Novel Polymeric Micelles-Coated Magnetic Nanoparticles for In Vivo Bioimaging of Liver: Toxicological Profile and Contrast Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Animals
2.3. Experimental Design
2.4. Biochemical Analysis
2.5. Histology
2.6. Nuclear Magnetic Relaxation Dispersion (NMRD) Profile and Relaxivity Measurements
2.7. Biodistribution of MNP-DSPE-PEG in Mice Liver Evaluated by Relaxometry
2.8. Magnetic Resonance Imaging (MRI) Analysis of Nanoparticle Distribution in the Liver Tissue
2.9. Preparation of Liver Tissue Total Extract
2.10. Measurement of Enzymatic Activities
2.11. Quantification of Reduced Glutathione (GSH) Concentration
2.12. Lipid Peroxidation Assay
2.13. Measurement of Oxidative Modifications of Proteins
2.14. Statistical Analyses
3. Results
3.1. Variation of AST, ALT and GGT Enzymatic Activities in Mice Blood Serum
3.2. Iron Biodistribution in Liver Tissue
3.2.1. Histochemistry Studies
3.2.2. Relaxometric Studies
3.3. Efficacy of MNP-DSPE-PEG as Contrast Agents
3.4. Variation of Enzymatic Activities in Mouse Liver Tissue
3.5. Variation of Intracellular GSH Content
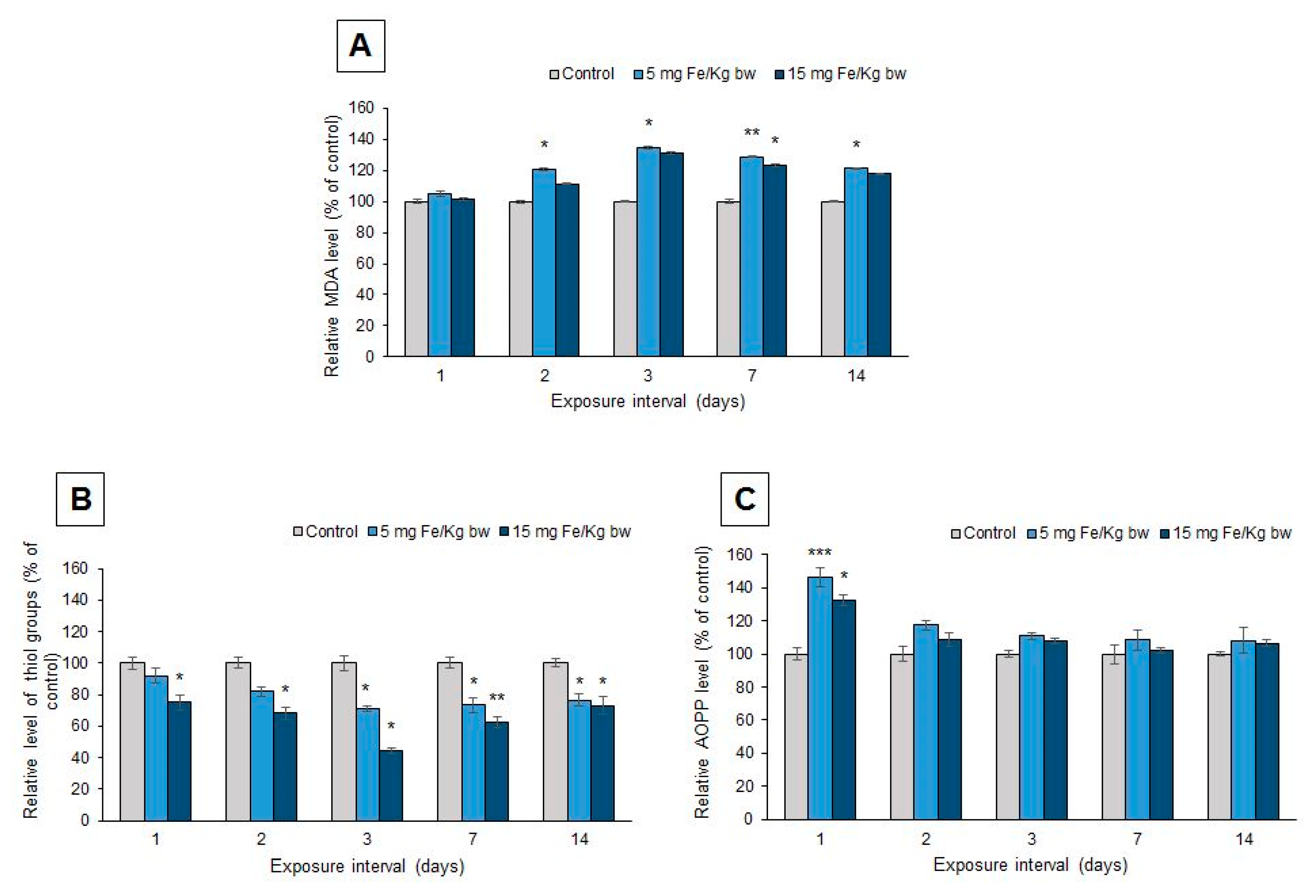
3.6. Oxidative Modifications
4. Discussions
Author Contributions
Conflicts of Interest
References
- Madamsetty, V.S.; Mukherjee, A.; Mukherjee, S. Recent Trends of the Bio-Inspired Nanoparticles in Cancer Theranostic. Front. Pharmacol. 2019, 10, 1264. [Google Scholar] [CrossRef]
- El Sayed, M. Clinical Applications of Magnetic Nanoparticles; Thanh, N., Ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Blyakhman, F.A.; Buznikov, N.A.; Sklyar, T.F.; Safronov, A.P.; Golubeva, E.V.; Svalov, A.V.; Sokolov, S.Y.; Melnikov, G.Y.; Orue, I.; Kurlyandskaya, G.V. Mechanical, Electrical and Magnetic Properties of Ferrogels with Embedded Iron Oxide Nanoparticles Obtained by Laser Target Evaporation: Focus on Multifunctional Biosensor Applications. Sensors 2018, 18, 872. [Google Scholar] [CrossRef]
- Van de Walle, A.; Perez, J.E.; Abou-Hassan, A.; Hemadi, M.; Luciani, N.; Wilhelm, C. Magnetic nanoparticles in regenerative medicine: What of their fate and impact in stem cells? Mater. Today Nano 2020, 11, 100084. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knuchel, R.; Kiessling, F.; Lammers, T. Iron Oxide Nanoparticles: Diagnostic, Therapeutic and Theranostic Applications. Adv. Drug. Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Kucheryavy, P.; He, J.; John, V.T.; Maharjan, P.; Spinu, L.; Goloverda, G.Z.; Kolesnichenko, V.L. Superparamagnetic Iron Oxide Nanoparticles with Variable Size and an Iron Oxidation State as Prospective Imaging Agents. Langmuir 2013, 29, 710–716. [Google Scholar] [CrossRef]
- Grossman, J.H.; McNeil, S.E. Nanotechnology in cancer medicine. Phys. Today 2012, 65, 38–42. [Google Scholar] [CrossRef]
