Graphitic Carbon Nitride for Photocatalytic Air Treatment
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation and Characterization
2.2. Photodegradation Process Setup—NO and Acetaldehyde
2.3. EPR Measurements
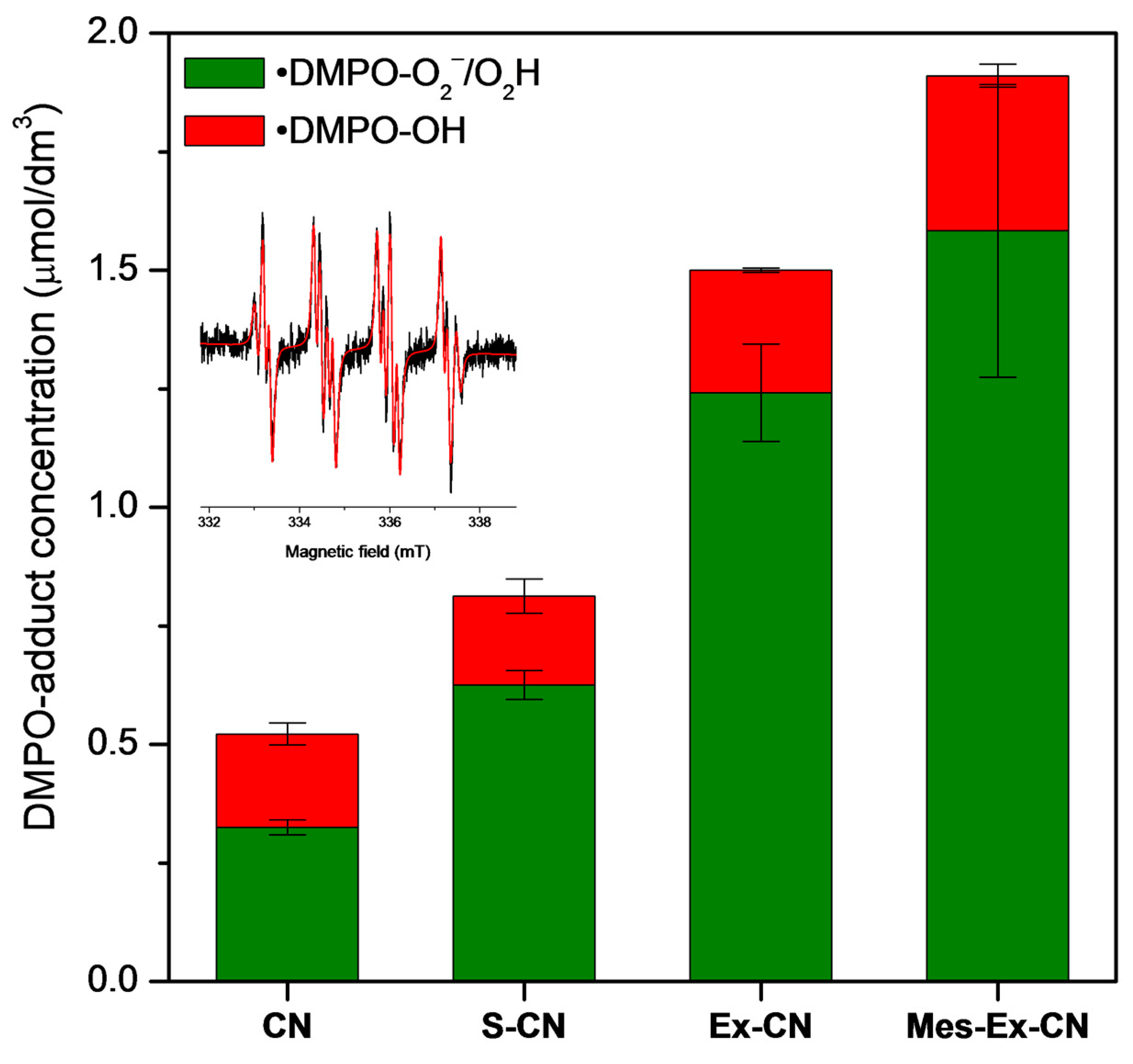
3. Results
3.1. Acetaldehyde Removal
3.2. NOx Removal
3.3. Mechanism of Photocatalytic Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Praus, P.; Svoboda, L.; Ritz, M.; Troppová, I.; Šihor, M.; Kočí, K. Graphitic carbon nitride: Synthesis, characterization and photocatalytic decomposition of nitrous oxide. Mater. Chem. Phys. 2017, 193, 438–446. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Malloy, C.D.; Marr, J.S. Sick-building syndrome. Lancet 1997, 349, 1013–1016. [Google Scholar] [CrossRef]
- ISO 22197-2: 2011. Fine Ceramics, Advanced Technical Ceramics—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials—Part 2: Removal of Acetaldehyde; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Dvoranová, D.; Brezová, V.; Dimotikali, D.; Trapalis, C. Selective removal of organic and inorganic air pollutants by adjusting the g-C3N4/TiO2 ratio. Catal. Today 2019. [Google Scholar] [CrossRef]
- Mills, A.; Hill, C.; Robertson, P.K.J. Overview of the current ISO tests for photocatalytic materials. J. Photochem. Photobiol. A Chem. 2012, 237, 7–23. [Google Scholar] [CrossRef]
- ISO 22197-1: 2016 (en). Fine Ceramics, Advanced Technical Ceramics—Test Method for Air-Purification Performance of Semiconducting Photocatalytic Materials—Part 1: Removal of Nitric Oxide; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Praus, P.; Smýkalová, A.; Foniok, K.; Velíšek, P.; Cvejn, D.; Žádný, J.; Storch, J. Post-synthetic derivatization of graphitic carbon nitride with methanesulfonyl chloride: Synthesis, characterization and photocatalysis. Nanomaterials 2020, 10, 193. [Google Scholar] [CrossRef] [Green Version]
- Svoboda, L.; Praus, P.; Lima, M.J.; Sampaio, M.J.; Matýsek, D.; Ritz, M.; Dvorský, R.; Faria, J.L.; Silva, C.G. Graphitic carbon nitride nanosheets as highly efficient photocatalysts for phenol degradation under high-power visible LED irradiation. Mater. Res. Bull. 2018, 100, 322–332. [Google Scholar] [CrossRef]
- Krýsa, J.; Baudys, M.; Vislocka, X.; Neumann-Spallart, M. Composite photocatalysts based on TiO2– carbon for air pollutant removal: Aspects of adsorption. Catal. Today 2020, 340, 34–39. [Google Scholar] [CrossRef]
- Paušová, Š.; Riva, M.; Baudys, M.; Krýsa, J.; Barbieriková, Z.; Brezová, V. Composite materials based on active carbon/TiO2 for photocatalytic water purification. Catal. Today 2019, 328, 178–182. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Dvoranová, D.; Mazúr, M.; Papailias, I.; Giannakopoulou, T.; Trapalis, C.; Brezová, V. EPR investigations of G-C3N4/TiO2 nanocomposites. Catalysts 2018, 8, 47. [Google Scholar] [CrossRef] [Green Version]
- Hollmann, D.; Karnahl, M.; Tschierlei, S.; Kailasam, K.; Schneider, M.; Radnik, J.; Grabow, K.; Bentrup, U.; Junge, H.; Beller, M.; et al. Structure–activity relationships in bulk polymeric and sol–gel-derived carbon nitrides during photocatalytic hydrogen production. Chem. Mater. 2014, 26, 1727–1733. [Google Scholar] [CrossRef]
