Preparation of Doxorubicin-Loaded Amphiphilic Poly(D,L-Lactide-Co-Glycolide)-b-Poly(N-Acryloylmorpholine) AB2 Miktoarm Star Block Copolymers for Anticancer Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
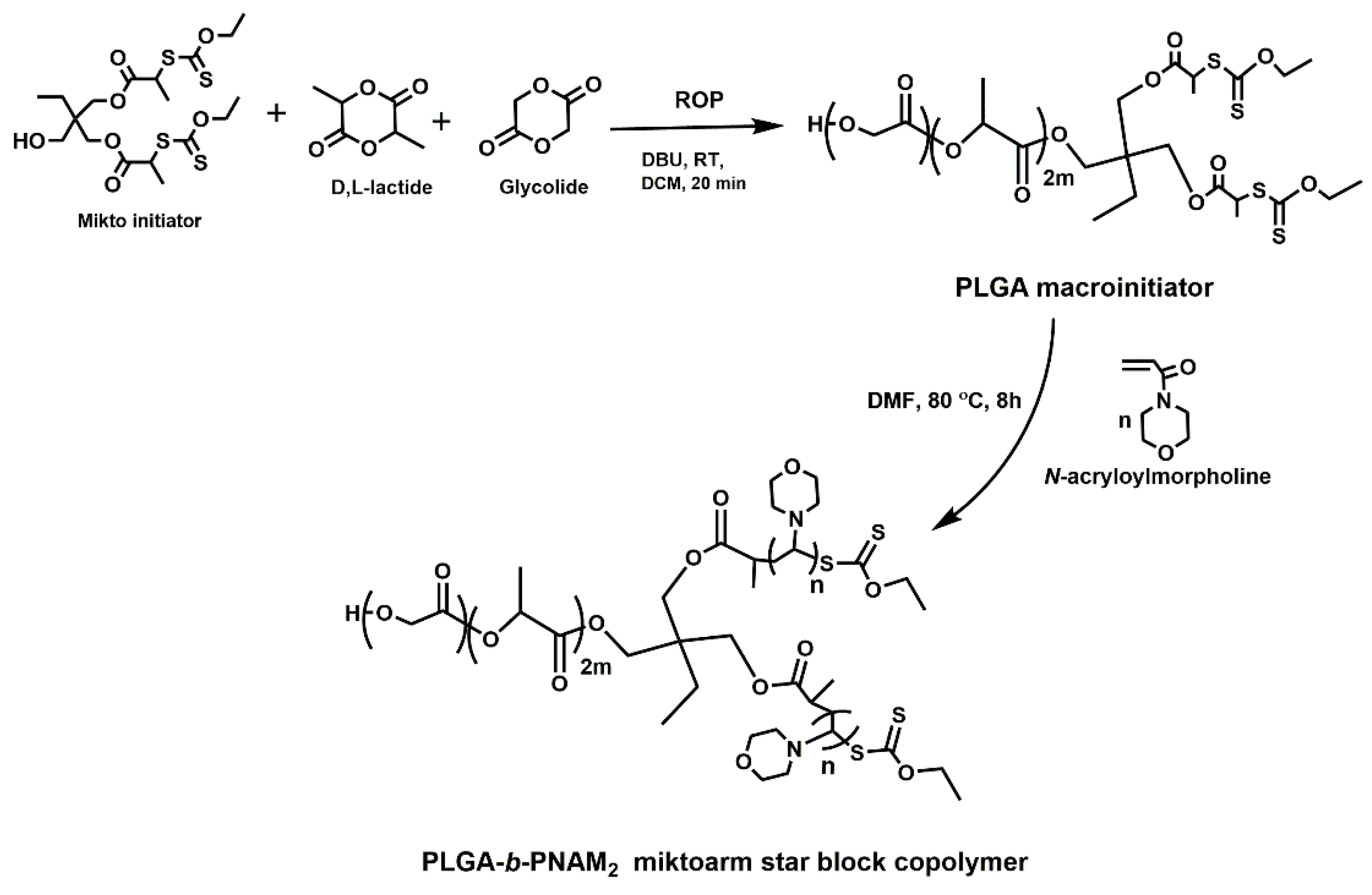
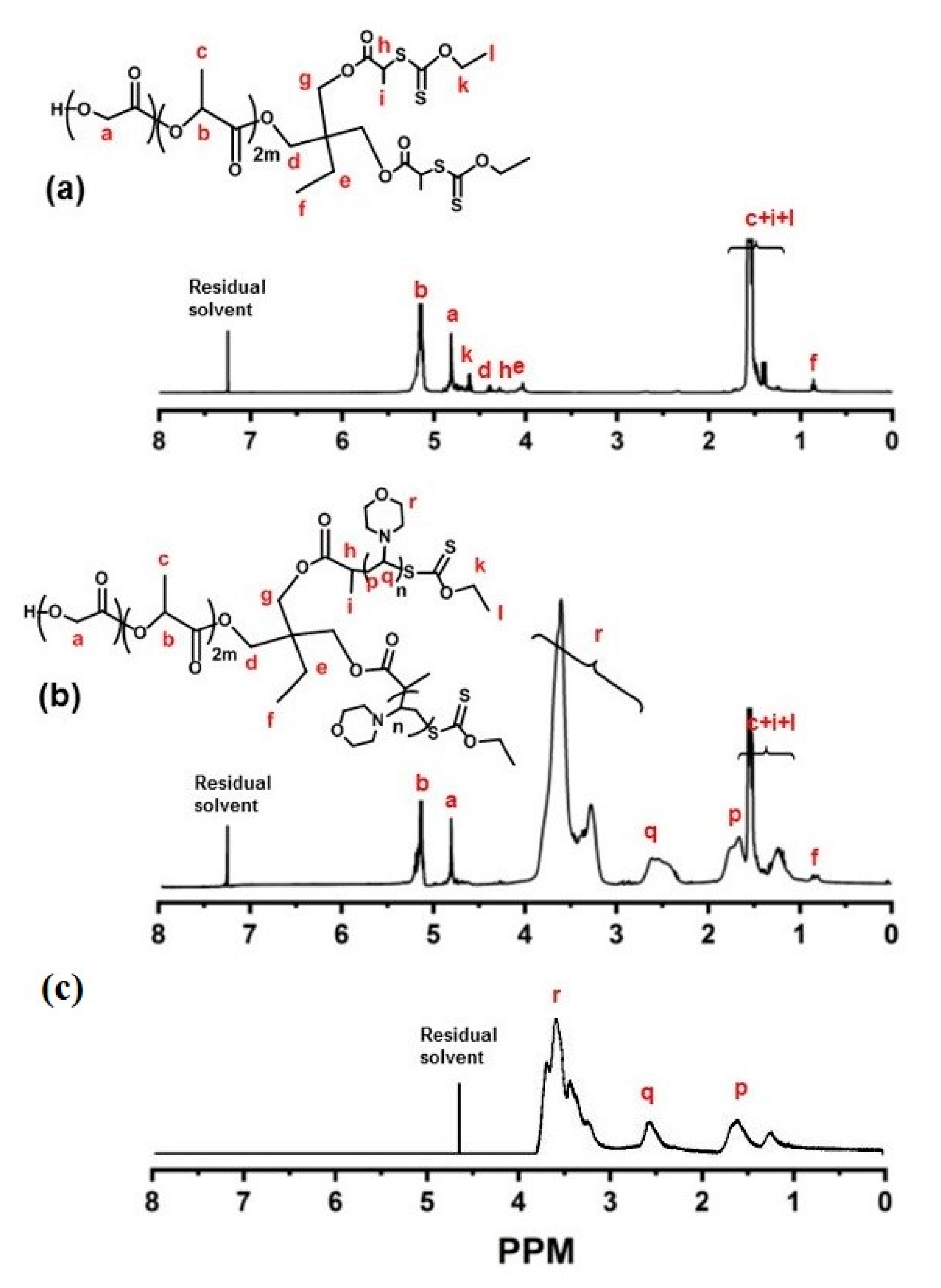
2.1. Synthesis of PLGA-b-PNAM2, the AB2-Type Miktoarm Star Block Copolymers
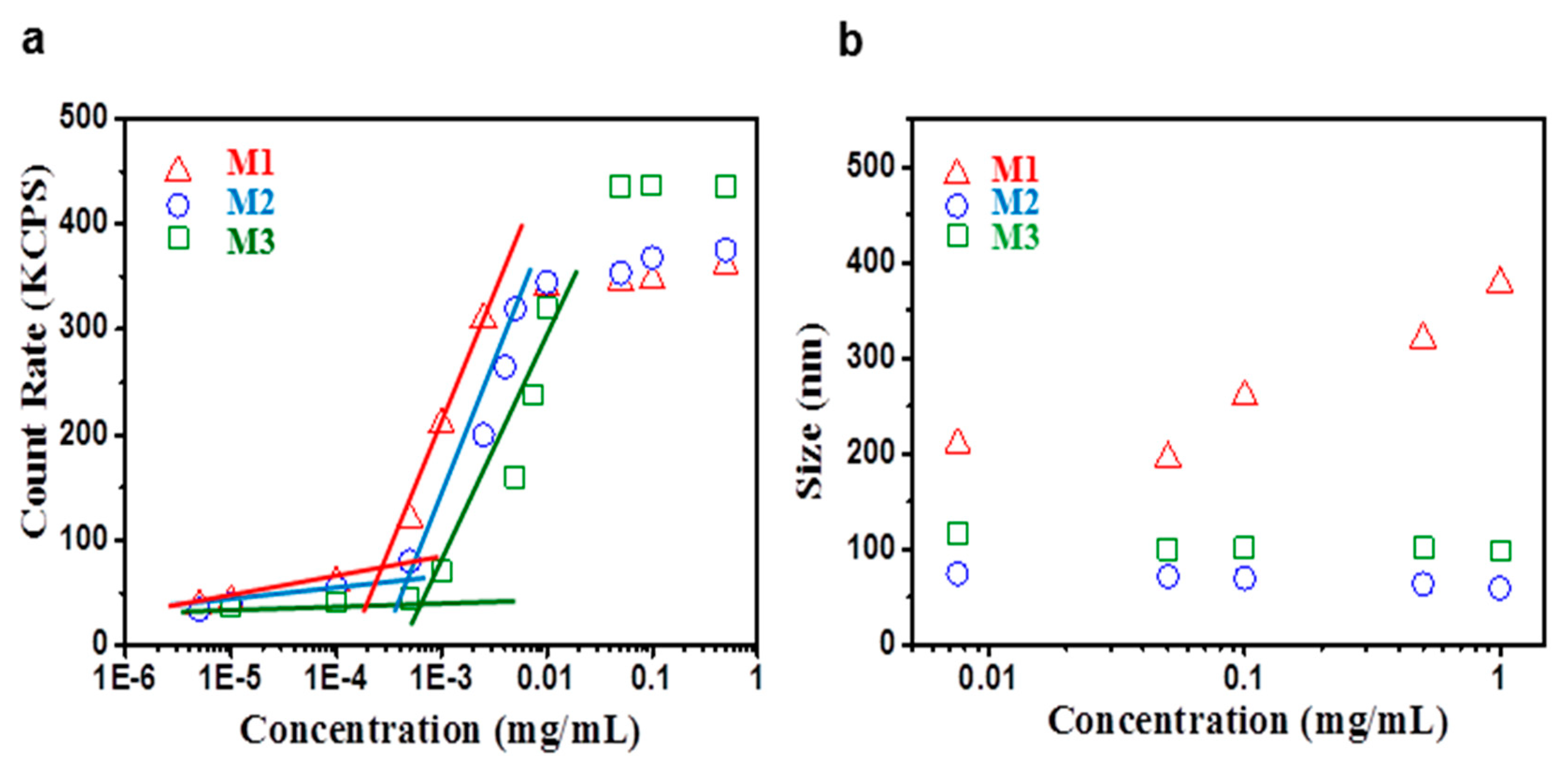
2.2. Self-Assembly Study of Miktoarm PLGA-b-PNAM2 Amphiphilic Star Block Copolymers
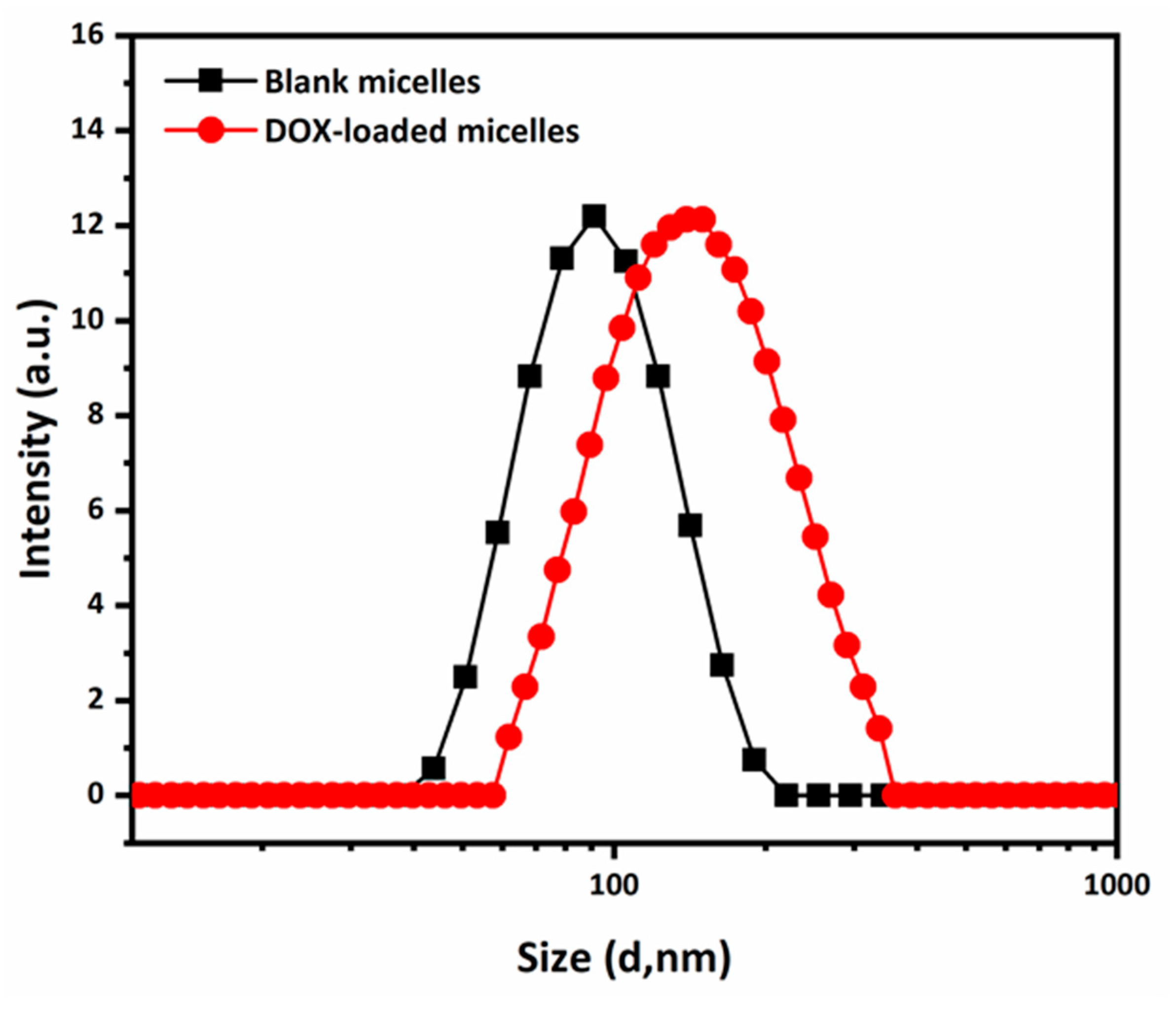
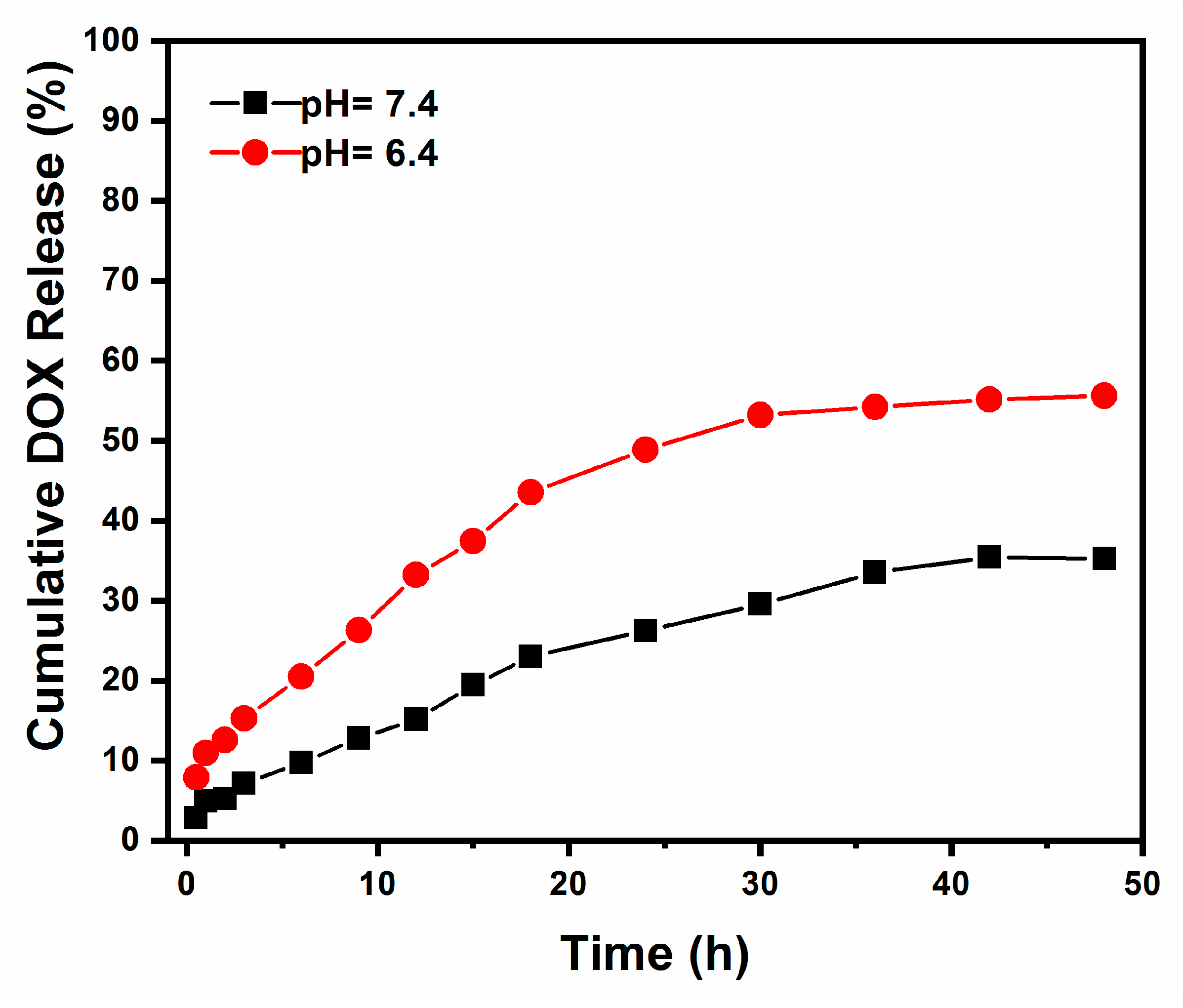
2.3. In Vitro Drug Loading and Release Study
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of 2-Ethyl-2-(Hydroxyl Methyl) Propane-1, 3-Diyl Bis(2-((Ethoxy Carbonothioyl) Thio) Propanoate) (Miktoarm Initiator)
4.3. Synthesis of PLGA Copolymer by ROP
4.4. Synthesis of PLGA-b-PNAM2 Miktoarm Star Block Copolymers by RAFT Polymerization (M1)
4.5. In Vitro Drug Loading and Release Study
4.6. Characterization
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, H.; Zhao, Y.; Wu, Y.; Hu, Y.L.; Nan, K.; Nie, G.; Chen, H. Enhanced anti-tumor efficacy by co-delivery of doxorubicin and paclitaxel with amphiphilic methoxy PEG-PLGA copolymer nanoparticles. Biomaterials 2011, 32, 8281–8290. [Google Scholar] [CrossRef]
- Zhu, Z.; Xie, C.; Liu, Q.; Zhen, X.; Zheng, X.; Wu, W.; Li, R.; Ding, Y.; Jiang, X.; Liu, B. The effect of hydrophilic chain length and iRGD on drug delivery from poly(epsilon-caprolactone)-poly(N-vinylpyrrolidone) nanoparticles. Biomaterials 2011, 32, 9525–9535. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [Green Version]