- Mülhopt, S.; Diabaté, S.; Dilger, M.; Adelhelm, C.; Anderlohr, C.; Bergfeldt, T.; Gómez de la Torre, J.; Jiang, Y.; Valsami-Jones, E.; Langevin, D.; et al. Characterization of Nanoparticle Batch-To-Batch Variability. Nanomaterials 2018, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Z.; Li, X.; Zhang, Y.; Yin, M.; Li, J.; Song, H.; Shi, J.; Ling, D.; Wang, L.; et al. Deciphering active biocompatibility of iron oxide nanoparticles from their intrinsic antagonism. Nano. Res. 2018, 11, 2746–2755. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef]
- Peng, X.; Chen, H.; Huang, J.; Mao, H.; Shin, D. Targeted magnetic iron oxide nanoparticles for tumor imaging and therapy. In Biomedical Engineering—From Theory to Applications; IntechOpen: Rijeka, Croatia, 2011; pp. 203–224. [Google Scholar]
- Kekutia, S.H.; Saneblidze, L.; Mikelashvili, V.; Markhulia, J.; Tatarashvili, R.; Daraselia, D.; Japaridze, D. A New Method For The Synthesis Of Nanoparticles for Biomedical Applications. Eur. Chem. Bull. 2015, 4, 33–36. [Google Scholar]
- Medeiros, S.F.; Filizzola, J.O.C.; Fonseca, V.F.M.; Oliveira, P.F.M.; Silva, T.M.; Elaissari, A.; Santos, A.M. Synthesis and characterization of stable aqueous dispersion of functionalized double-coated iron oxide nanoparticles. Mater. Lett. 2015, 160, 522–525. [Google Scholar] [CrossRef]
- Huang, J.; Zhong, X.; Wang, L.; Yang, L.; Mao, H. Improving the Magnetic Resonance Imaging Contrast and Detection Methods with Engineered Magnetic Nanoparticles. Theranostics 2012, 2, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Hwang, H.M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Zhang, B.; Wang, L.; Wang, J.; Li, X.; Yang, G.; Gao, F. Superparamagnetic iron oxide nanoparticles coated with different polymers and their MRI contrast effects in the mouse brains. Appl. Surf. Sci. 2015, 326, 32–38. [Google Scholar] [CrossRef]
- Zavisova, V.; Koneracka, M.; Kovac, J.; Kubovcikova, M.; Antal, I.; Kopcansky, P.; Bednarikova, M.; Muckova, M. The cytotoxicity of iron oxide nanoparticles with different modifications evaluated in vitro. J. Magn. Magn. Mater. 2015, 380, 85–89. [Google Scholar] [CrossRef]
- Pisciotti, M.L.; Lima, E., Jr.; Vasquez Mansilla, M.; Tognoli, V.E.; Troiani, H.E.; Pasa, A.A.; Creczynski-Pasa, T.B.; Silva, A.H.; Gurman, P.; Colombo, L.; et al. In vitro and In vivo experiments with iron oxide nanoparticles functionalized with dextran or polyethylene glycol for medical applications: Magnetic targeting. J. Biomed. Mater. Res. 2014, 102, 860–868. [Google Scholar] [CrossRef]
- Yousuf, S.; Enoch, I.V.M.V.; Paulraj, M.S.; Dhanaraj, P. Chromenoneconjugated magnetic iron oxide nanoparticles. Toward conveyable DNA binders. Colloids Surf. B 2015, 135, 448–457. [Google Scholar] [CrossRef]