- Dvoranová, D.; Barbieriková, Z.; Mazúr, M.; García-López, E.I.; Marcì, G.; Lušpai, K.; Brezová, V. EPR investigations of polymeric and H2O2-modified C3N4-based photocatalysts. J. Photochem. Photobiol. A Chem. 2019, 375, 100–113. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs thermal exfoliation of g-3N4 for NOx removal under visible light irradiation. Appl. Catal. B Environ. 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, G.; Tian, Y.; Yan, L.; Deng, M.; Yang, B.; Kang, Z.; Sun, H. Hydroxyl decorated g-3N4 nanoparticles with narrowed bandgap for high efficient photocatalyst design. Appl. Catal. B Environ. 2019, 244, 262–271. [Google Scholar] [CrossRef]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 224–245. [Google Scholar] [CrossRef] [Green Version]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef] [Green Version]
- Alberti, A.; Macciantelli, D. Electron Paramagnetic Resonance: A Practitioner’s Toolkit; Brustolon, M., Giamello, E., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009; 287p. [Google Scholar]
- Paušová, Š.; Pacileo, L.; Baudys, M.; Hrubantová, A.; Neumann-Spallart, M.; Dvoranová, D.; Brezova, V.; Krýsa, J. Active carbon/TiO2 composites for photocatalytic decomposition of benzoic acid in water and toluene in air. Catal. Today 2020. [Google Scholar] [CrossRef]
- Nikokavoura, A.; Trapalis, C. Graphene and g-C3N4 based photocatalysts for NOx removal: A review. Appl. Surf. Sci. 2018, 430, 18–52. [Google Scholar] [CrossRef]












| Sample Name | Preparation | BET Surface Area (m2/g) | Phase Composition (XRD, XRF) |
|---|---|---|---|
| TiO2 P25 | (Producer: Evonik), flame hydrolysis of TiCl4 | 45 | 70% anatase 30% rutile |
| CG100 | (Producer: Precheza), sulphate process | 70–110 * | 100% anatase |
| CN | Thermal treatment of melamine | 11 ** | — |
| Ex-CN | Thermal treatment of melamine following thermal exfoliation | 90 ** | — |
| S-CN | Thermal treatment of thiourea | 20 ** | Sulphur content XRF 0.22 (weight %) ** |
| Mes-Ex-CN | Ex-CN with modification using methanesulfonyl | 67 ** | Sulphur content XRF 0.56 (weight %) ** |
| Standard | ISO 22197-1 | ISO 22197-2 |
|---|---|---|
| pollutant | NOx | acetaldehyde |
| inlet concentration (ppm) | 1 | 5 |
| flow rate (dm3/min) | 3 | 1 |
| UV irradiance (mW/cm2) | 1 | 1 |
| analysis | chemiluminescence analyzer | GC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baudys, M.; Paušová, Š.; Praus, P.; Brezová, V.; Dvoranová, D.; Barbieriková, Z.; Krýsa, J. Graphitic Carbon Nitride for Photocatalytic Air Treatment. Materials 2020, 13, 3038. https://doi.org/10.3390/ma13133038
Baudys M, Paušová Š, Praus P, Brezová V, Dvoranová D, Barbieriková Z, Krýsa J. Graphitic Carbon Nitride for Photocatalytic Air Treatment. Materials. 2020; 13(13):3038. https://doi.org/10.3390/ma13133038
Chicago/Turabian StyleBaudys, Michal, Šárka Paušová, Petr Praus, Vlasta Brezová, Dana Dvoranová, Zuzana Barbieriková, and Josef Krýsa. 2020. "Graphitic Carbon Nitride for Photocatalytic Air Treatment" Materials 13, no. 13: 3038. https://doi.org/10.3390/ma13133038





