated Graphene Quantum Dots Thin Film for Potential Optical Detection of Heavy Metal Ions
Abstract
:1. Introduction
2. Experimental Details
2.1. Reagent and Materials
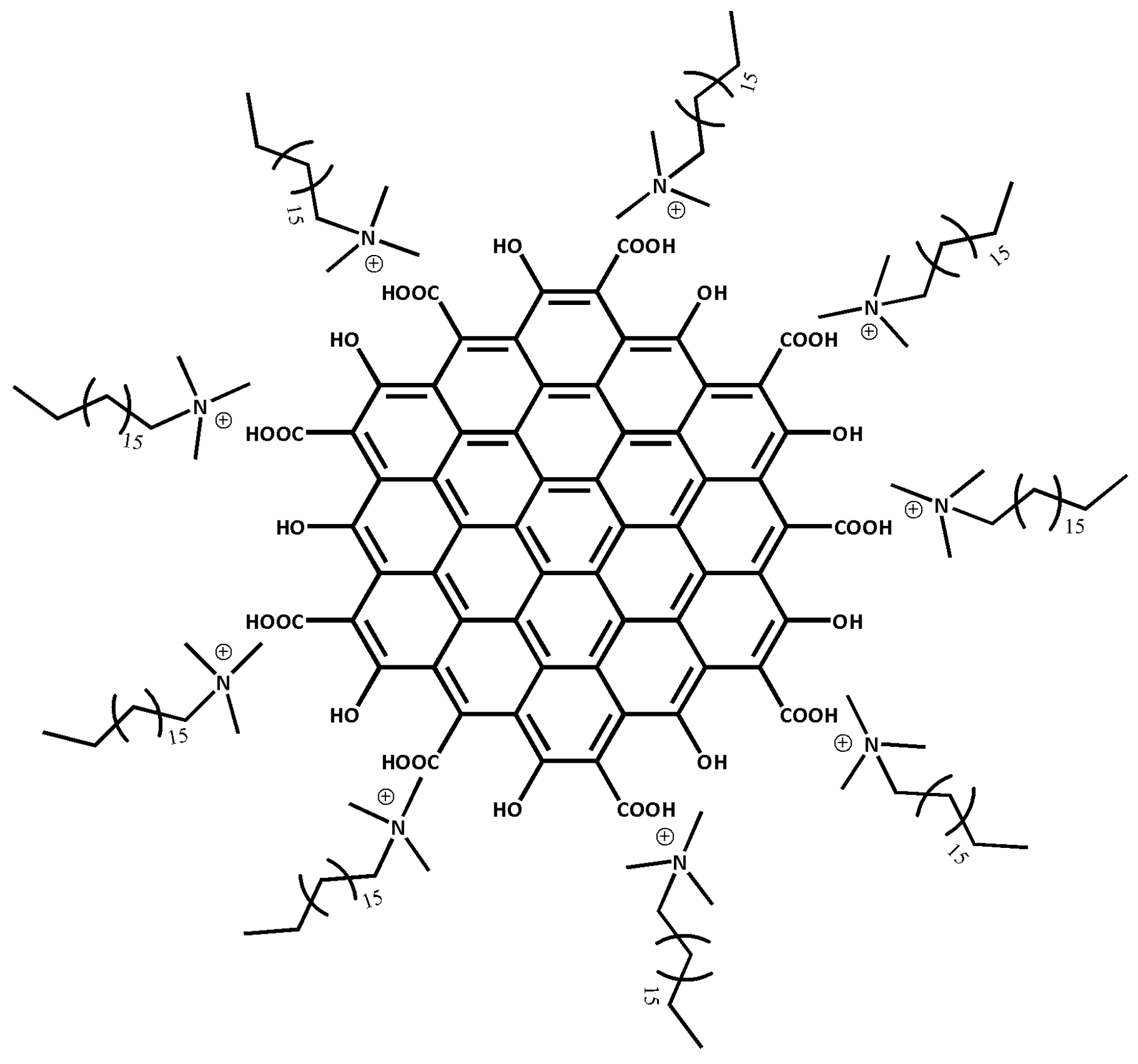
2.2. Preparation of Chemical
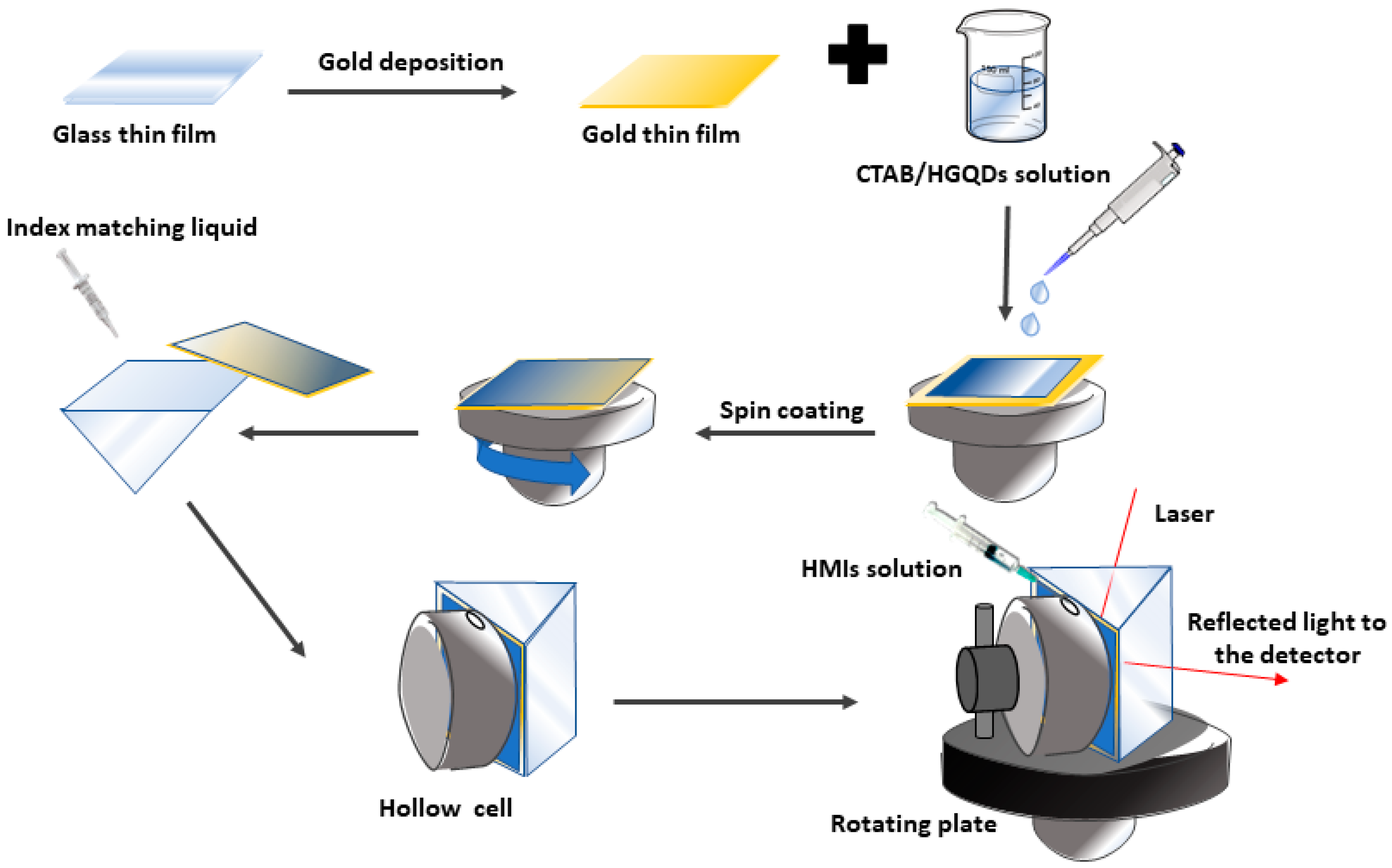
2.3. CTAB/HGQDs Thin Film Preparation
2.4. Structural and Optical Instrumentations
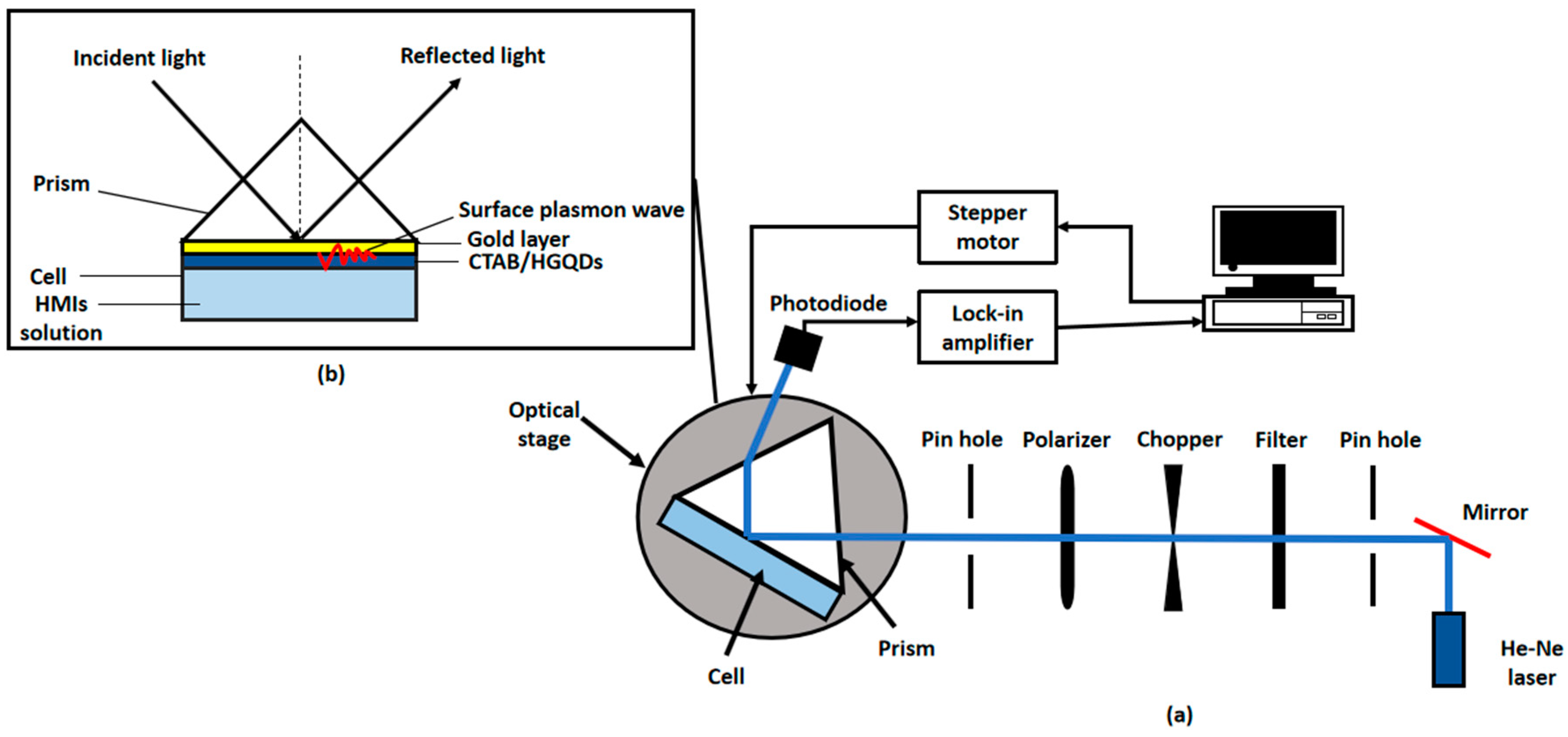
2.5. Surface Plasmon Resonance (SPR) Technique
3. Results and Discussion
3.1. FTIR Analysis
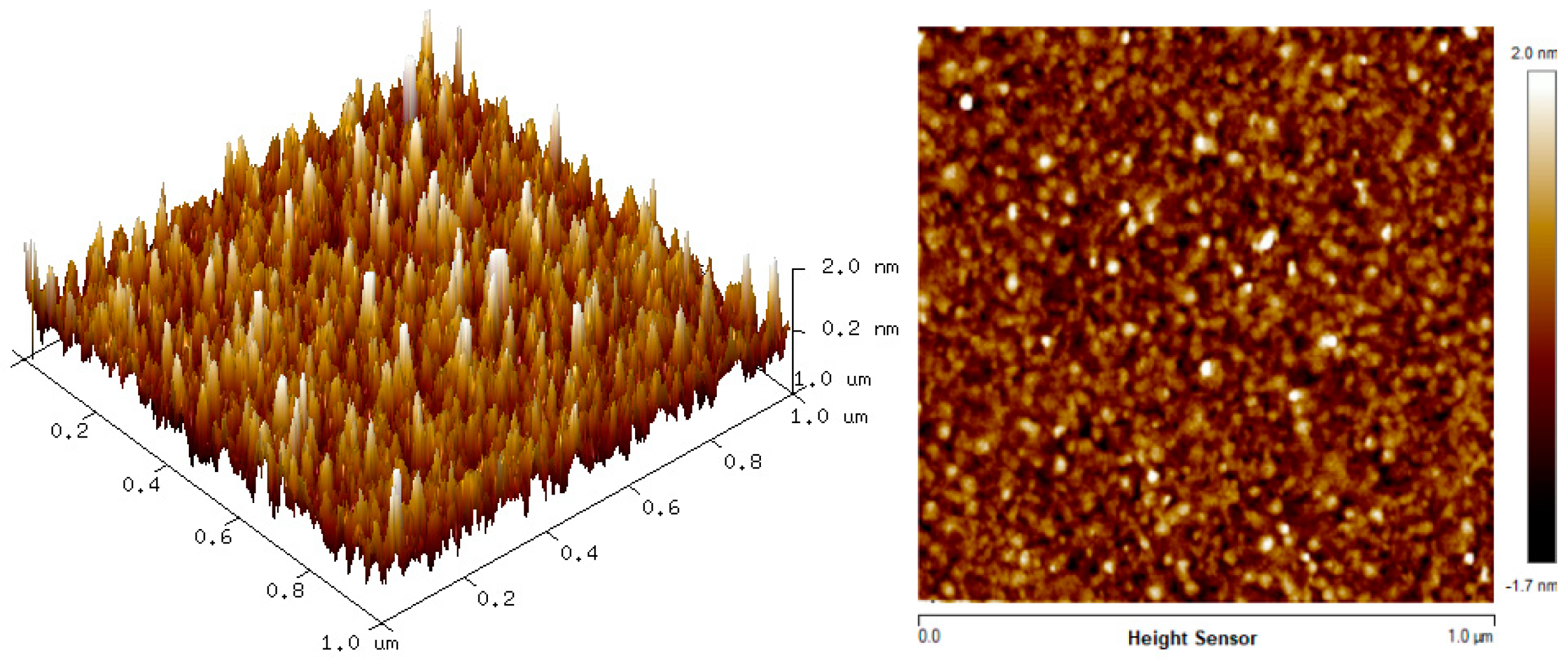
3.2. Surface Morphology
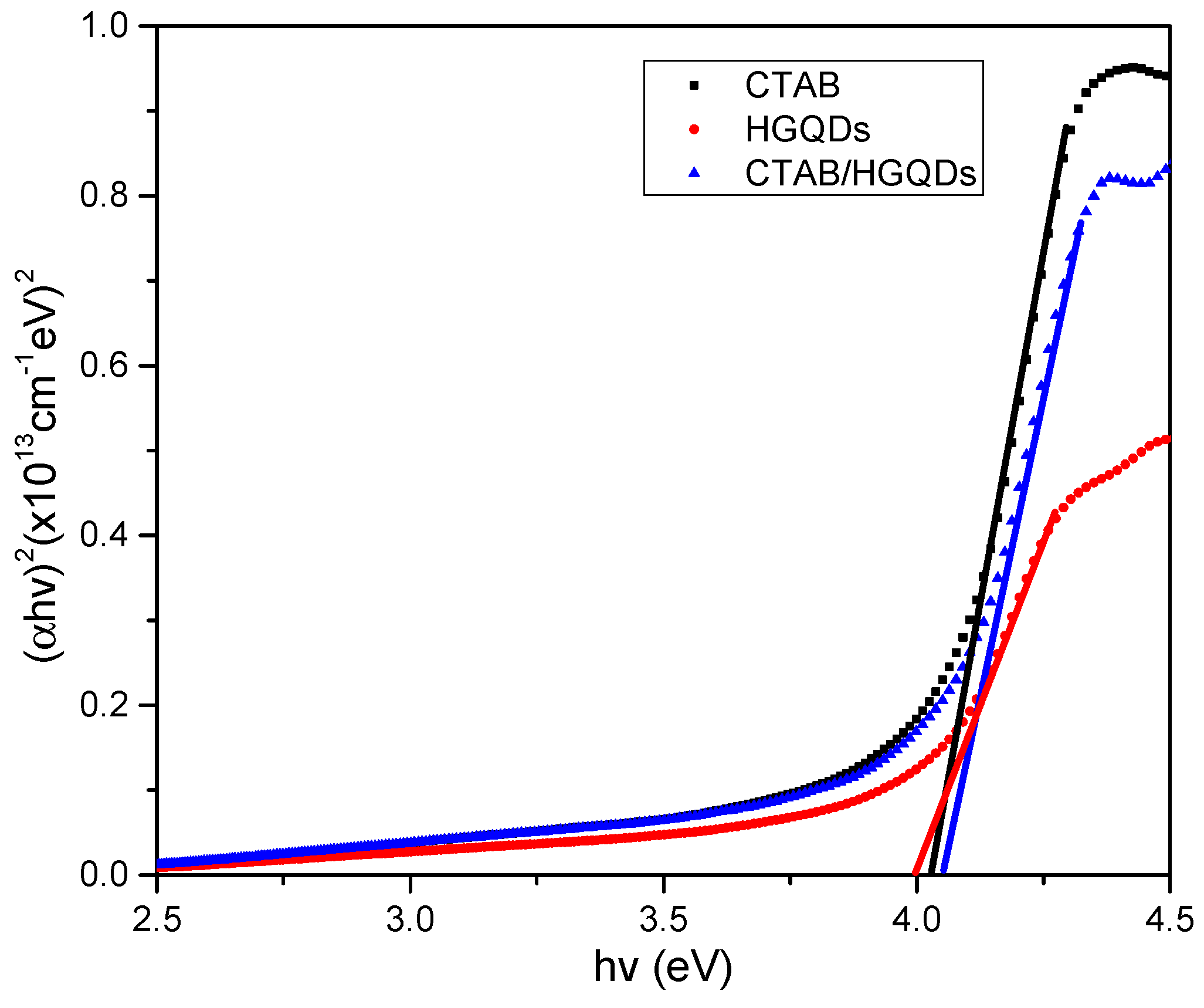
3.3. UV-Vis Absorption
3.4. Photoluminescence Emission
3.5. SPR Test of CTAB/HGQDs Thin Film
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saleviter, S.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Abdullah, J.; Zaid, M.H.M. Structural and optical studies of cadmium sulfide quantum dot-graphene oxide-chitosan nanocomposite thin film as a novel SPR spectroscopy active layer. J. Nanomater. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Biju, V.; Itoh, T.; Anas, A.; Sujith, A.; Ishikawa, M.; Abdulaziz, A. Semiconductor quantum dots and metal nanoparticles: Syntheses, optical properties, and biological applications. Anal. Bioanal. Chem. 2008, 391, 2469–2495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Coleman, J.J. Semiconductor quantum dots. Curr. Opin. Solid State Mater. Sci. 2016, 20, 352–360. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and graphene quantum dots for optoelectronic and energy devices: A review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum dots in bioanalysis: A review of applications across various platforms for fluorescence spectroscopy and imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef] [Green Version]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 178, 802–812. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Wang, C.; Liu, K.; Zhu, R.; Qiang, H.; Lin, Y. Synthesis of nitrogen-doped and amino acid-functionalized graphene quantum dots from glycine, and their application to the fluorometric determination of ferric ion. Microchim. Acta 2014, 182, 763–770. [Google Scholar] [CrossRef]
- Sun, X.; Peng, Y.; Lin, Y.; Cai, L.; Li, F.; Liu, B. G-quadruplex formation enhancing energy transfer in self-assembled multilayers and fluorescence recognize for Pb2+ ions. Sens. Actuators B Chem. 2018, 255, 2121–2125. [Google Scholar] [CrossRef]