- Scialabba, C.; Licciardi, M.; Mauro, N.; Rocco, F.; Ceruti, M.; Giammona, G. Inulin-based polymer coated SPIONs as potential drug delivery systems for targeted cancer therapy. Eur. J. Pharm. Biopharm. 2014, 88, 695–705. [Google Scholar] [CrossRef]
- Penon, O.; Marín, M.J.; Amabilino, D.B.; Russell, D.A.; Pérez-García, L. Iron oxide nanoparticles functionalized with novel hydrophobic and hydrophilic porphyrins as potential agents for photodynamic therapy. J. Colloid Interface Sci. 2016, 462, 154–165. [Google Scholar] [CrossRef]
- Chen, T.J.; Cheng, T.H.; Chen, C.Y.; Hsu, S.; Cheng, T.L.; Liu, G.C.; Wang, Y.M. Targeted Herceptin–dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. J. Biol. Inorg. Chem. 2009, 14, 253–260. [Google Scholar] [CrossRef]
- Do, M.A.; Yoon, G.J.; Yeum, J.H.; Han, M.; Chang, Y.; Choi, J.H. Polyethyleneimine-mediated synthesis of superparamagnetic iron oxide nanoparticles with enhanced sensitivity in T2 magnetic resonance imaging. Colloids Surf. B 2014, 122, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.T.; Halaney, D.L.; Sokolov, K.V.; Ma, L.L.; Shipley, H.J.; Mahajan, S.; Louden, C.L.; Asmis, R.; Milner, T.E.; Johnston, K.P.; et al. Excretion and toxicity of gold–iron nanoparticles. Nanomed. Nanotechnol. 2013, 9, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Yu-Shen, L.; Si-Han, W.; Hung, Y.; Yi-Hsin, C.; Chang, C.; Meng-Liang, L.; Chih- Pin, T.; Chung-Yuan, M. Multifunctional composite nanoparticles: Magnetic, luminescent, and mesoporous. Chem. Mater. 2006, 18, 5170–5172. [Google Scholar]
- Liu, Y.; Yang, K.; Cheng, L.; Zhu, J.; Ma, X.; Xu, H.; Li, Y.; Guo, L.; Gu, H.; Liu, Z. PEGylated Fe Pt@Fe2O3 core-shell magnetic nanoparticles. Potential theranostic applications and In vivo toxicity studies. Nanomedicine 2013, 9, 1077–1088. [Google Scholar] [CrossRef]
- Couvreur, P.; Reddy, L.H.; Mangenot, S.; Poupaert, J.H.; Lepetre-Mouelhi, S.; Pili, B.; Bourgaux, C.; Amenitsch, H.; Ollivon, M. Discovery of new hexagonal supramolecular nanostructures formed by squalenoylation of an anticancer nucleoside analog. Small 2008, 4, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Khemtong, C.; Kessinger, C.W.; Gao, J. Polymeric Nanomedicine for Cancer MR Imaging and Drug Delivery. Chem. Commun. (Camb.) 2009, 24, 3497–3510. [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Maity, D.; Yong, C.X.; Chuang, K.-H.; Ding, J.; Feng, S.-S. Vitamin E (d-alpha-tocopheryl-co-poly(ethylene glycol) 1000 succinate) micelles-superparamagnetic iron oxide nanoparticles for enhanced thermotherapy and MRI. Biomaterials 2011, 32, 5663–5672. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Liu, G.; Deng, Z.; Wu, S.; Li, P.; Xu, Z.; Xu, H.; Chu, P.K. Magnetite-loaded fluorine-containing polymeric micelles for magnetic resonance imaging and drug delivery. Biomaterials 2012, 33, 3013–3024. [Google Scholar] [CrossRef]