- Verma, G.; Hassan, P.A. Self assembled materials: Design strategies and drug delivery perspectives. Phys. Chem. Chem. Phys. 2013, 15, 17016–17028. [Google Scholar] [CrossRef]
- Khanna, K.; Varshney, S.; Kakkar, A. Miktoarm star polymers: Advances in synthesis, self-assembly, and applications. Polym. Chem. 2010, 1, 1171. [Google Scholar] [CrossRef]
- Altintas, O.; Schulze-Suenninghausen, D.; Luy, B.; Barner-Kowollik, C. ABC-type miktoarm star terpolymers accessed by H-bonding driven supramolecular self-assembly. Eur. Polym. J. 2015, 62, 409–417. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Modular Approaches to Star and Miktoarm Star Polymers by ATRP of Cross-Linkers. Macromol. Symp. 2010, 291, 12–16. [Google Scholar] [CrossRef]
- Isono, T.; Otsuka, I.; Kondo, Y.; Halila, S.; Fort, S.; Rochas, C.; Satoh, T.; Borsali, R.; Kakuchi, T. Sub-10 nm Nano-Organization in AB2- and AB3-Type Miktoarm Star Copolymers Consisting of Maltoheptaose and Polycaprolactone. Macromolecules 2013, 46, 1461–1469. [Google Scholar] [CrossRef]
- Babin, J.; Leroy, C.; Lecommandoux, S.; Borsali, R.; Gnanou, Y.; Taton, D. Towards an easy access to amphiphilic rod-coil miktoarm star copolymers. Chem. Commun. 2005, 15, 1993–1995. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.K.; Zainol, I.; Ng, C.H.; Ooi, I.H. Miktoarm star polymers nanocarrier: Synthesis, characterisation, and in-vitro drug release study. J. Polym. Res. 2019, 26, 79. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Park, H.; Kim, J.; Park, D.; Pramanick, S.; Kim, D.H.; Kim, W.J. Miktoarm Amphiphilic Block Copolymer with Singlet Oxygen-Labile Stereospecific β-Aminoacrylate Junction: Synthesis, Self-Assembly, and Photodynamically Triggered Drug Release. Biomacromolecules 2018, 19, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Bates, M.W.; Barbon, S.M.; Levi, A.E.; Lewis, R.M.; Beech, H.K.; Vonk, K.M.; Zhang, C.; Fredrickson, G.H.; Hawker, C.J.; Bates, C.M. Synthesis and Self-Assembly of ABn Miktoarm Star Polymers. ACS Macro Lett. 2020, 9, 396–403. [Google Scholar] [CrossRef]
- Zhu, M.-M.; Song, F.; Nie, W.-C.; Wang, X.-L.; Wang, Y.-Z. A facile chemoenzymatic synthesis of amphiphilic miktoarm star copolymers from a sugar core and their potential for anticancer drug delivery. Polymer 2016, 93, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Soliman, G.M.; Redon, R.; Sharma, A.; Mejia, D.; Maysinger, D.; Kakkar, A. Miktoarm star polymer based multifunctional traceable nanocarriers for efficient delivery of poorly water soluble pharmacological agents. Macromol. Biosci. 2014, 14, 1312–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Zhang, X.; Peng, H.; Zhang, M.; Zhang, X.; Liu, Z.; Ma, L.; Wei, H. Optimization of Amphiphilic Miktoarm Star Copolymers for Anticancer Drug Delivery. ACS Biomater. Sci. Eng. 2018, 4, 2903–2910. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Y.; Chen, Y.; Li, S.; He, B. Synthesis and crystallization of well-defined biodegradable miktoarm star PEG-PCL-PLLA copolymer. Mater. Lett. 2016, 171, 83–86. [Google Scholar] [CrossRef]