- Sukumaran, L. A study of graphene. Int. J. Educ. Manag. Eng. 2014, 4, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Li, J.; Guo, Q.; Nie, G. An ultrasensitive electrochemiluminescence assay for Hg2+ through graphene quantum dots and poly(5-formylindole) nanocomposite. Sens. Actuators B Chem. 2019, 282, 824–830. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Wu, Z.; Du, Z.; Tang, Z.; Wu, X. Graphene quantum dots with high yield and high quality synthesized from low cost precursor of aphanitic graphite. Nanomaterials 2020, 10, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.-F.; Dai, L. Recent advances in graphene quantum dots for fluorescence bioimaging from cells through tissues to animals. Part. Part. Syst. Charact. 2014, 32, 515–523. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.-W.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.Y.; Jiang, H.X.; Li, X.; Teng, K.S.; Luk, C.M.; Zeng, S.; Hao, J.; et al. Deep ultraviolet photoluminescence of water-soluble self-passivated graphene quantum dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef]
- Pan, D.; Guo, L.; Zhang, J.; Xi, C.; Xue, Q.; Huang, H.; Li, J.; Zhang, Z.; Yu, W.; Chen, Z.; et al. Cutting sp2 clusters in graphene sheets into colloidal graphene quantum dots with strong green fluorescence. J. Mater. Chem. 2012, 22, 3314–3318. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene quantum dots derived from carbon fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Zhao, Y.; Shi, G.; Deng, L.; Hou, Y.; Qu, L. An electrochemical avenue to green-luminescent graphene quantum dots as potential electron-acceptors for photovoltaics. Adv. Mater. 2010, 23, 776–780. [Google Scholar] [CrossRef]
- Ma, H.; Wu, D.; Cui, Z.; Li, Y.; Zhanga, Y.; Du, B.; Wei, Q. Graphene-based optical and electrochemical biosensors: A review. Anal. Lett. 2013, 46, 1–17. [Google Scholar] [CrossRef]
- Fan, T.; Zeng, W.; Tang, W.; Yuan, C.; Tong, S.; Cai, K.; Liu, Y.; Huang, W.; Min, Y.; Epstein, A. Controllable size-selective method to prepare graphene quantum dots from graphene oxide. Nanoscale Res. Lett. 2015, 10, 55. [Google Scholar] [CrossRef] [Green Version]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M.; Saleviter, S. Development of graphene quantum dots-based optical sensor for toxic metal ion detection. Sensors 2019, 19, 3850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical properties of chitosan/hydroxyl-functionalized graphene quantum dots thin film for potential optical detection of ferric (III) ion. Opt. Laser Technol. 2019, 120, 105724. [Google Scholar] [CrossRef]