- Gao, G.H.; Lee, J.W.; Nguyen, M.K.; Im, G.H.; Yang, J.; Heo, H.; Jeon, P.; Parkn, T.G.; Lee, J.H.; Lee, D.S. pH-responsive polymeric micelle based on PEG-poly(β-amino ester)/(amido amine) as intelligent vehicle for magnetic resonance imaging in detection of cerebral ischemic area. J. Control. Release 2011, 155, 11–17. [Google Scholar] [CrossRef]
- Li, H.; Yan, K.; Shang, Y.; Shrestha, L.; Liao, R.; Liu, F.; Li, P.; Xu, H.; Xu, Z.; Chu, P.K. Folate-bovine serum albumin functionalized polymeric micelles loaded with superparamagnetic iron oxide nanoparticles for tumor targeting and magnetic resonance imaging. Acta Biomater. 2015, 15, 117–126. [Google Scholar] [CrossRef]
- Yokoyama, M. Clinical Applications of Polymeric Micelle Carrier Systems in Chemotherapy and Image Diagnosis of Solid Tumors. J. Exp. Clin. Med. 2011, 3, 151–158. [Google Scholar] [CrossRef]
- Qu, J.B.; Shao, H.H.; Jing, G.L.; Huang, F. PEG-chitosan-coated iron oxide nanoparticles with high saturated magnetization as carriers of 10-hydroxycamptothecin: Preparation, characterization and cytotoxicity studies. Colloids Surf. B 2013, 102, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Ebrahimi, P.; Rad, H.A.; Asgari, M. Optimization of preparation of chitosan-coated iron oxide nanoparticles for biomedical applications by chemometrics approaches. Int. Nano. Lett. 2013, 3, 48. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Savitha, S.; Wu, C.-J.; Lin, F.-H.; Stobiński, l. Carbon encapsulated iron oxide nanoparticles surface engineered with polyethylene glycolfolic acid to induce selective hyperthermia in folate over expressed cancer cells. Int. J. Pharm. 2015, 480, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hałupka-Bryl, M.; Bednarowicz, M.; Dobosz, B.; Krzyminiewski, R.; Zalewski, T.; Wereszczyńska, B.; Nowaczyk, G.; Jarek, M.; Nagasaki, Y. Doxorubicin loaded PEG-b-poly(4 vinylbenzylphosphonate) coated magnetic iron oxide nanoparticles for targeted drug delivery. J. Magn. Magn. Mater. 2015, 384, 320–327. [Google Scholar] [CrossRef]
- Yan, H.; Guo, M.; Liu, K. Multifunctional magnetic hybrid nanoparticles as a nanomedical platform for cancer targeted imaging and therapy. In Biomedical Science, Engineering and Technology; IntechOpen: Rijeka, Croatia, 2012; pp. 283–300. [Google Scholar]
- Predoi, D.; Andronescu, E.; Radu, M.; Munteanu, M.C.; Dinischiotu, A. Synthesis and characterization of biocompatible maghemite nanoparticles. Dig. J. Nanomater. Biostruct. 2010, 5, 779–786. [Google Scholar]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and polymer polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture. Int. J. Mol. Sci. 2012, 13, 5554–5570. [Google Scholar] [CrossRef]
