Diselenide Core Cross-Linked Micelles of Poly(Ethylene Oxide)-b-Poly(Glycidyl Methacrylate) Prepared through Alkyne-Azide Click Chemistry as a Near-Infrared Controlled Drug Delivery System
Abstract
:1. Introduction
2. Results and Discussion
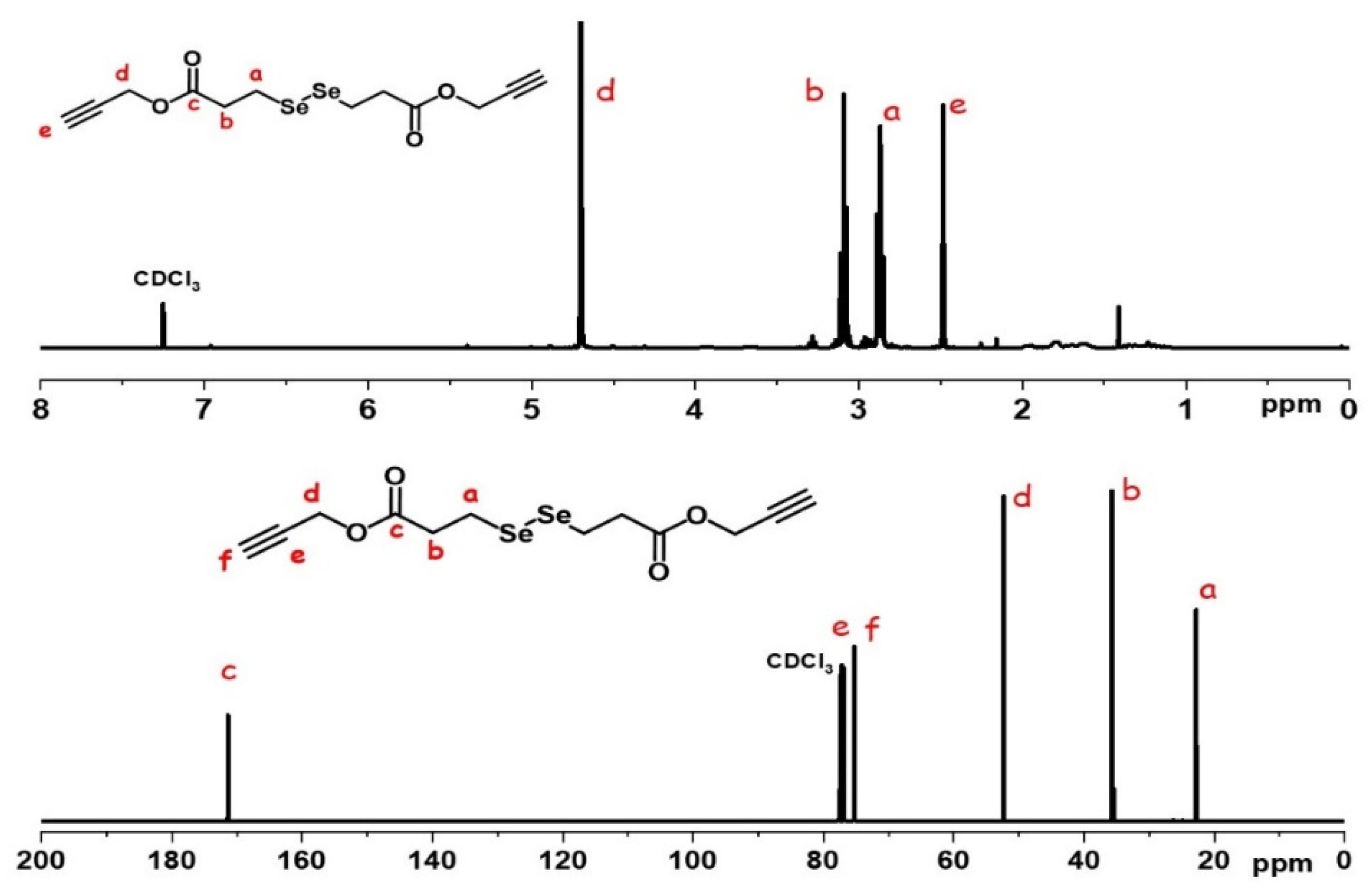
2.1. Preparation of Diselenide Cross-Linkers
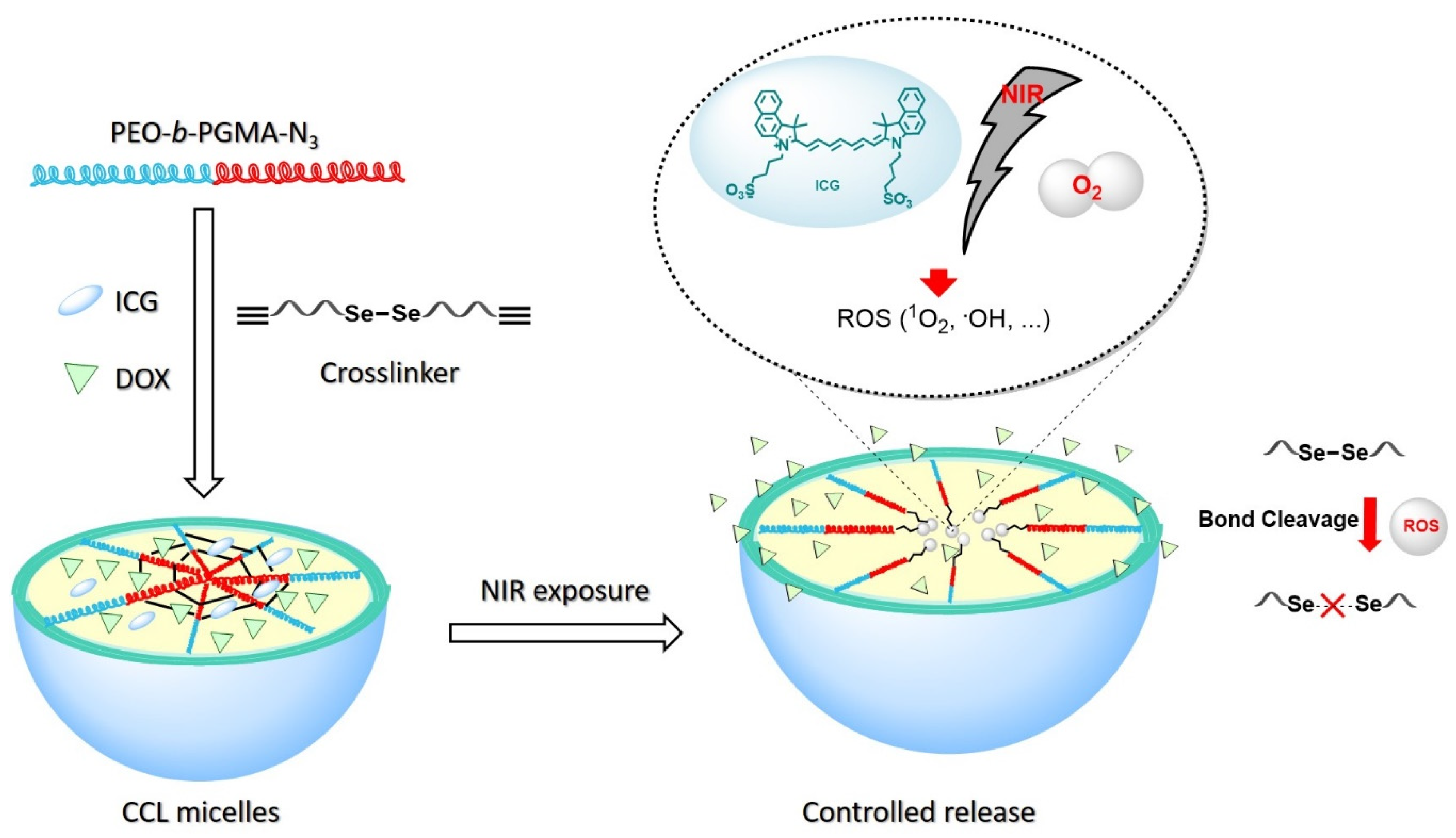
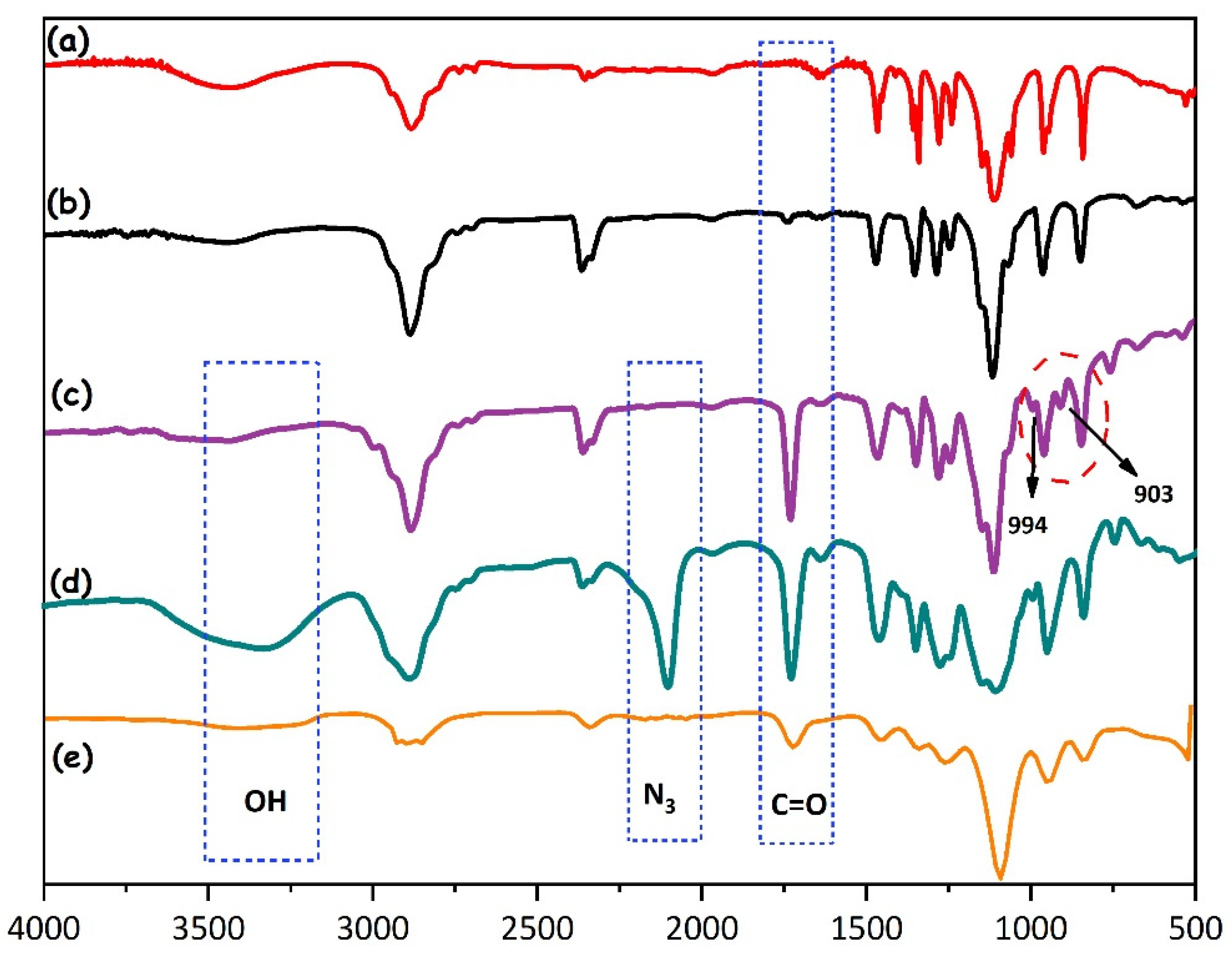
2.2. Preparation and Characterization of CCL Micelles
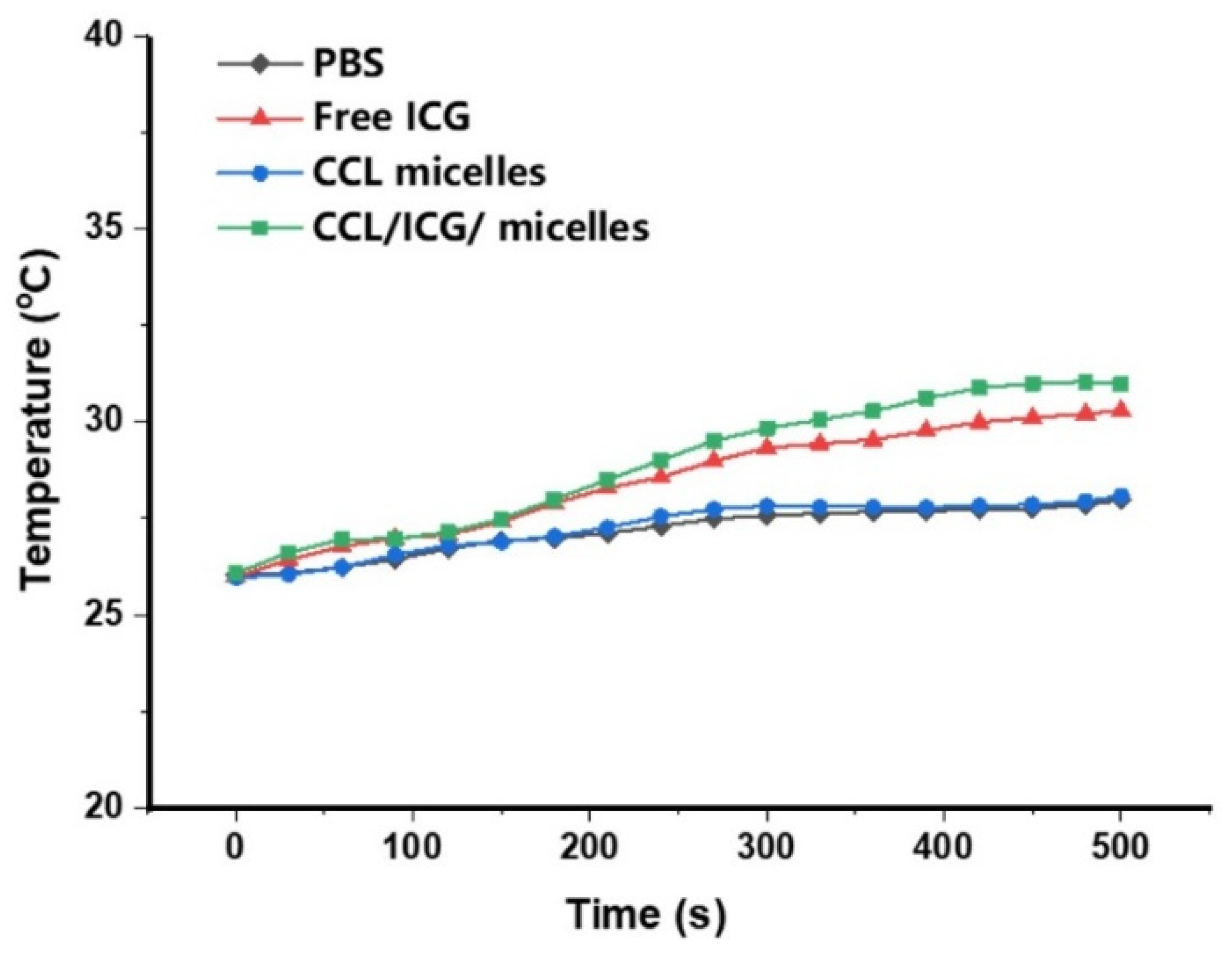
2.3. NIR Light Irradiation to the CCL Micelles
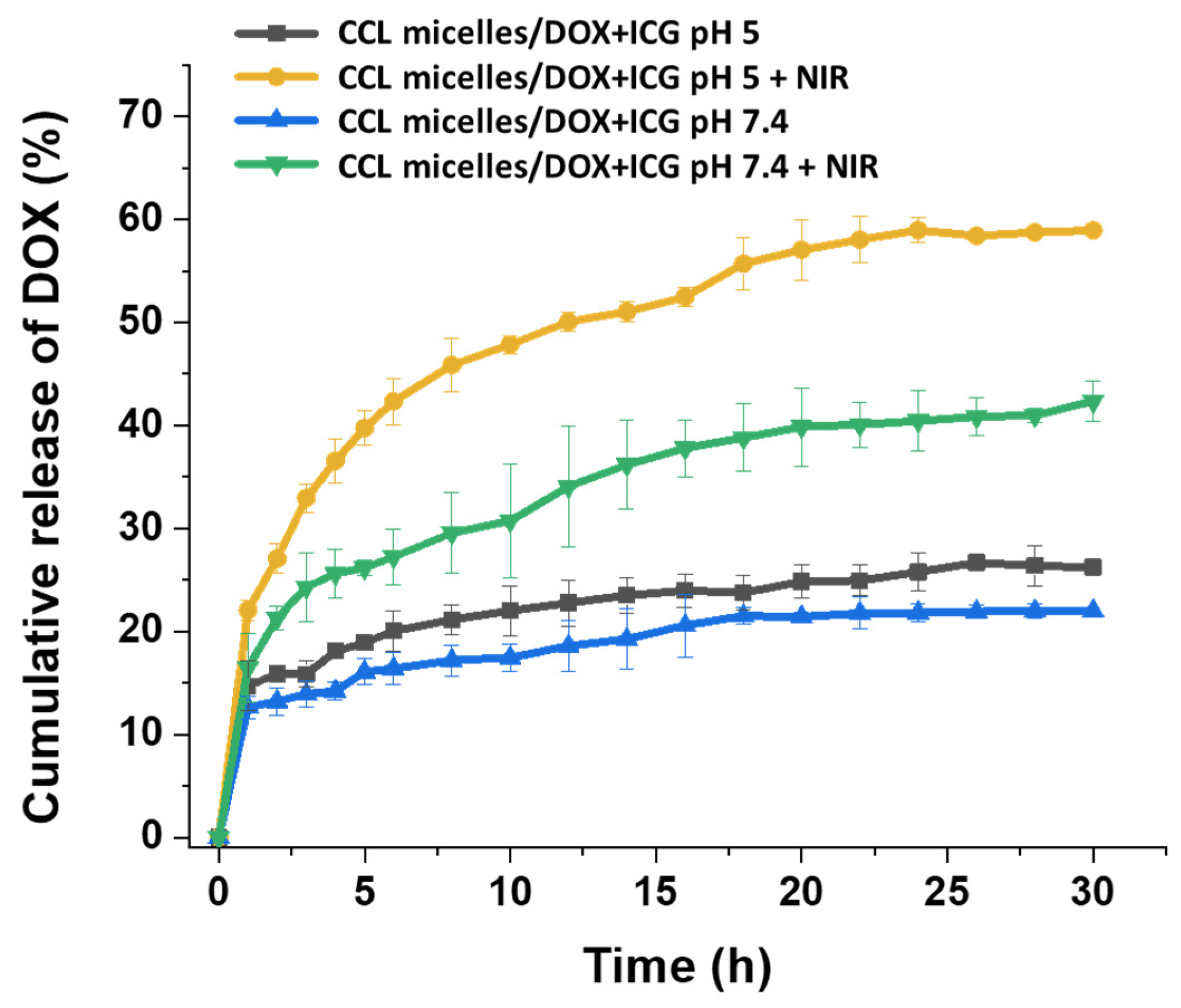
2.4. Drug Release Behavior
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Diselenide Cross-Linkers
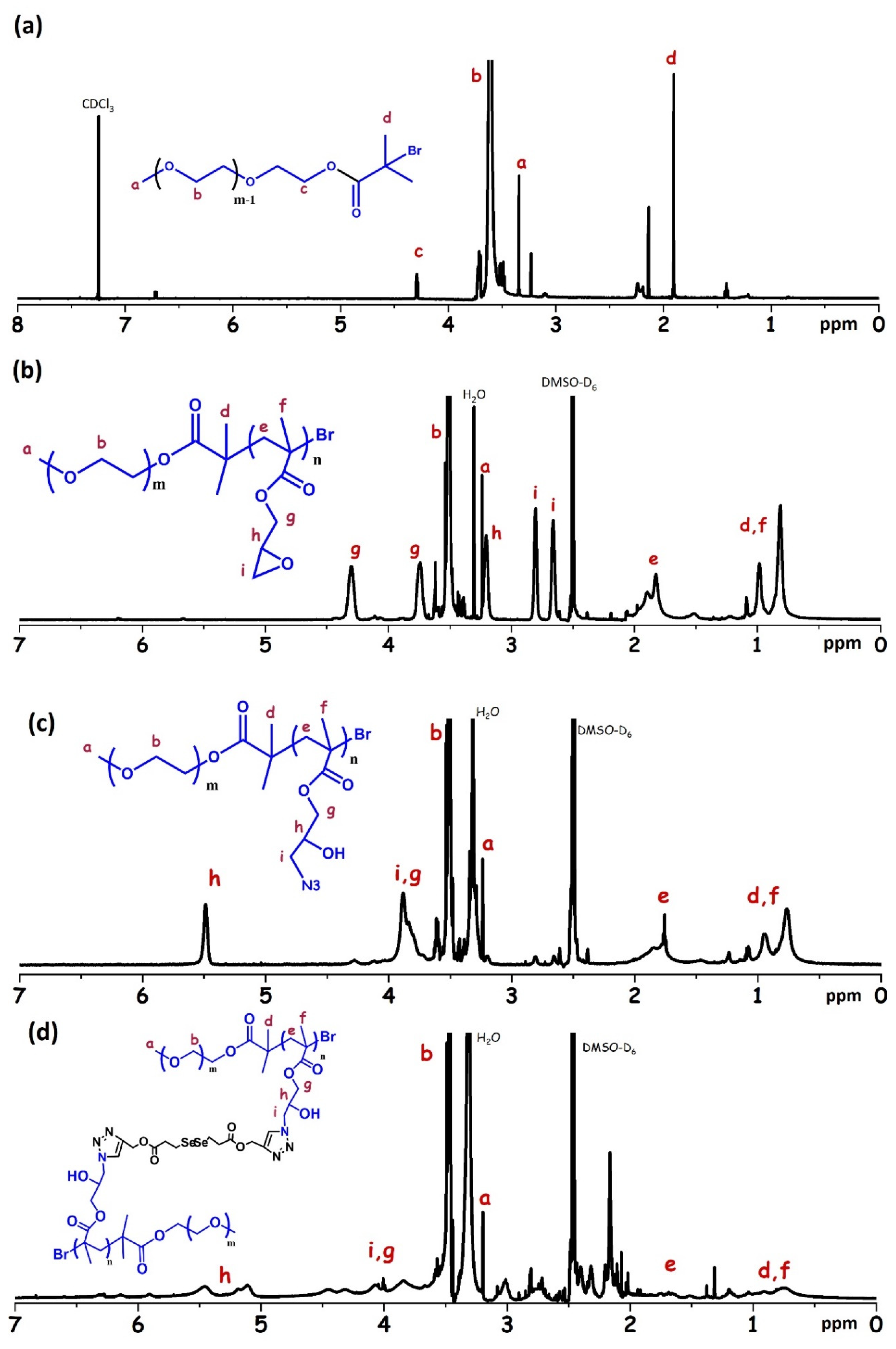
4.3. Preparation of PEO-Br Macroinitiator and PEO-b-PGMA
4.4. Preparation of PEO-b-PGMA-N3
4.5. Preparation of Diselenide CCL Micelles
4.6. Determination of the Critical Micelles Concentration
4.7. Drug Release Behavior
4.8. Characterization
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kataoka, K.; Harada, A.; Nagasaki, Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv. Drug Deliv. Rev. 2012, 64, 37–48. [Google Scholar] [CrossRef]