- Yang, T.; Cai, F.; Zhang, X.; Huang, Y. Nitrogen and sulfur codoped graphene quantum dots as a new fluorescent probe for Au3+ ions in aqueous media. RSC Adv. 2015, 5, 107340–107347. [Google Scholar] [CrossRef]
- Xia, C.; Hai, X.; Chen, X.-W.; Wang, J. Simultaneously fabrication of free and solidified N, S-doped graphene quantum dots via a facile solvent-free synthesis route for fluorescent detection. Talanta 2017, 168, 269–278. [Google Scholar] [CrossRef]
- Geethalakshmi, K.R.; Otero, R.C.; Ng, T.Y. Tunable optical properties of OH-functionalised graphene quantum dots. J. Mater. Chem. C 2016, 4, 8429–8438. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Wu, H.; Shang, P.; Zeng, X.; Chi, Y. Immobilizing water-soluble graphene quantum dots with gold nanoparticles for low potential electrochemiluminescence immunosensor. Nanoscale 2015, 7, 16366–16371. [Google Scholar] [CrossRef]
- Gao, X.X.; Zhou, X.; Ma, Y.F.; Wang, C.P.; Chu, F.X. A fluorometric and colorimetric dual-mode sensor based on nitrogen and iron co-doped graphene quantum dots for detection of ferric ions in biological fluids and cellular imaging. New J. Chem. 2018, 42, 14751–14756. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Hou, X.; Sun, Z.; Jiang, Y.; Dong, Z.; Tao, Q.; Man, J.; Cao, Y. Coal-derived nitrogen, phosphorus and sulfur co-doped graphene quantum dots: A promising ion fluorescent probe. Appl. Surf. Sci. 2018, 445, 519–526. [Google Scholar] [CrossRef]
- Lundstedt, A.; Papadakis, R.; Li, H.; Han, Y.; Jorner, K.; Bergman, J.; Leifer, K.; Grennberg, H.; Ottosson, H. White-light photoassisted covalent functionalization of graphene using 2-propanol. Small Methods 2017, 1, 1700214. [Google Scholar] [CrossRef]
- Bi, N.; Chen, Y.; Qi, H.; Zheng, X.; Chen, Y.; Liao, X.; Zhang, H.; Tian, Y. A sensitive localized surface plasmon resonance sensor for determining mercury(II) ion using noble metal nanoparticles as probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 95, 276–281. [Google Scholar] [CrossRef]
- Liu, X.; Li, N.; Xu, M.; Jiang, C.; Wang, J.; Song, G.; Wang, Y. Dual sensing performance of 1,2-squaraine for the colorimetric detection of Fe3+ and Hg2+ ions. Materials 2018, 11, 1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Fan, Q.; Li, J.; Yu, L.; Qu, L.-B. A novel and green CTAB-functionalized graphene nanosheets electrochemical sensor for Sudan I determination. Sens. Actuators B Chem. 2014, 203, 759–765. [Google Scholar] [CrossRef]