- Yoon, K.; Kang, H.C.; Li, L.; Cho, H.; Park, M.-K.; Lee, E.; Bae, Y.H.; Huh, K.M. Amphiphilic poly(ethylene glycol)-poly(ε-caprolactone) AB2 miktoarm copolymers for self-assembled nanocarrier systems: Synthesis, characterization, and effects of morphology on antitumor activity. Polym. Chem. 2015, 6, 531–542. [Google Scholar] [CrossRef]
- Soliman, G.M.; Sharma, R.; Choi, A.O.; Varshney, S.K.; Winnik, F.M.; Kakkar, A.K.; Maysinger, D. Tailoring the efficacy of nimodipine drug delivery using nanocarriers based on A2B miktoarm star polymers. Biomaterials 2010, 31, 8382–8392. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Kang, S.-W.; Bae, Y.H. Polymersome Formation from AB2Type 3-Miktoarm Star Copolymers. Macromolecules 2009, 42, 7456–7464. [Google Scholar] [CrossRef]
- Erdogan, T.; Ozyurek, Z.; Hizal, G.; Tunca, U. Facile synthesis of AB2-type miktoarm star polymers through the combination of atom transfer radical polymerization and ring-opening polymerization. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 2313–2320. [Google Scholar] [CrossRef]
- Shi, Y.; Fu, Z.; Li, B.; Zhang, L.; Cai, X.; Zhang, D. Synthesis of AB2 miktoarm star-shaped copolymers by combining stable free radical polymerization and atom transfer radical polymerization. Eur. Polym. J. 2007, 43, 2612–2619. [Google Scholar] [CrossRef]
- Ramesh, K.; Thangagiri, B.; Mishra, A.K.; Ahn, B.-H.; Gal, Y.-S.; Lim, K.T. AB2-type miktoarm poly(l-lactide)-b-poly(N-acryloylmorpholine) amphiphilic star block copolymers as nanocarriers for drug delivery. React. Funct. Polym. 2018, 132, 112–119. [Google Scholar] [CrossRef]
