Effect of Alkaline-Basic Electrolytes on the Capacitance Performance of Biomass-Derived Carbonaceous Materials
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
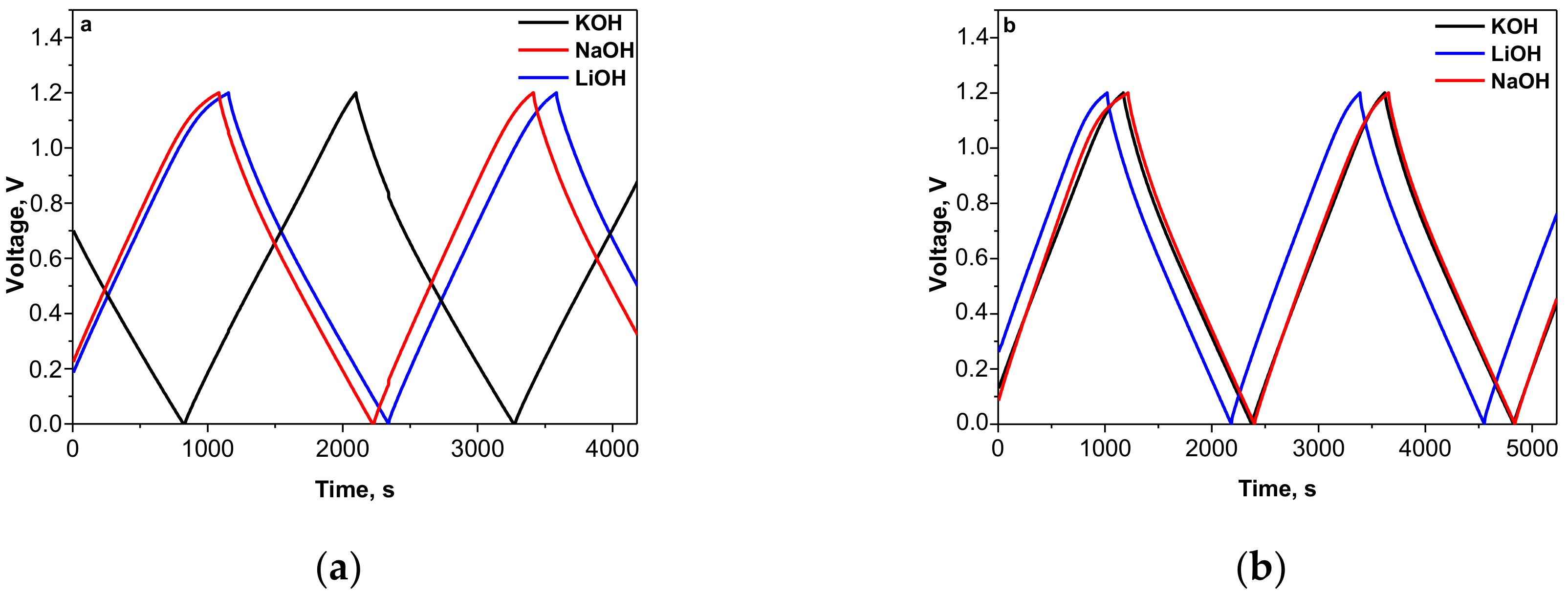
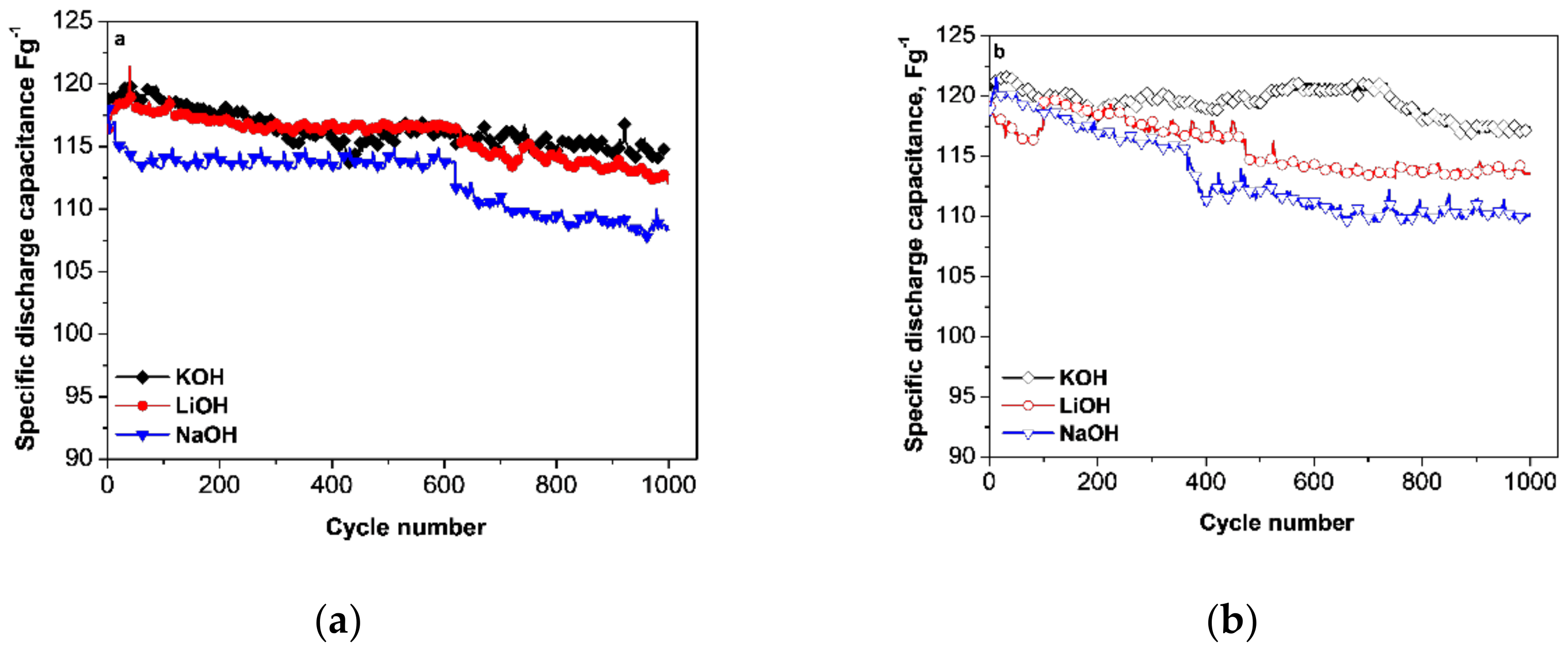
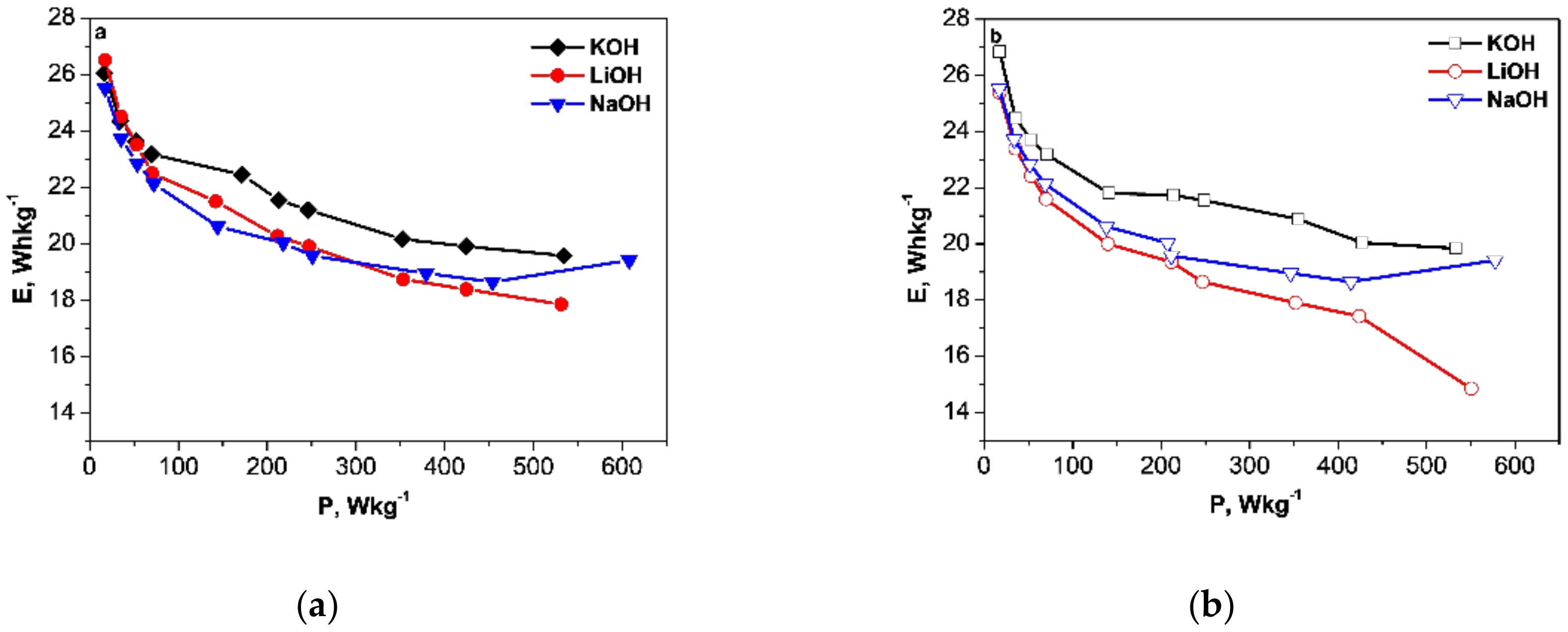
3.1. Capacitance Performance of Biomass-Derived Carbon
3.2. Postmortem Characterization of Carbon Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poonam; Sharma, K.; Arora, A.; Tripathi, S. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Fic, K.; Platek, A.; Piwek, J.; Frackowiak, E. Sustainable materials for electrochemical capacitors. Mater. Today 2018, 21, 437–454. [Google Scholar] [CrossRef]
- Milanova, V.; Markova, I.; Piski, M.; Stankulov, T.; Petrov, T.; Denev, I. Synthesis and study of carbon-based nanocomposites with Co-Sn nanoparticles for electrode material. J. Chem. Technol. Metall. 2015, 50, 288–298. [Google Scholar]
- Forse, A.; Merlet, C.; Griffin, J.; Grey, C. New Perspectives on the Charging Mechanisms of Supercapacitors. J. Am. Chem. Soc. 2016, 138, 5731–5744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Mirzaeian, M.; Abbas, Q.; Ogwu, A.; Hall, P.; Jirandehi, F. Electrode and electrolyte materials for electrochemical capacitors. Int. J. Hydrogen Energy 2017, 42, 25565–25587. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Zhao, X. Biomass-derived carbon electrode materials for supercapacitors. Sust. Energy Fuels 2017, 1, 1265–1281. [Google Scholar] [CrossRef]