- Selvi, S.S.T.; Linet, J.M.; Sagadevan, S. Influence of CTAB surfactant on structural and optical properties of CuS and CdS nanoparticles by hydrothermal route. J. Exp. Nanosci. 2018, 13, 130–143. [Google Scholar] [CrossRef] [Green Version]
- Yao, P.J.; Wang, J.; Du, H.Y.; Zhao, L.; Yan, W.P. Effect of CTAB concentration on synthesis and sensing properties of perovskite oxide via a hydrothermal process. Key Eng. Mater. 2013, 543, 330–333. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, F.; Tong, M.; Hou, Y. Removal of arsenate by cetyltrimethylammonium bromide modified magnetic nanoparticles. J. Hazard. Mater. 2012, 227, 461–468. [Google Scholar] [CrossRef]
- Leng, Y.; Qian, S.; Wang, Y.; Lu, C.; Ji, X.; Lu, Z.; Lin, H. Single-indicator-based multidimensional sensing: detection and identification of heavy metal ions and understanding the foundations from experiment to simulation. Sci. Rep. 2016, 6, 25354. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Gou, H.; Al-Ogaidi, I.; Wu, N. Nanostructured sensors for detection of heavy metals: A review. ACS Sustain. Chem. Eng. 2013, 1, 713–723. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Chik, C.E.N.C.E.; Mahdi, M.A. Development of an optical sensor based on surface plasmon resonance phenomenon for diagnosis of dengue virus E-protein. Sens. Bio-Sens. Res. 2018, 20, 16–21. [Google Scholar] [CrossRef]
- Bottari, G.; Herranz, M.Á.; Wibmer, L.; Volland, M.; Rodríguez-Pérez, L.; Guldi, D.M.; Hirsch, A.; Martin, N.; D’Souza, F.; Torres, T. Chemical functionalization and characterization of graphene-based materials. Chem. Soc. Rev. 2017, 46, 4464–4500. [Google Scholar] [CrossRef] [Green Version]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.; Hobza, P.; Zboril, R.; Kim, H.R. Functionalization of graphene: covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Assali, M.; Mousa, A.; Thiab, S.; Al-Ott, M.; Taha, A.A.; Faroun, M.; Gomez, P.R. Non-covalent functionalization of graphene sheets with surfactants and their antibacterial activity. Palest. Med. Pharm. J. 2016, 1, 65–72. [Google Scholar]
- Haar, S. Supramolecular Approaches to Graphene: Generation of Functional Hybrid Assemblies; Université de Strasbourg: Strasbourg, France, 2015. [Google Scholar]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Zaid, M.H.M.; Daniyal, W.M.E.M.M.; Mahdi, M.A. Sensitive surface plasmon resonance performance of cadmium sulfide quantum dots-amine functionalized graphene oxide based thin film towards dengue virus E-protein. Opt. Laser Technol. 2019, 114, 204–208. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Anas, N.A.A.; Ramdzan, N.S.M.; Mahdi, M.A. Optical and structural properties of cadmium sulphide quantum dots based thin films as potential sensing material for dengue virus E-protein. Results Phys. 2018, 11, 734–739. [Google Scholar] [CrossRef]