- Varna, M.; Ratajczak, P.; Ferreira, I.; Leboeuf, C.; Bousquet, G.; Janin, A. In vivo Distribution of Inorganic Nanoparticles in Preclinical Models. J. Biomater. Nanobiotechnol. 2012, 3, 269–279. [Google Scholar] [CrossRef]
- Mandarano, G.; Lodhia, J.; Eu, P.; Ferris, N.J.; Davidson, R.; Cowell, S.F. Development and use of iron oxide nanoparticles (Part 2): The application of iron oxide contrast agents in MRI. Biomed. Imaging Interv. J. 2010, 6, e13. [Google Scholar] [CrossRef]
- Gu, L.; Fang, R.; Sailor, M.; Park, J.H. In vivo clearance and toxicity of monodisperse iron oxide nanocrystals. J. Am. Chem. Soc. 2012, 6, 4947–4954. [Google Scholar] [CrossRef]
- Harney, A.S.; Meade, T.J. Molecular imaging of in vivo gene expression. Future Med. Chem. 2010, 2, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Pujalté, I.; Passagne, I.; Brouillaud, B.; Tréguer, M.; Durand, E.; Ohayon-Courtes, C.; L’Azou, B. Cytotoxicity and oxidative stress induced by different metallic nanoparticles on human kidney cells. Part. Fibre Toxicol. 2011, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Hohnholt, M.C.; Geppert, M.; Dringen, R. Treatment with iron oxide nanoparticles induces ferritin synthesis but not oxidative stress in oligodendroglial cells. Acta Biomater. 2011, 7, 3946–3954. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, L.; Tanwir, F.; Babadi, V.Y. In vitro toxicity of iron oxide nanoparticle: Oxidative damages on Hep G2 cells. Exp. Toxicol. Pathol. 2015, 67, 197–203. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Wu, C.-H.; Aronstam, R.S. Toxicity of Transition Metal Oxide Nanoparticles: Recent Insights from in vitro Studies. Materials 2010, 3, 4842–4859. [Google Scholar] [CrossRef]
- Tournebize, J.; Sapin-Minet, A.; Bartosz, G.; Leroy, P.; Boudier, A. Pitfalls of assays devoted to evaluation of oxidative stress induced by inorganic nanoparticles. Talanta 2013, 116, 753–763. [Google Scholar] [CrossRef]
- Radu Balas, M.; Din Popescu, I.M.; Hermenean, A.; Cinteză, O.L.; Burlacu, R.; Ardelean, A.; Dinischiotu, A. Exposure to iron oxide nanoparticles coated with phospholipid-based polymeric micelles induce biochemical and histopathological pulmonary changes in mice. Int. J. Mol. Sci. 2015, 16, 29417–29435. [Google Scholar] [CrossRef]
- Cinteza, L.O.; Ohulchansky, T.Y.; Sahoo, Y.; Bergey, E.J.; Pandey, R.K.; Prasad, P.N. Diacyllipid micelle-based nanocarrier for magnetically guided delivery of drugs in photodynamic therapy. Mol. Pharm. 2006, 3, 415–423. [Google Scholar] [CrossRef]
- Burtea, C.; Laurent, S.; Vander Elst, L.; Muller, R.N. Contrast Agents—Magnetic Resonance; Handb Exp Pharmacol, (185 Pt 1); Semmler, W., Schwaiger, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 135–165. [Google Scholar]