- Rösler, A.; Vandermeulen, G.W.; Klok, H.A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. 2010, 6, 714–729. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2012, 64, 246–255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Ko, N.R.; Oh, J.K. Recent advances in stimuli-responsive degradable block copolymer micelles: synthesis and controlled drug delivery applications. Chem. Commn. 2012, 48, 7542–7552. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Cao, X.T.; Kim, Y.H.; Gal, Y.S.; Lim, K.T. Block copolymeric micelles of poly (ethylene oxide)-b-poly (glycidyl methacrylate) for pH-triggered drug release. Mol. Cryst. Liq. Cryst. 2017, 644, 145–151. [Google Scholar] [CrossRef]
- Han, D.; Tong, X.; Zhao, Y. Fast photodegradable block copolymer micelles for burst release. Macromolecules 2011, 44, 437–439. [Google Scholar] [CrossRef]
- Tang, R.; Ji, W.; Panus, D.; Palumbo, R.N.; Wang, C. Block copolymer micelles with acid-labile ortho ester side-chains: synthesis, characterization, and enhanced drug delivery to human glioma cells. J. Control. Release 2011, 151, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, S.P.; Sougrat, R.; Hooghan, B.; Anjum, D.H.; Behzad, A.R.; Zhao, L.; Pradeep, N.; Pinnau, I.; Vainio, U.; Peinemann, K.V. Ultraporous films with uniform nanochannels by block copolymer micelles assembly. Macromolecules 2010, 43, 8079–8085. [Google Scholar] [CrossRef]
- Miller, T.; Breyer, S.; van Colen, G.; Mier, W.; Haberkorn, U.; Geissler, S.; Voss, S.; Weigandt, M.; Goepferich, A. Premature drug release of polymeric micelles and its effects on tumor targeting. Int. J. Pharm. 2013, 445, 117–124. [Google Scholar] [CrossRef]
- Li, J.; Sun, L.; Liu, Y.; Yao, H.; Jiang, S.; Li, Y.; Zhang, Y. To reduce premature drug release while ensuring burst intracellular drug release of solid lipid nanoparticle-based drug delivery system with clathrin modification. Nanomedicine 2019, 15, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Iman, M.; Varkouhi, A.K.; Rijcken, C.J.; Schiffelers, R.M.; Etrych, T.; Ulbrich, K.; van Nostrum, C.F.; Lammers, T.; Storm, G.; et al. Core-cross-linked polymeric micelles with controlled release of covalently entrapped doxorubicin. Biomaterials 2010, 31, 7797–7804. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cheng, Y.; Wang, B. Dual-Responsive Boronate Cross-linked Micelles for Targeted Drug Delivery. Angew. Chem. Int. Ed. 2012, 51, 5293–5295. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Jiang, Y.; Cheng, R.; Meng, F.; Zhong, Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: promises, progress and prospects. Nano Today 2012, 7, 467–480. [Google Scholar] [CrossRef]
- Sun, Q.; Bi, H.; Wang, Z.; Li, C.; Wang, X.; Xu, J.; Zhu, H.; Zhao, R.; He, F.; Gai, S.; et al. Hyaluronic acid-targeted and pH-responsive drug delivery system based on metal-organic frameworks for efficient antitumor therapy. Biomaterials 2019, 223, 119473. [Google Scholar] [CrossRef]
- Cao, X.T.; Le, C.M.Q.; Lim, K.T.; Thi, H.H.P.; Kim, G.D.; Gal, Y.S. Redox-responsive core cross-linked prodrug micelles prepared by click chemistry for pH-triggered doxorubicin delivery. Express Polym. Lett. 2017, 11, 832–845. [Google Scholar] [CrossRef]
- Long, W.; Ouyang, H.; Wan, W.; Yan, W.; Zhou, C.; Huang, H.; Liu, M.; Zhang, X.; Feng, Y.; Wei, Y. “Two in one”: Simultaneous functionalization and DOX loading for fabrication of nanodiamond-based pH responsive drug delivery system. Mater. Sci. Eng. C 2020, 108, 110413. [Google Scholar] [CrossRef]
- Laskar, P.; Somani, S.; Campbell, S.J.; Mullin, M.; Keating, P.; Tate, R.J.; Irving, C.; Leung, H.Y.; Dufès, C. Camptothecin-based dendrimersomes for gene delivery and redox-responsive drug delivery to cancer cells. Nanoscale 2019, 11, 20058–20071. [Google Scholar] [CrossRef]
- Tao, B.; Yin, Z. Redox-Responsive Coordination Polymers of Dopamine-Modified Hyaluronic Acid with Copper and 6-Mercaptopurine for Targeted Drug Delivery and Improvement of Anticancer Activity against Cancer Cells. Polymers 2020, 12, 1132. [Google Scholar] [CrossRef]
- Oh, K.S.; Hwang, C.; Lee, H.Y.; Song, J.S.; Park, H.J.; Lee, C.K.; Song, I.; Lim, T.H. Preclinical studies of ropivacaine extended-release from a temperature responsive hydrogel for prolonged relief of pain at the surgical wound. Int. J. Pharm. 2019, 558, 225–230. [Google Scholar] [CrossRef]