- Homola, J. On the sensitivity of surface plasmon resonance sensors with spectral interrogation. Sens. Actuators B Chem. 1997, 41, 207–211. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Yang, R.; Qu, L.; Harrington, P.B. A sensitive electrochemical chlorophenols sensor based on nanocomposite of ZnSe quantum dots and cetyltrimethylammonium bromide. Anal. Chim. Acta 2013, 804, 76–83. [Google Scholar] [CrossRef]
- Fen, Y.W.; Mahmood, W.; Yunus, M.; Yusof, N.A. Detection of mercury and copper ions using surface plasmon resonance optical sensor. Sens. Mater. 2011, 23, 325. [Google Scholar]
- Fen, Y.W.; Yunus, W.M.M. Surface plasmon resonance spectroscopy as an alternative for sensing heavy metal ions: A review. Sens. Rev. 2013, 33, 305–314. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Abdullah, J.; Sadrolhosseini, A.R.; Omar, N.A.S. Design and analysis of surface plasmon resonance optical sensor for determining cobalt ion based on chitosan-graphene oxide decorated quantum dots-modified gold active layer. Opt. Express 2019, 27, 32294–32307. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Al-Rekabi, S.H.; Mahdi, M.A.; Omar, N.A.S. Incorporation of surface plasmon resonance with novel valinomycin doped chitosan-graphene oxide thin film for sensing potassium ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 111–115. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Ramdzan, N.S.M.; Nakajima, H.; Mahdi, M.A. Enhancing the sensitivity of a surface plasmon resonance-based optical sensor for zinc ion detection by the modification of a gold thin film. RSC Adv. 2019, 9, 41729–41736. [Google Scholar] [CrossRef] [Green Version]
- Elfeky, S.; Mahmoud, S.E.; Youssef, A.F. Applications of CTAB modified magnetic nanoparticles for removal of chromium (VI) from contaminated water. J. Adv. Res. 2017, 8, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xie, J.; Jiang, D.; Wei, X.; Chen, M. Modifiers-assisted formation of nickel nanoparticles and their catalytic application to p-nitrophenol reduction. CrystEngComm 2013, 15, 560–569. [Google Scholar] [CrossRef]