- Boujibar, O.; Ghosh, A.; Achak, O.; Chafik, T.; Ghamouss, F. A high energy storage supercapacitor based on nanoporous activated carbonelectrode made from Argan shells with excellent ion transport in aqueous and non-aqueous electrolytes. J. Energy Storage 2019, 26, 100958. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zhou, J.; Liang, T. Biomass-Derived Porous Carbon Materials for Supercapacitor. Front. Chem. 2019, 7, 274. [Google Scholar] [CrossRef] [Green Version]
- Kalderis, D.; Bethanis, S.; Paraskeva, P.; Diamadopoulos, E. Production of Activated Carbon From Bagasse and Rice Husk by a Single-Stage Chemical Activation Method at Low Retention Times. Bioresour Technol. 2008, 99, 6809–6816. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Shen, S.; Bao, B.; Wu, L. Functional Biomass Carbons with Hierarchical Porous Structure for Supercapacitor Electrode Materials. Electrochim. Acta 2015, 180, 241–251. [Google Scholar] [CrossRef]
- Fan, L.; Chen, T.; Song, W.; Li, X.; Zhang, S. High nitrogen-containing cotton derived 3D porous carbon frameworks for high-performance supercapacitors. Sci. Rep. 2015, 5, 15388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Jang, Y.; Jeong, H. Bamboo-based activated carbon for supercapacitor applications. Curr Appl Phys. 2014, 14, 1616–1620. [Google Scholar] [CrossRef]