- Wang, J.; Huang, N.; Peng, Q.; Cheng, X.; Li, W. Temperature/pH dual-responsive and luminescent drug carrier based on PNIPAM-MAA/lanthanide-polyoxometalates for controlled drug delivery and imaging in HeLa cells. Mater. Chem. Phys. 2020, 239, 121994. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y. A novel pH/temperature-responsive hydrogel based on tremella polysaccharide and poly (N-isopropylacrylamide). Colloids Surf. 2020, 586, 124270. [Google Scholar] [CrossRef]
- Yan, T.; Li, F.; Qi, S.; Tian, J.; Tian, R.; Hou, J.; Luo, Q.; Dong, Z.; Xu, J.; Liu, J. Light-responsive vesicles for enantioselective release of chiral drugs prepared from a supra-amphiphilic M-helix. Chem. Commun. 2020, 56, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, R.; Yang, H.; Bao, C.; Fan, J.; Wang, C.; Lin, Q.; Zhu, L. Light-responsive polymersomes with a charge-switch for targeted drug delivery. J. Mater. Chem. B. 2020, 8, 727–735. [Google Scholar] [CrossRef]
- Salma, S.A.; Patil, M.P.; Kim, D.W.; Le, C.M.Q.; Ahn, B.H.; Kim, G.D.; Lim, K.T. Near-infrared light-responsive, diselenide containing core-cross-linked micelles prepared by the Diels–Alder click reaction for photocontrollable drug release application. Polym. Chem. 2018, 9, 4813–4823. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta Gen. Subject 2015, 1850, 1642–1660. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, K.; Liu, H. Biocompatibility selenium nanoparticles with an intrinsic oxidase-like activity. J. Nano Res. 2016, 18, 74. [Google Scholar] [CrossRef]
- Siboro, S.A.P.; Anugrah, D.S.B.; Jeong, Y.T.; Yoo, S.I.; Lim, K.T. Systematic investigation to the effects of near-infrared light exposure on polymeric micelles of poly (ethylene glycol)-block-poly (styrene-alt-maleic anhydride) loaded with indocyanine green. Polym. Degrad. Stabil. 2019, 167, 241–249. [Google Scholar] [CrossRef]
- Le, C.M.; Thi, H.H.P.; Cao, X.T.; Kim, G.D.; Oh, C.W.; Lim, K.T. Redox-responsive core cross-linked micelles of poly (ethylene oxide)-b-poly (furfuryl methacrylate) by Diels-Alder reaction for doxorubicin release. J. Polym. Sci. 2016, 54, 3741–3750. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siboro, S.A.P.; Salma, S.A.; Kim, H.-R.; Jeong, Y.T.; Gal, Y.-S.; Lim, K.T. Diselenide Core Cross-Linked Micelles of Poly(Ethylene Oxide)-b-Poly(Glycidyl Methacrylate) Prepared through Alkyne-Azide Click Chemistry as a Near-Infrared Controlled Drug Delivery System. Materials 2020, 13, 2846. https://doi.org/10.3390/ma13122846
Siboro SAP, Salma SA, Kim H-R, Jeong YT, Gal Y-S, Lim KT. Diselenide Core Cross-Linked Micelles of Poly(Ethylene Oxide)-b-Poly(Glycidyl Methacrylate) Prepared through Alkyne-Azide Click Chemistry as a Near-Infrared Controlled Drug Delivery System. Materials. 2020; 13(12):2846. https://doi.org/10.3390/ma13122846
Chicago/Turabian StyleSiboro, Sonita A.P., Sabrina Aufar Salma, Hyeung-Rak Kim, Yeon Tae Jeong, Yeong-Soon Gal, and Kwon Taek Lim. 2020. "Diselenide Core Cross-Linked Micelles of Poly(Ethylene Oxide)-b-Poly(Glycidyl Methacrylate) Prepared through Alkyne-Azide Click Chemistry as a Near-Infrared Controlled Drug Delivery System" Materials 13, no. 12: 2846. https://doi.org/10.3390/ma13122846