- Sun, X.; Xu, C.; Wu, G.; Ye, Q.; Wang, C. Poly(Lactic-co-Glycolic Acid): Applications and Future Prospects for Periodontal Tissue Regeneration. Polymers 2017, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.; Singh, S.; Mitra, K.; Chattopadhyay, D.; Misra, N.; Ray, B. Self-assembly of Novel Poly(d,l-Lactide-co-Glycolide)-b-Poly(N-Vinylpyrrolidone) (PLGA-b-PNVP) Amphiphilic Diblock Copolymers. Colloid Polym. Sci. 2016, 294, 399–407. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Chen, T.; Xian, W.; Zhang, H.; Wu, L.; Zhu, W.; Zeng, Q. Nanoscale cationic micelles of amphiphilic copolymers based on star-shaped PLGA and PEI cross-linked PEG for protein delivery application. J. Mater. Sci. Mater. Med. 2019, 30, 93. [Google Scholar] [CrossRef]
- Shen, H.; Niu, Y.; Hu, X.; Yang, F.; Wang, S.; Wu, D. A biomimetic 3D microtubule-orientated poly(lactide-co-glycolide) scaffold with interconnected pores for tissue engineering. J. Mater. Chem. B 2015, 3, 4417–4425. [Google Scholar] [CrossRef]
- Chaduc, I.; Reynaud, E.; Dumas, L.; Albertin, L.; D’Agosto, F.; Lansalot, M. From well-defined poly( N -acryloylmorpholine)-stabilized nanospheres to uniform mannuronan- and guluronan-decorated nanoparticles by RAFT polymerization-induced self-assembly. Polymer 2016, 106, 218–228. [Google Scholar] [CrossRef]
- Rivas, B.L.; Maureira, A.; Geckeler, K.E. Novel water-soluble acryloylmorpholine copolymers: Synthesis, characterization, and metal ion binding properties. J. Appl. Polym. Sci. 2006, 101, 180–185. [Google Scholar] [CrossRef]