- Andre, S.; Ansciauxa, E.; Saidi, E.; Larbanoix, L.; Stanicki, D.; Nonclercq, D.; Vander Elst, L.; Laurent, S.; Muller, R.N.; Burtea, C. Validation by Magnetic Resonance Imaging of the Diagnostic Potential of a Heptapeptide-Functionalized Imaging Probe Targeted to Amyloid-β and Able to Cross the Blood-Brain Barrier. J. Alzheimers Dis. 2017, 60, 1547–1565. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Beutler, E. Red Cell Metabolism. In A Manual of Biochemical Methods, 3rd ed.; Grune and Stratton: Orlando, FL, USA, 1984; pp. 68–73. [Google Scholar]
- Del Rio, D.; Pellegrini, N.; Colombi, B.; Bianchi, M.; Serafini, M.; Torta, F.; Tegoni, M.; Musci, M.; Brighenti, F. Rapid fluorimetric method to detect total plasma malondialdehyde with mild derivatization conditions. Clin. Chem. 2003, 49, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Riener, C.; Kada, G.; Gruber, H.J. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4,4′-dithiodipyridine. Anal. Bioanal. Chem. 2002, 373, 266–276. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Nguyen, A.T.; Descamp, S.; Latsha, B. Microtitre plate assay for phagocyte derived taurine chloroaminea. J. Clin. Lab. Anal. 1992, 6, 47–53. [Google Scholar] [CrossRef]
- Popescu, I.M.; Cinteza, L.O.; Hermenean, A.; Dinischiotu, A. In vivo exposure of mice spleen to magnetite nanoparticles encapsulated in phospholipid polymeric micelles; an oxidative stress and structural approach. Dig. J. Nanomater. Biostruct. 2015, 10, 871–881. [Google Scholar]
- Lei, L.; Ling-Ling, J.; Yun, Z.; Gang, L. Toxicity of superparamagnetic iron oxide nanoparticles: Research strategies and implications for nanomedicine. Chin. Phys. B 2013, 22, 127503. [Google Scholar] [CrossRef]
- Li, J.; Chang, X.; Chen, X.; Gu, Z.; Zhao, F.; Chai, Z.; Zhao, Y. Toxicity of inorganic nanomaterials in biomedical imaging. Biotechnol. Adv. 2014, 32, 727–743. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Siontas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative paradigm. Nano. Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Chaves, N.L.; Estrella-Lopis, I.; Bottner, J.; Lopes, C.A.P.; Guido, B.C.; de Sousa, A.R.; Bao, S.N. Exploring cellular uptake of iron oxide nanoparticles associated with rhodium citrate in breast cancer cells. Int. J. Nanomed. 2017, 12, 5511–5523. [Google Scholar] [CrossRef]
- Kostevsek, N. A Review on the Optimal Design of Magnetic Nanoparticle-Based T2 MRI contrast Agents. Magnetochemistry 2020, 6, 11. [Google Scholar] [CrossRef]
- Kandasamy, G.; Maity, D. Recent advances in supermagnetic iron oxide nanoparticles (SPIONs) for in vitro and in vivo cancer nanotheranostics. Int. J. Pharm. 2015, 496, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Laurent, S.; Jo, Y.; Roch, A.; Mikhaylova, M.; Bhujwalla, Z.; Muller, R.; Muhammed, M. A High-Performance Magnetic Resonance Imaging T2 Contrast Agent. Adv. Mater. 2007, 19, 1874–1878. [Google Scholar] [CrossRef]