- Kostoglou, N.; Koczwara, C.; Prehal, C.; Terziyska, V.; Babic, B.; Matovic, B.; Constantinides, G.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.; et al. Nanoporous activated carbon cloth as a versatile material for hydrogen adsorption, selective gas separation and electrochemical energy storage. Nano Energy 2017, 40, 49–64. [Google Scholar] [CrossRef]
- Phattharasupakun, N.; Wutthiprom, J.; Suktha, P.; Iamprasertkun, P.; Chanlek, N.; Shepherd, C.; Hadzifejzovic, E.; Moloney, M.; Foord, J.; Sawangphruk, M. High-performance supercapacitors of carboxylate-modified hollow carbon nanospheres coated on flexible carbon fibre paper: Effects of oxygen-containing group contents, electrolytes and operating temperature. Electrochim. Acta 2017, 238, 64–73. [Google Scholar] [CrossRef]
- Calvo, E.G.; Rey-Raap, N.; Arenillas, A.; Menéndez, J.A. The effect of the carbon surface chemistry and electrolyte pH on the energy storage of supercapacitors. RSC Adv. 2014, 4, 32398–32404. [Google Scholar] [CrossRef] [Green Version]
- Forse, A.C.; Griffin, J.M.; Merlet, C.; Bayley, P.M.; Wang, H.; Simon, P.; Grey, C.P. NMR Study of Ion Dynamics and Charge Storage in Ionic Liquid Supercapacitors. C. P. J. Am. Chem. Soc. 2015, 137, 7231–7242. [Google Scholar] [CrossRef]
- Luo, Z.-X.; Xing, Y.-Z.; Liu, S.; Ling, Y.-C.; Kleinhammes, A.; Wu, Y. Dehydration of Ions in Voltage-Gated Carbon Nanopores Observed by in Situ NMR. J. Phys. Chem. Lett. 2015, 6, 5022–5026. [Google Scholar] [CrossRef]