- Guivar, J.A.R.; Sanches, E.A.; Magon, C.J.; Fernandes, E.G.R. Preparation and characterization of cetyltrimethylammonium bromide (CTAB)-stabilized Fe3O4 nanoparticles for electrochemistry detection of citric acid. J. Electroanal. Chem. 2015, 755, 158–166. [Google Scholar] [CrossRef]
- Su, G.; Yang, C.; Zhu, J.-J. Fabrication of gold nanorods with tunable longitudinal surface plasmon resonance peaks by reductive dopamine. Langmuir 2015, 31, 817–823. [Google Scholar] [CrossRef]
- Jin, S.H.; Ali, Y.; Jun, G.H.; Hong, S.H.; Jeon, S. Tuning the photoluminescence of graphene quantum dots through the charge transfer effect of functional groups. ACS Nano 2013, 7, 1239–1245. [Google Scholar] [CrossRef]
- Du, F.-P.; Cao, N.-N.; Zhang, Y.-F.; Fu, P.; Wu, Y.-G.; Lin, Z.-D.; Shi, R.; Amini, A.; Cheng, C. PEDOT:PSS/graphene quantum dots films with enhanced thermoelectric properties via strong interfacial interaction and phase separation. Sci. Rep. 2018, 8, 6441. [Google Scholar] [CrossRef] [Green Version]
- Kausar, A. Technical imprint of polymer nanocomposite comprising graphene quantum dot. Polym. Technol. Mater. 2019, 58, 597–617. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Maroofi, S.M.; Mazarji, M.; Nabi-Bidhendi, G. Preparation of modified reduced graphene oxide nanosheet with cationic surfactant and its dye adsorption ability from colored wastewater. J. Surfactants Deterg. 2017, 20, 1085–1093. [Google Scholar] [CrossRef]
- Zamiranvari, A.; Solati, E.; Dorranian, D. Effect of CTAB concentration on the properties of graphene nanosheet produced by laser ablation. Opt. Laser Technol. 2017, 97, 209–218. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H.; Wang, C.; Zhang, J.; Zhang, Z. Adsorption of hexavalent chromium from aqueous solutions by graphenemodified with cetyltrimethylammonium bromide. J. Colloid Interface Sci. 2013, 394, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cai, X.; Shen, F. Preparation and property of UV-curable polyurethane acrylate film filled with cationic surfactant treated graphene. Appl. Surf. Sci. 2016, 379, 433–439. [Google Scholar] [CrossRef]
- Azizi, S.; Belhaj, M.; Zargouni, S.; Dridi, C. Correlation between composition, morphology and optical properties of PVK:n-ZnO:CTAB thin films. Appl. Phys. A 2017, 123, 123. [Google Scholar] [CrossRef]
- Lai, S.K.; Luk, C.M.; Tang, L.; Teng, K.S.; Lau, S.P. Photoresponse of polyaniline-functionalized graphene quantum dots. Nanoscale 2015, 7, 5338–5343. [Google Scholar] [CrossRef] [Green Version]
- Qian, F.; Li, X.; Tang, L.; Lai, S.K.; Lu, C.; Lau, S.P. Potassium doping: Tuning the optical properties of graphene quantum dots. AIP Adv. 2016, 6, 075116. [Google Scholar] [CrossRef] [Green Version]