- Savelyeva, X.; Lessard, B.H.; Marić, M. Amphiphilic Poly(4-acryloylmorpholine)/Poly[2-(N-carbazolyl)ethyl acrylate] Random and Block Copolymers Synthesized by NMP. Macromol. React. Eng. 2012, 6, 200–212. [Google Scholar] [CrossRef]
- Fares, M.M.; Al-Shboul, A.M. Stimuli pH-responsive (N-vinyl imidazole-co-acryloylmorpholine) hydrogels; mesoporous and nanoporous scaffolds. J. Biomed. Mater. Res. Part A 2012, 100, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, H.; Luo, Y.L.; Tang, W. Redox-Responsive Self-Assembly Micelles from Poly(N-acryloylmorpholine-block-2-acryloyloxyethyl ferrocenecarboxylate) Amphiphilic Block Copolymers as Drug Release Carriers. ACS Appl. Mater. Interfaces 2017, 9, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Samarajeewa, S.; Shrestha, R.; Li, Y.; Wooley, K.L. Degradability of poly(lactic acid)-containing nanoparticles: Enzymatic access through a cross-linked shell barrier. J. Am. Chem. Soc. 2012, 134, 1235–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Feng, Q.; He, Y.; Yan, F.; Chen, L.; Zhao, Y. Dual functionalized poly(vinylidene fluoride) membrane with acryloylmorpholine and argatroban to improve antifouling and hemocompatibility. J. Biomed. Mater. Res. Part A 2017, 105, 178–188. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakayama, M.; Itoga, K.; Yamato, M.; Okano, T. Micropatterned thermoresponsive polymer brush surfaces for fabricating cell sheets with well-controlled orientational structures. Biomacromolecules 2011, 12, 1414–1418. [Google Scholar] [CrossRef]
