Effect of Isomeric Amine Chain Extenders and Crosslink Density on the Properties of Liquid Crystal Elastomers
Abstract
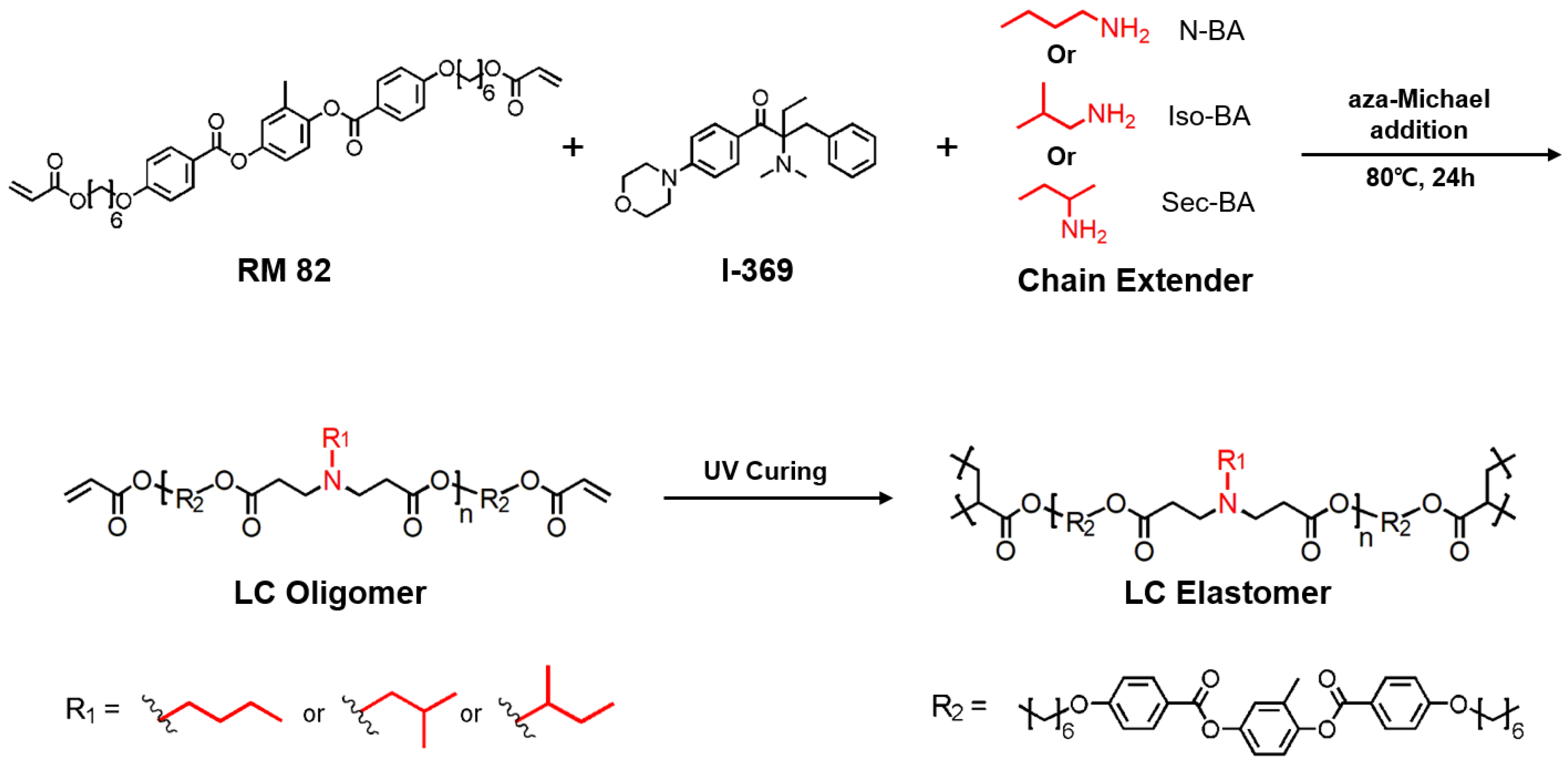
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Cells
2.3. Synthesis of Surface-Aligned Liquid Crystal Elastomers
2.4. Synthesis of Polydomain Liquid Crystal Elastomers
2.5. Gel Fraction
2.6. Characterization
3. Results and Discussion
3.1. Synthesis of LCEs
3.2. Molecular Characterization
3.3. Thermal, Viscoelastic, and Mechanical Properties
3.4. Thermal Actuation Properties
3.5. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- White, T.J.; Broer, D.J. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015, 14, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, R.S.; Kim, H.; Boothby, J.M.; Ware, T.H. Liquid crystal elastomer actuators: Synthesis, alignment, and applications. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 395–411. [Google Scholar] [CrossRef]
- Ohm, C.; Brehmer, M.; Zentel, R. Liquid crystalline elastomers as actuators and sensors. Adv. Mater. 2010, 22, 3366–3387. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.M.; Donovan, B.R.; White, T.J. Materials as machines. Adv. Mater. 2020, 32, 1906564. [Google Scholar] [CrossRef]
- Kowalski, B.A.; Guin, T.C.; Auguste, A.D.; Godman, N.P.; White, T.J. Pixelated polymers: Directed self assembly of liquid crystalline polymer networks. ACS Macro Lett. 2017, 6, 436–441. [Google Scholar] [CrossRef]
- Modes, C.; Warner, M. Shape-programmable materials. Phys. Today 2016, 69, 32–38. [Google Scholar] [CrossRef]
- He, Q.; Wang, Z.; Song, Z.; Cai, S. Bioinspired design of vascular artificial muscle. Adv. Mater. Technol. 2019, 4, 1800244. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Wang, Z.; Wang, Y.; Minori, A.; Tolley, M.T.; Cai, S. Electrically controlled liquid crystal elastomer–based soft tubular actuator with multimodal actuation. Sci. Adv. 2019, 5, eaax5746. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, Z.; Yang, Y.; Chen, Q.; Qian, X.; Wu, Y.; Liang, H.; Xu, Y.; Wei, Y.; Ji, Y. Seamless multimaterial 3D liquid-crystalline elastomer actuators for next-generation entirely soft robots. Sci. Adv. 2020, 6, eaay8606. [Google Scholar] [CrossRef] [Green Version]
- Rogóż, M.; Zeng, H.; Xuan, C.; Wiersma, D.S.; Wasylczyk, P. Light-driven soft robot mimics caterpillar locomotion in natural scale. Adv. Opt. Mater. 2016, 4, 1689–1694. [Google Scholar] [CrossRef]
- Liu, D.; Bastiaansen, C.W.M.; den Toonder, J.M.J.; Broer, D.J. Photo-switchable surface topologies in chiral nematic coatings. Angew. Chem. Int. Ed. 2012, 51, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.E.; Ji, Y.; Torras, N.; Zinoviev, K.; Terentjev, E.M. Carbon-nanotube sensitized nematic elastomer composites for IR-visible photo-actuation. Soft Matter 2012, 8, 1570–1574. [Google Scholar] [CrossRef]