- Jung, H.; Park, B.; Lee, C.; Cho, J.; Suh, J.; Park, J.; Kim, Y.; Kim, J.; Cho, G.; Cho, H. Dual MRI T1 and T2(*) contrast with size-controlled iron oxide nanoparticles. Nanomedicine 2014, 10, 1679–1689. [Google Scholar] [CrossRef]
- Pourcelle, V.; Laurent, S.; Welle, A.; Vriamont, N.; Stanicki, D.; Vander Elst, L.; Muller, R.N.; Marchand-Brynaert, J. Functionalization of the PEG corona of nanoparticles by clip photochemistry in water: Application to the grafting of RGD ligands on PEGylated USPIO imaging agent. Bioconjug. Chem. 2015, 26, 822–829. [Google Scholar] [CrossRef]
- Arsalani, N.; Fattahi, H.; Laurent, S.; Burtea, C.; Vander Elst, L.; Muller, R.N. Polyglycerol-grafted superparamagnetic iron oxide nanoparticles: Highly efficient MRI contrast agent for liver and kidney imaging and potential scaffold for cellular and molecular imaging. Contrast Media Mol. Imaging 2012, 7, 185–194. [Google Scholar] [CrossRef]
- Zhu, H.; Tao, J.; Wang, W.; Zhou, Y.; Li, P.; Li, Z.; Yan, K.; Wu, S.; Yeung, K.W.K.; Xu, Z.; et al. Magnetic, fluorescent, and thermo-responsive Fe3O4/rare earth incorporated poly(St-NIPAM) core–shell colloidal nanoparticles in multimodal optical/magnetic resonance imaging probes. Biomaterials 2013, 34, 2296–2306. [Google Scholar] [CrossRef]
- Yan, K.; Li, H.; Li, P.; Zhu, H.; Shen, J.; Yi, C.; Wu, S.; Yeung, K.W.K.; Xu, Z.; Xu, H.; et al. Self-assembled magnetic fluorescent polymeric micelles for magnetic resonance and optical imaging. Biomaterials 2014, 35, 344–355. [Google Scholar] [CrossRef]
- Ahmad, T.; Rhee, I.; Hong, S.; Chang, Y.; Lee, J. Silica-coated Iron-oxide Nanoparticles Synthesized as a T2 Contrast Agent for Magnetic Resonance Imaging by Using the Reverse Micelle Method. J. Korean Phys. Soc. 2010, 57, 1545–1549. [Google Scholar]
- Arbab, A.S.; Wilson, L.B.; Ashari, P.; Jordan, E.K.; Lewis, B.K.; Frank, J.A. A model of lysosomal metabolis of dextran coated superparamagnetic iron oxide (SPION) nanoparticles: Implications for cellular magnetic resonance imaging. NMR Biomed. 2005, 18, 383–389. [Google Scholar] [CrossRef]
- Chen, T.; Furst, A.; Chien, P.K. The Effects of Cadmium and Iron on Catalase Activities in Tubifex. J. Am. Coll. Toxicol. 1994, 13, 112–120. [Google Scholar] [CrossRef]
- Domellöf, M.; Dewey, K.G.; Cohen, R.J.; Lönnerdal, B.; Hermell, O. Iron supplements reduce erythrocyte copper-zinc superoxide dismutase activity in term, breastfed infants. Acta Paedriat. 2005, 94, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Vance, C.K.; Miller, A.F. A simple proposal that can explain the inactivity of metal-substituted superoxide dismutase. J. Am. Chem. Soc. 1998, 120, 461–467. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Abdelhalim, M.A.K.; Al-Ayed, M.S.; Alhomida, A.S. Effect of gold nanoparticles on glutathione and malondialdehyde levels in liver, lung and heart of rats. Saudi J. Biol. Sci. 2012, 19, 461–464. [Google Scholar] [CrossRef]