- Chado, G.R.; Holland, E.N.; Tice, A.K.; Stoykovich, M.P.; Kaar, J.L. Modification of Lipase with Poly(4-acryloylmorpholine) Enhances Solubility and Transesterification Activity in Anhydrous Ionic Liquids. Biomacromolecules 2018, 19, 1324–1332. [Google Scholar] [CrossRef]
- Ramesh, K.; Mishra, A.K.; Patel, V.K.; Vishwakarma, N.K.; Biswas, C.S.; Paira, T.K.; Mandal, T.K.; Maiti, P.; Misra, N.; Ray, B. Synthesis of well-defined amphiphilic poly(d,l-lactide)-b-poly(N-vinylpyrrolidone) block copolymers using ROP and xanthate-mediated RAFT polymerization. Polymer 2012, 53, 5743–5753. [Google Scholar] [CrossRef]
- Mayadunne, R.T.A.; Jeffery, J.; Moad, G.; Rizzardo, E. Living Free Radical Polymerization with Reversible Addition−Fragmentation Chain Transfer (RAFT Polymerization): Approaches to Star Polymers. Macromolecules 2003, 36, 1505–1513. [Google Scholar] [CrossRef]
- Boschmann, D.; Vana, P. Z-RAFT star polymerizations of acrylates: Star coupling via intermolecular chain transfer to polymer. Macromolecules 2007, 40, 2683–2693. [Google Scholar] [CrossRef]
- Lu, J.; Owen, S.C.; Shoichet, M.S. Stability of Self-Assembled Polymeric Micelles in Serum. Macromolecules 2011, 44, 6002–6008. [Google Scholar] [CrossRef]
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional polymeric micelles for delivery of drugs and siRNA. Front. Pharmacol. 2014, 5, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, K.; Anugrah, D.S.B.; Lim, K.T. Supramolecular poly(N-acryloylmorpholine)-b-poly(d,l-lactide) pseudo-block copolymer via host-guest interaction for drug delivery. React. Funct. Polym. 2018, 131, 12–21. [Google Scholar] [CrossRef]