- Abdulla, H.S.; Abbo, A.I. Optical and electrical properties of thin films of polyaniline and polypyrrole. Int. J. Electrochem. Sci. 2012, 7, 10666–10678. [Google Scholar]
- Ganesan, K.P.; Anandhan, N.; Dharuman, V.; Sami, P.; Pannerselvam, R.; Marimuthu, T. Electrochemically modified crystal orientation, surface morphology and optical properties using CTAB on Cu2O thin films. Results Phys. 2017, 7, 82–86. [Google Scholar] [CrossRef]
- Kolomenskii, A.A.; Gershon, P.D.; Schuessler, H.A. Sensitivity and detection limit of concentration surface-plasmon resonance. Appl. Opt. 1997, 36, 6539–6547. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Label-free optical spectroscopy for characterizing binding properties of highly sensitive nanocrystalline cellulose-graphene oxide based nanocomposite towards nickel ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 212, 25–31. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Characterization of the optical properties of heavy metal ions using surface plasmon resonance technique. Opt. Photonics J. 2011, 1, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Kamil, Y.M.; Daniyal, W.M.E.M.M.; Abdullah, J.; Mahdi, M.A. Experimental evaluation on surface plasmon resonance sensor performance based on sensitive hyperbranched polymer nanocomposite thin films. Sens. Actuators A Phys. 2020, 303, 111830. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of Pb2+ based on immobilized p-tert-butylcalix[4]arene-tetrakis in chitosan thin film as an active layer. Sens. Actuators B Chem. 2012, 171, 287–293. [Google Scholar] [CrossRef]
- Wu, C.-M.; Lin, L.-Y. Immobilization of metallothionein as a sensitive biosensor chip for the detection of metal ions by surface plasmon resonance. Biosens. Bioelectron. 2004, 20, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Sadrolhosseini, A.R.; Noor, A.; Moksin, M.M.; Abdi, M.M.; Mohammadi, A. Application of polypyrrole-chitosan layer for detection of Zn (II) and Ni (II) in aqueous solutions using surface plasmon resonance. Int. J. Polym. Mater. 2013, 62, 284–287. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.; Noor, A.; Bahrami, A.; Lim, H.N.; Talib, Z.A.; Mahdi, M.A. Application of polypyrrole multi-walled carbon nanotube composite layer for detection of mercury, lead and iron ions using surface plasmon resonance technique. PLoS ONE 2014, 9, e93962. [Google Scholar] [CrossRef] [Green Version]