- Priprem, A.; Mahakunakorn, P.; Thomas, C.; Thomas, I. Cytotoxicity studies of superparamagnetic iron oxide nanoparticles in macrophage and liver cells. Am. J. Nanotechnol. 2010, 1, 78–85. [Google Scholar] [CrossRef]
- Ma, P.; Luo, Q.; Chen, J.; Gan, Y.; Du, J.; Ding, S.; Xi, Z.; Yang, X. Intraperitoneal injection of magnetic Fe3O4-nanoparticle induces hepatic and renal tissue injury via oxidative stress in mice. Int. J. Nanomed. 2012, 7, 4809–4818. [Google Scholar]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef]
- Fernandez-Urrusuno, R.; Fattal, E.; Feger, J.; Couvreur, P.; Therond, P. Evaluation of hepatic antioxidant systems after intravenous administration of polymeric nanoparticles. Biomaterials 1997, 18, 511–517. [Google Scholar] [CrossRef]

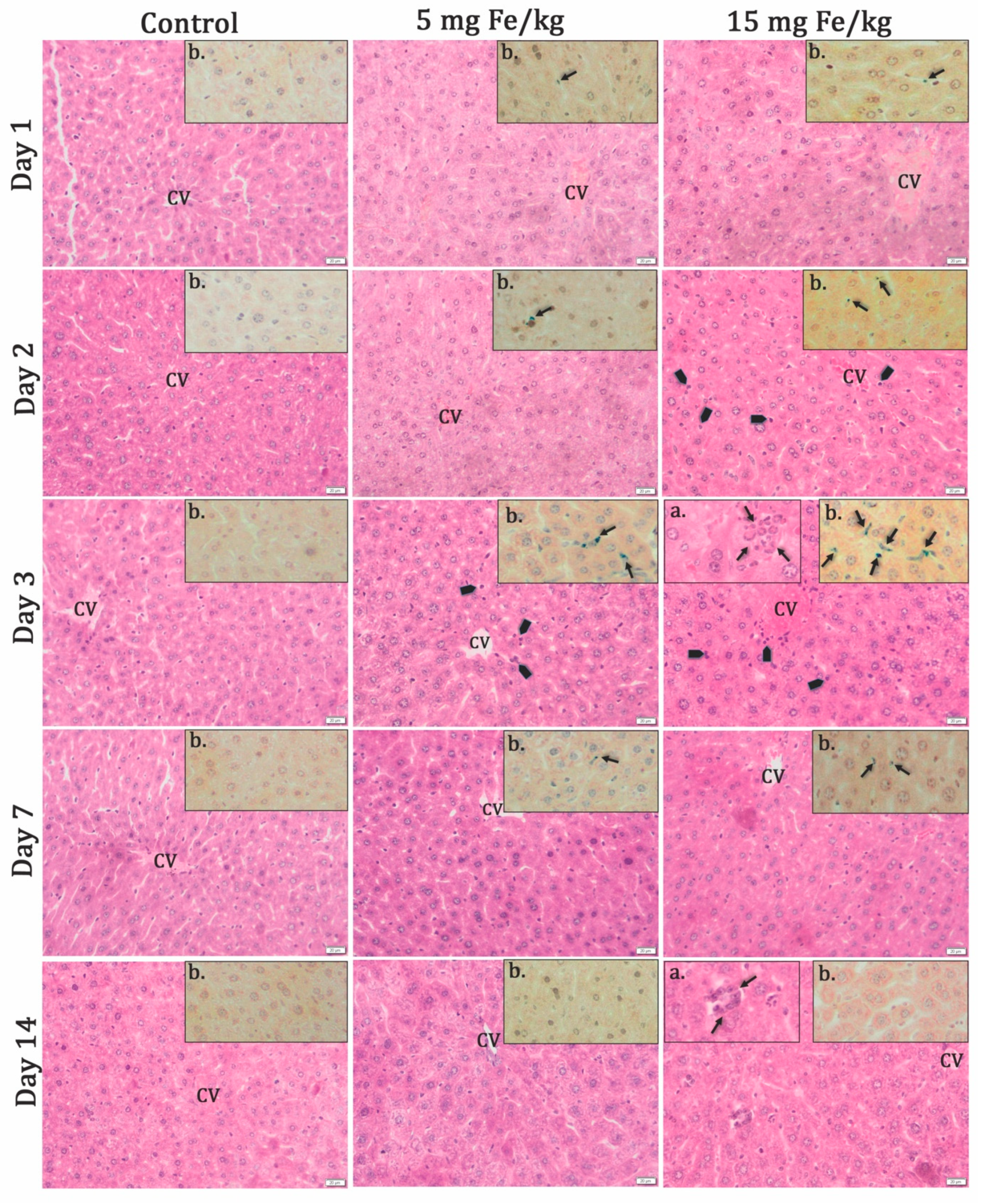

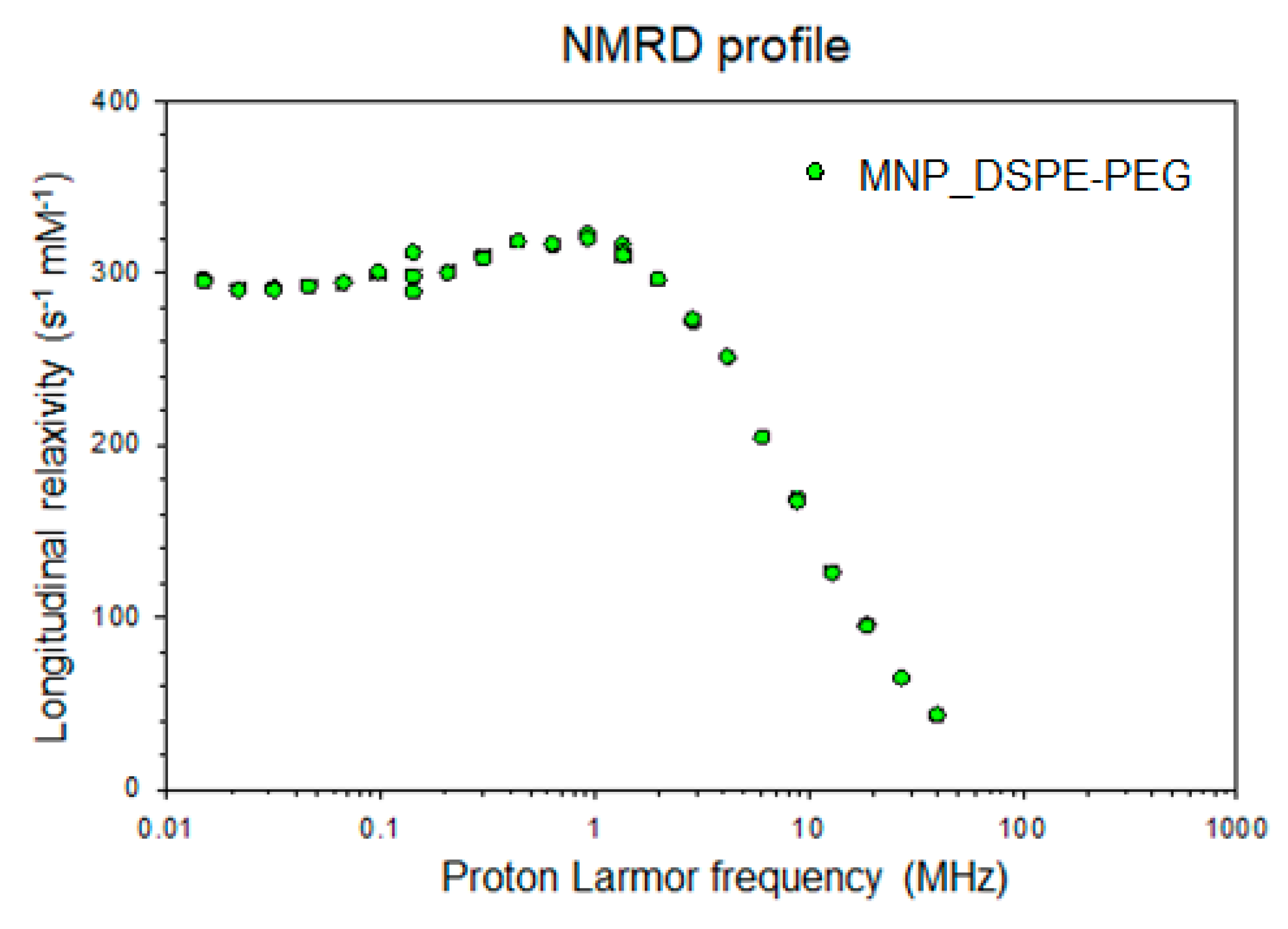
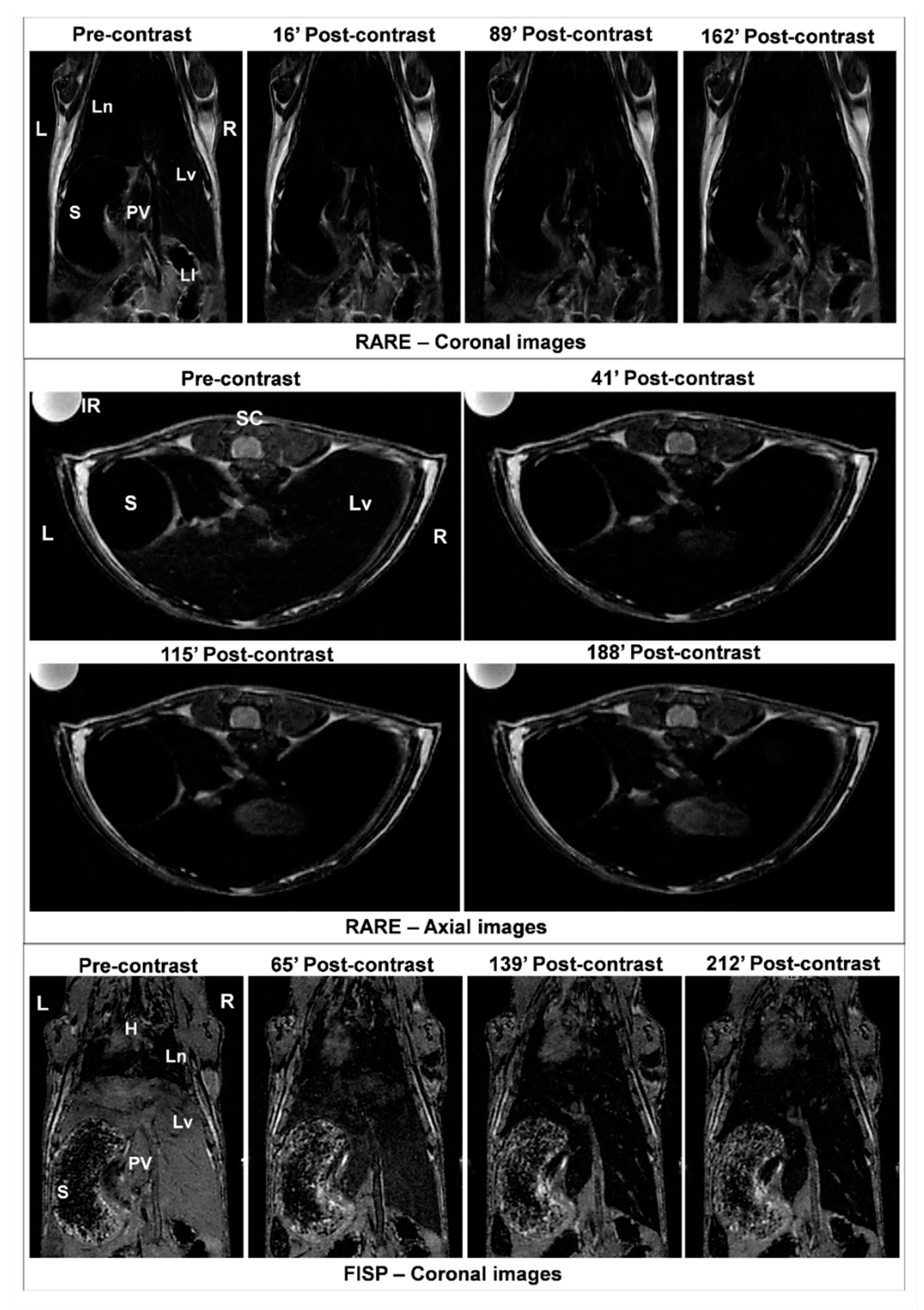
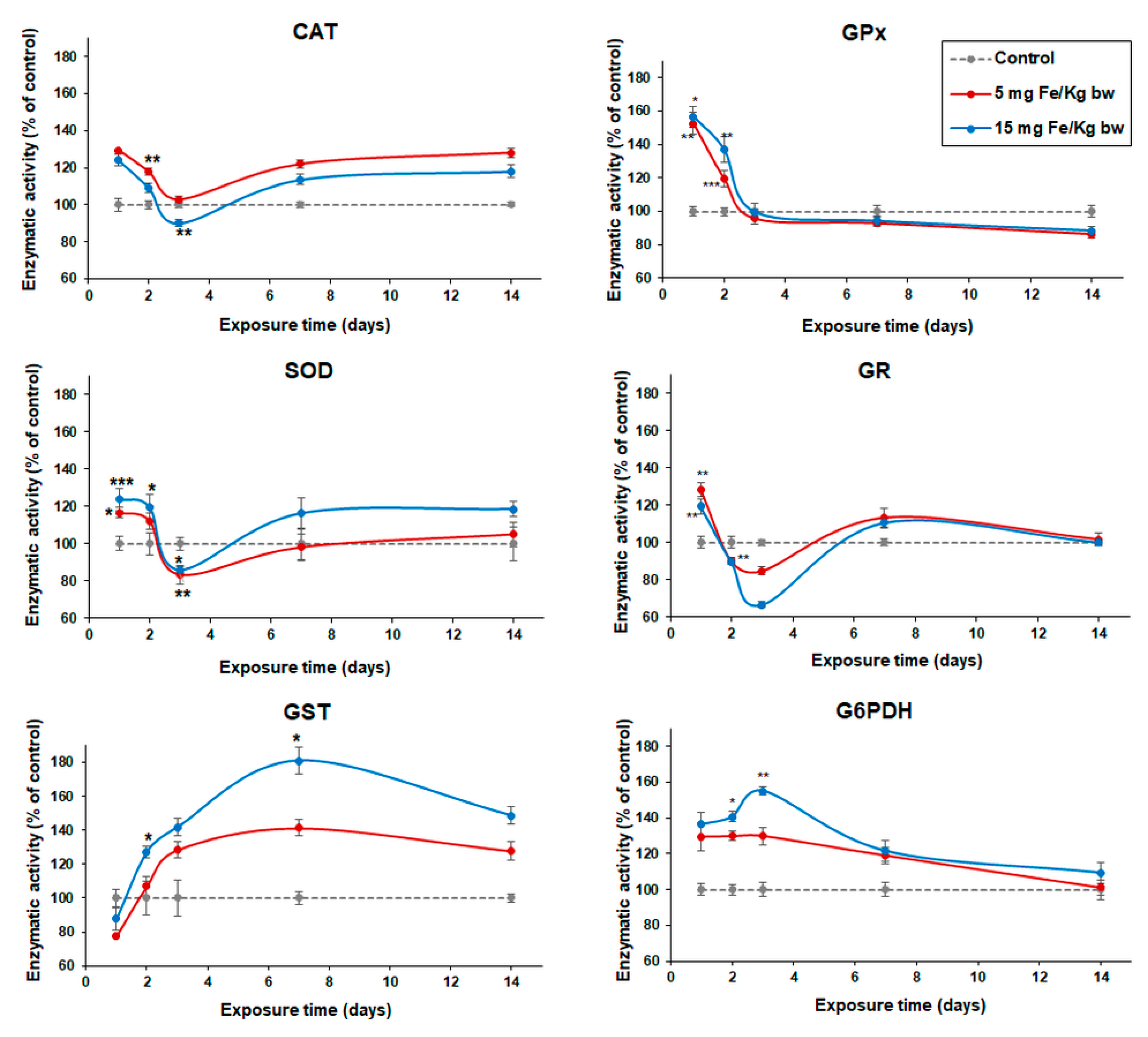

| Parameters | r1 (s−1 mM−1) | r2 (s−1 mM−1) | r2/r1 (s−1 mM−1) |
|---|---|---|---|
| 20 MHz | 15.39 | 151.23 | 9.83 |
| 60 MHz | 3.85 | 183.48 | 47.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu Din, I.M.; Balas, M.; Hermenean, A.; Vander Elst, L.; Laurent, S.; Burtea, C.; Cinteza, L.O.; Dinischiotu, A. Novel Polymeric Micelles-Coated Magnetic Nanoparticles for In Vivo Bioimaging of Liver: Toxicological Profile and Contrast Enhancement. Materials 2020, 13, 2722. https://doi.org/10.3390/ma13122722
Popescu Din IM, Balas M, Hermenean A, Vander Elst L, Laurent S, Burtea C, Cinteza LO, Dinischiotu A. Novel Polymeric Micelles-Coated Magnetic Nanoparticles for In Vivo Bioimaging of Liver: Toxicological Profile and Contrast Enhancement. Materials. 2020; 13(12):2722. https://doi.org/10.3390/ma13122722
Chicago/Turabian StylePopescu Din, Ioana Mihaela, Mihaela Balas, Anca Hermenean, Luce Vander Elst, Sophie Laurent, Carmen Burtea, Ludmila Otilia Cinteza, and Anca Dinischiotu. 2020. "Novel Polymeric Micelles-Coated Magnetic Nanoparticles for In Vivo Bioimaging of Liver: Toxicological Profile and Contrast Enhancement" Materials 13, no. 12: 2722. https://doi.org/10.3390/ma13122722
APA StylePopescu Din, I. M., Balas, M., Hermenean, A., Vander Elst, L., Laurent, S., Burtea, C., Cinteza, L. O., & Dinischiotu, A. (2020). Novel Polymeric Micelles-Coated Magnetic Nanoparticles for In Vivo Bioimaging of Liver: Toxicological Profile and Contrast Enhancement. Materials, 13(12), 2722. https://doi.org/10.3390/ma13122722