- Hu, C.-C.; Wang, C.-C. Effects of electrolytes and electrochemical pretreatments on the capacitive characteristics of activated carbon fabrics for supercapacitors. J. Power Sources 2004, 125, 299–308. [Google Scholar] [CrossRef]
- Andreas, H.A.; Conway, B.E. Examination of the double-layer capacitance of an high specific-area C-cloth electrode as titrated from acidic to alkaline pHs. Electrochim. Acta 2006, 51, 6510–6520. [Google Scholar] [CrossRef]
- Beguin, F.; Friebe, M.; Jurewicz, K.; Vix-Gurtel, C.; Dentzer, J.; Frackowiak, E. State of hydrogen electrochemically stored using nanoporous carbons as negative electrode materials in an aqueous medium. Carbon 2006, 44, 2392–2398. [Google Scholar] [CrossRef]
- Demarconnay, L.; Raymundo-Piñero, E.; Beguin, F. A symmetric carbon/carbon supercapacitor operating at 1.6 V by using a neutral aqueous solution. Electrochem. Commun. 2010, 12, 1275–1278. [Google Scholar] [CrossRef]
- Fic, K.; Lota, G.; Meller, M.; Frackowiak, E. Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ. Sci. 2012, 5, 5842–5850. [Google Scholar] [CrossRef]
- Electrochemical Capacitor Performance: Influence of Aqueous Electrolytes. Available online: https://www.intechopen.com/books/supercapacitors-theoretical-and-practical-solutions/electrochemical-capacitor-performance-influence-of-aqueous-electrolytes (accessed on 30 June 2020).