- Sanson, C.; Schatz, C.; Le Meins, J.-F.; Soum, A.; Thévenot, J.; Garanger, E.; Lecommandoux, S. A simple method to achieve high doxorubicin loading in biodegradable polymersomes. J. Control. Release 2010, 147, 428–435. [Google Scholar] [CrossRef]


| Run | Miktoarm Polymer | NAM b (Equiv) | Conv. c (%) | Mn(NMR) d (g/mol) | Mn(GPC) e (g/mol) | Đ |
|---|---|---|---|---|---|---|
| PLGA | PLGA26–macroinitiator | – | 88 | 4800 | 5300 | 1.20 |
| M1 | PLGA26–b–(PNAM21)2 | 50 | 90 | 10,760 | 11,600 | 1.38 |
| M2 | PLGA26–b–(PNAM40)2 | 100 | 86 | 16,140 | 17,400 | 1.40 |
| M3 | PLGA26–b–(PNAM81)2 | 200 | 82 | 25,800 | 28,000 | 1.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramesh, K.; Mishra, A.K.; Kim, J.K.; Jeong, Y.T.; Gal, Y.-S.; Lim, K.T. Preparation of Doxorubicin-Loaded Amphiphilic Poly(D,L-Lactide-Co-Glycolide)-b-Poly(N-Acryloylmorpholine) AB2 Miktoarm Star Block Copolymers for Anticancer Drug Delivery. Materials 2020, 13, 3713. https://doi.org/10.3390/ma13173713
Ramesh K, Mishra AK, Kim JK, Jeong YT, Gal Y-S, Lim KT. Preparation of Doxorubicin-Loaded Amphiphilic Poly(D,L-Lactide-Co-Glycolide)-b-Poly(N-Acryloylmorpholine) AB2 Miktoarm Star Block Copolymers for Anticancer Drug Delivery. Materials. 2020; 13(17):3713. https://doi.org/10.3390/ma13173713
Chicago/Turabian StyleRamesh, Kalyan, Avnish Kumar Mishra, Jin Kon Kim, Yeon Tae Jeong, Yeong-Soon Gal, and Kwon Taek Lim. 2020. "Preparation of Doxorubicin-Loaded Amphiphilic Poly(D,L-Lactide-Co-Glycolide)-b-Poly(N-Acryloylmorpholine) AB2 Miktoarm Star Block Copolymers for Anticancer Drug Delivery" Materials 13, no. 17: 3713. https://doi.org/10.3390/ma13173713