- Babakhanova, G.; Krieger, J.; Li, B.-X.; Turiv, T.; Kim, M.-H.; Lavrentovich, O.D. Cell alignment by smectic liquid crystal elastomer coatings with nanogrooves. J. Biomed. Mater. Res. Part A 2020, 108, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Küpfer, J.; Finkelmann, H. Nematic liquid single crystal elastomers. Macromol. Rapid Commun. 1991, 12, 717–726. [Google Scholar] [CrossRef]
- Donnio, B.; Wermter, H.; Finkelmann, H. A simple and versatile synthetic route for the preparation of main-chain, liquid-crystalline elastomers. Macromolecules 2000, 33, 7724–7729. [Google Scholar] [CrossRef]
- Li, M.-H.; Keller, P.; Yang, J.; Albouy, P.-A. An artificial muscle with lamellar structure based on a nematic triblock copolymer. Adv. Mater. 2004, 16, 1922–1925. [Google Scholar] [CrossRef]
- Fleischmann, E.-K.; Forst, F.R.; Zentel, R. Liquid-crystalline elastomer fibers prepared in a microfluidic device. Macromol. Chem. Phys. 2014, 215, 1004–1011. [Google Scholar] [CrossRef]
- Ahn, S.-k.; Deshmukh, P.; Kasi, R.M. Shape memory behavior of side-chain liquid crystalline polymer networks triggered by dual transition temperatures. Macromolecules 2010, 43, 7330–7340. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Saed, M.; Nair, D.P.; Gong, T.; Reed, S.M.; Bowman, C.N. Tailorable and programmable liquid-crystalline elastomers using a two-stage thiol–acrylate reaction. RSC Adv. 2015, 5, 18997–19001. [Google Scholar] [CrossRef]
- Ware, T.H.; Perry, Z.P.; Middleton, C.M.; Iacono, S.T.; White, T.J. Programmable liquid crystal elastomers prepared by thiol–ene photopolymerization. ACS Macro Lett. 2015, 4, 942–946. [Google Scholar] [CrossRef]
- Saed, M.O.; Ambulo, C.P.; Kim, H.; De, R.; Raval, V.; Searles, K.; Siddiqui, D.A.; Cue, J.M.O.; Stefan, M.C.; Shankar, M.R.; et al. Molecularly-engineered, 4D-printed liquid crystal elastomer actuators. Adv. Funct. Mater. 2019, 29, 1806412. [Google Scholar] [CrossRef]
- Yu, L.; Shahsavan, H.; Rivers, G.; Zhang, C.; Si, P.; Zhao, B. Programmable 3D shape changes in liquid crystal polymer networks of uniaxial orientation. Adv. Funct. Mater. 2018, 28, 1802809. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, H.; He, Q.; Cai, S. Reprogrammable, reprocessible, and self-healable liquid crystal elastomer with exchangeable disulfide bonds. ACS Appl. Mater. Interfaces 2017, 9, 33119–33128. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.; Verduzco, R. Direct shape programming of liquid crystal elastomers. Soft Matter 2019, 15, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Merkel, D.R.; Traugutt, N.A.; Visvanathan, R.; Yakacki, C.M.; Frick, C.P. Thermomechanical properties of monodomain nematic main-chain liquid crystal elastomers. Soft Matter 2018, 14, 6024–6036. [Google Scholar] [CrossRef]