- Cennamo, N.; Alberti, G.; Pesavento, M.; D’Agostino, G.; Quattrini, F.; Biesuz, R.; Zeni, L. A simple small size and low cost sensor based on surface plasmon resonance for selective detection of Fe(III). Sensors 2014, 14, 4657–4671. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Noor, A.; Moksin, M.M.; Abdi, M.M.; Soleimani, H.; Abas, A.F.; Talib, Z.A. Polypyrrole thin film sensor base surface plasmon resonance for detection of Cu(II) and Fe(III) in aqueous solution. In AIP Conference Proceedings; University Park, MD, USA, 2012; Volume 1482, pp. 200–204. [Google Scholar]
- Ganesharajah, M.; Koneswaran, M. Citrate capped silver nanoparticles as optical sensor for ferric ions. Int. J. Nanotechnol. Med. Eng. 2018, 3, 63–70. [Google Scholar]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Exploration of surface plasmon resonance for sensing copper ion based on nanocrystalline cellulose-modified thin film. Opt. Express 2018, 26, 34880–34893. [Google Scholar] [CrossRef]








| Materials | HMIs | Detection Limit | References |
|---|---|---|---|
| metallothionein (MT) | Zn2+, Ni2+, Cd2+ | ~2 µM | [74] |
| polypyrrole-chitosan | Zn2+, Ni2+ | Zn2+: 15 μM Ni2+: 17 μM | [75] |
| polypyrrole-multiwalled carbon nanotube | Fe2+ | 1.79 μM | [76] |
| deferoxamine self-assembled monolayer | Fe3+ | 2.1 µM | [77] |
| polypyrrole | Cu2+, Fe3+ | ~1.8 µM | [78] |
| citrate-capped silver nanoparticles as | Fe3+ | 13 mM | [79] |
| CTAB/HGQDs | Fe3+, Zn2+, Ni2+ | ~1.8 µM | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anas, N.A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M. Investigating the Properties of Cetyltrimethylammonium Bromide/Hydroxylated Graphene Quantum Dots Thin Film for Potential Optical Detection of Heavy Metal Ions. Materials 2020, 13, 2591. https://doi.org/10.3390/ma13112591
Anas NAA, Fen YW, Yusof NA, Omar NAS, Ramdzan NSM, Daniyal WMEMM. Investigating the Properties of Cetyltrimethylammonium Bromide/Hydroxylated Graphene Quantum Dots Thin Film for Potential Optical Detection of Heavy Metal Ions. Materials. 2020; 13(11):2591. https://doi.org/10.3390/ma13112591
Chicago/Turabian StyleAnas, Nur Ain Asyiqin, Yap Wing Fen, Nor Azah Yusof, Nur Alia Sheh Omar, Nur Syahira Md Ramdzan, and Wan Mohd Ebtisyam Mustaqim Mohd Daniyal. 2020. "Investigating the Properties of Cetyltrimethylammonium Bromide/Hydroxylated Graphene Quantum Dots Thin Film for Potential Optical Detection of Heavy Metal Ions" Materials 13, no. 11: 2591. https://doi.org/10.3390/ma13112591