- Karamanova, B.; Stoyanova, A.; Schipochka, M.; Girginov, C.A.; Stoyanova, R. On the cycling stability of biomass-derived carbons as electrodes in supercapacitors. J. Alloys Compd. 2019, 803, 882–890. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, S.; Yan, X.; Lyu, M.; Wang, L.; Bell, J. 2-Methylimidazole-Derived Ni–Co Layered Double Hydroxide Nanosheets as High Rate Capability and High Energy Density Storage Material in Hybrid Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 15510–15524. [Google Scholar] [CrossRef]
- Huang, J.; Xu, P.; Cao, D.; Zhou, X.; Wang, G. Asymmetric supercapacitors based on β-Ni(OH)2 nanosheets and activated carbon with high energy density. J. Power Sources 2014, 246, 371. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, H.; Fu, Y.; Abe, T.; Ogumi, Z. Temperature effects on the electrochemical behavior of spinel LiMn2O4 in quaternary ammonium-based ionic liquid electrolyte. J. Phys. Chem. 2005, 109, 13676–13684. [Google Scholar] [CrossRef]
- Garcia-Gomez, A.; Barranco, V.; Moreno-Fernandez, G.; Ibañez, J.; Centeno, T.; Rojo, J. Correlation between Capacitance and Porosity in Microporous Carbon Monoliths. J. Phys. Chem. C 2015, 17, 15687–15690. [Google Scholar] [CrossRef] [Green Version]
- Frackowiak, E.; Beguin, F. Carbon materials for the electrochemical storage of energy in Capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Song, S.; Ma, F.; Wu, G.; Ma, D.; Geng, W.; Wan, J. Facile self-templating large scale preparation of biomass-derived 3D hierarchical porous carbon for advanced supercapacitors. J. Mater. Chem. A 2015, 3, 18154–18162. [Google Scholar] [CrossRef]
- Ganguly, A.; Sharma, S.; Papakonstantinou, P.; Hamilton, P.J. Probing the Thermal Deoxygenation of Graphene Oxide Using High-Resolution In Situ X-ray-Based Spectroscopies. J. Phys. Chem. C 2011, 115, 17009–17019. [Google Scholar]
- Reiche, S.; Blume, R.; Zhao, X.C.; Su, D.; Kunkes, E.; Behrens, M.; Schlögl, R. Reactivity of mesoporous carbon against water—An in-situ XPS study. Carbon 2014, 77, 175–183. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal Stability and Reducibility of Oxygen-Containing Functional Groups on Multiwalled Carbon Nanotube Surfaces: A Quantitative High-Resolution XPS and TPD/TPR Study. J. Phys. Chem. 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Clark, D.; Dilks, A. Esca applied to polymers. XXIII. RF glow discharge modification of polymers in pure oxygen and helium–oxygen mixtures. J. Polym. Sci. Pol. Chem. 1979, 17, 957–976. [Google Scholar] [CrossRef]
- Fang, Y.; Luo, B.; Jia, Y.; Li, X.; Wang, B.; Song, Q.; Kang, F.; Zhi, L. Renewing Functionalized Graphene as Electrodes for High-Performance Supercapacitors. Adv. Mater. 2012, 24, 6348–6355. [Google Scholar] [CrossRef]




| Type of Ion | Size of Bare Ion (Å) [23] | Size of Hydrated Ions (Å) [23] | Ionic Conductivity (S cm2mol−1) [23] | YP-50F | YP-80F | ||||
|---|---|---|---|---|---|---|---|---|---|
| iR, V | C, Fg−1 | CS, % | iR, V | C, Fg−1 | CS, % | ||||
| Li+ | 0.60 | 3.82 | 38.69 | 0.024 | 113 | 95.4 | 0.028 | 114 | 95.0 |
| Na+ | 0.95 | 3.58 | 50.11 | 0.026 | 108 | 92.1 | 0.028 | 110 | 91.5 |
| K+ | 1.33 | 3.31 | 73.50 | 0.023 | 115 | 96.6 | 0.026 | 117 | 96.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamanova, B.; Stoyanova, A.; Shipochka, M.; Veleva, S.; Stoyanova, R. Effect of Alkaline-Basic Electrolytes on the Capacitance Performance of Biomass-Derived Carbonaceous Materials. Materials 2020, 13, 2941. https://doi.org/10.3390/ma13132941
Karamanova B, Stoyanova A, Shipochka M, Veleva S, Stoyanova R. Effect of Alkaline-Basic Electrolytes on the Capacitance Performance of Biomass-Derived Carbonaceous Materials. Materials. 2020; 13(13):2941. https://doi.org/10.3390/ma13132941
Chicago/Turabian StyleKaramanova, Boryana, Antonia Stoyanova, Maria Shipochka, Svetlana Veleva, and Radostina Stoyanova. 2020. "Effect of Alkaline-Basic Electrolytes on the Capacitance Performance of Biomass-Derived Carbonaceous Materials" Materials 13, no. 13: 2941. https://doi.org/10.3390/ma13132941
APA StyleKaramanova, B., Stoyanova, A., Shipochka, M., Veleva, S., & Stoyanova, R. (2020). Effect of Alkaline-Basic Electrolytes on the Capacitance Performance of Biomass-Derived Carbonaceous Materials. Materials, 13(13), 2941. https://doi.org/10.3390/ma13132941