- Godman, N.P.; Kowalski, B.A.; Auguste, A.D.; Koerner, H.; White, T.J. Synthesis of elastomeric liquid crystalline polymer networks via chain transfer. ACS Macro Lett. 2017, 6, 1290–1295. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, X.; Yang, S. Instant locking of molecular ordering in liquid crystal elastomers by oxygen-mediated thiol–acrylate click reactions. Angew. Chem. Int. Ed. 2018, 57, 5665–5668. [Google Scholar] [CrossRef] [PubMed]
- McBride, M.K.; Martinez, A.M.; Cox, L.; Alim, M.; Childress, K.; Beiswinger, M.; Podgorski, M.; Worrell, B.T.; Killgore, J.; Bowman, C.N. A readily programmable, fully reversible shape-switching material. Sci. Adv. 2018, 4, eaat4634. [Google Scholar] [CrossRef] [Green Version]
- Ware, T.H.; McConney, M.E.; Wie, J.J.; Tondiglia, V.P.; White, T.J. Voxelated liquid crystal elastomers. Science 2015, 347, 982–984. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.-H.; Kim, D.-Y.; Jeong, K.-U.; Ahn, S.-k. Surface aligned main-chain liquid crystalline elastomers: Tailored properties by the choice of amine chain extenders. Macromolecules 2018, 51, 1141–1149. [Google Scholar] [CrossRef]
- Lee, J.; Guo, Y.; Choi, Y.-J.; Jung, S.; Seol, D.; Choi, S.; Kim, J.-H.; Kim, Y.; Jeong, K.-U.; Ahn, S.-k. Mechanically programmed 2D and 3D liquid crystal elastomers at macro- and microscale via two-step photocrosslinking. Soft Matter 2020, 16, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Yang, Y.; Chen, Q.; Terentjev, E.M.; Wei, Y.; Ji, Y. Mouldable liquid-crystalline elastomer actuators with exchangeable covalent bonds. Nat. Mater. 2014, 13, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.; Yang, Y.; Xu, Y.; Qian, X.; Wei, Y.; Ji, Y. Durable liquid-crystalline vitrimer actuators. Chem. Sci. 2019, 10, 3025–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saed, M.O.; Torbati, A.H.; Starr, C.A.; Visvanathan, R.; Clark, N.A.; Yakacki, C.M. Thiol-acrylate main-chain liquid-crystalline elastomers with tunable thermomechanical properties and actuation strain. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 157–168. [Google Scholar] [CrossRef]
- Traugutt, N.A.; Volpe, R.H.; Bollinger, M.S.; Saed, M.O.; Torbati, A.H.; Yu, K.; Dadivanyan, N.; Yakacki, C.M. Liquid-crystal order during synthesis affects main-chain liquid-crystal elastomer behavior. Soft Matter 2017, 13, 7013–7025. [Google Scholar] [CrossRef]
- Ware, T.H.; White, T.J. Programmed liquid crystal elastomers with tunable actuation strain. Polym. Chem. 2015, 6, 4835–4844. [Google Scholar] [CrossRef]
- Kim, H.; Boothby, J.M.; Ramachandran, S.; Lee, C.D.; Ware, T.H. Tough, shape-changing materials: Crystallized liquid crystal elastomers. Macromolecules 2017, 50, 4267–4275. [Google Scholar] [CrossRef]
- Saed, M.O.; Volpe, R.H.; Traugutt, N.A.; Visvanathan, R.; Clark, N.A.; Yakacki, C.M. High strain actuation liquid crystal elastomers via modulation of mesophase structure. Soft Matter 2017, 13, 7537–7547. [Google Scholar] [CrossRef]
- Lu, H.-F.; Wang, M.; Chen, X.-M.; Lin, B.-P.; Yang, H. Interpenetrating liquid-crystal polyurethane/polyacrylate elastomer with ultrastrong mechanical property. J. Am. Chem. Soc. 2019, 141, 14364–14369. [Google Scholar] [CrossRef]
- Guo, Y.; Lee, J.; Son, J.; Ahn, S.-k.; Carrillo, J.-M.Y.; Sumpter, B.G. Decoding liquid crystal oligomer phase transitions: Toward molecularly engineered shape changing materials. Macromolecules 2019, 52, 6878–6888. [Google Scholar] [CrossRef]
- Ahn, S.-k.; Ware, T.H.; Lee, K.M.; Tondiglia, V.P.; White, T.J. Photoinduced topographical feature development in blueprinted azobenzene-functionalized liquid crystalline elastomers. Adv. Funct. Mater. 2016, 26, 5819–5826. [Google Scholar] [CrossRef]
- Aramaki, K. Semi-quantitative evaluation of steric effect on corrosion inhibition efficiency of branched alkyl amines. Corros. Eng. 1977, 26, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Karasz, F.E.; MacKnight, W.J. The influence of stereoregularity on the glass transition temperatures of vinyl polymers. Macromolecules 1968, 1, 537–540. [Google Scholar] [CrossRef]
- Lee, K.M.; Koerner, H.; Vaia, R.A.; Bunning, T.J.; White, T.J. Relationship between the photomechanical response and the thermomechanical properties of azobenzene liquid crystalline polymer networks. Macromolecules 2010, 43, 8185–8190. [Google Scholar] [CrossRef]
- Thomsen, D.L.; Keller, P.; Naciri, J.; Pink, R.; Jeon, H.; Shenoy, D.; Ratna, B.R. Liquid crystal elastomers with mechanical properties of a muscle. Macromolecules 2001, 34, 5868–5875. [Google Scholar] [CrossRef]
- Zhan, P.; Zhang, W.; Jacobs, I.E.; Nisson, D.M.; Xie, R.; Weissen, A.R.; Colby, R.H.; Moulé, A.J.; Milner, S.T.; Maranas, J.K.; et al. Side chain length affects backbone dynamics in poly(3-alkylthiophene)s. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Galuska, L.; Qian, Z.; Zhang, S.; Huang, L.; Prine, N.; Li, T.; He, Y.; Hong, K.; Gu, X. The effect of side-chain branch position on the thermal properties of poly(3-alkylthiophenes). Polym. Chem. 2020, 11, 517–526. [Google Scholar] [CrossRef]







| Sample | Acryl/Amine Ratio a | Mn (g/mol) b | Tni (°C) c | Tg (°C) d | Td (°C) e | E// (MPa) f | E⊥ (MPa) g | G (%) h | |
|---|---|---|---|---|---|---|---|---|---|
| LC Mixture | LC Oligomer | LC elastomer | |||||||
| LCE-N1 | 1.81 | 1720 | 101 | 106 | 19 | 289 | 173 | 37 | 92 |
| LCE-N2 | 1.33 | 2610 | 98 | 105 | 7 | 273 | 35 | 10 | 89 |
| LCE-I1 | 1.47 | 1720 | 100 | 108 | 27 | 299 | 284 | 94 | 91 |
| LCE-I2 | 1.03 | 2610 | 98 | 106 | 14 | 290 | 43 | 19 | 89 |
| LCE-S1 | 1.01 | 1570 | 103 | 105 | 23 | 280 | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Choi, S.; Kang, B.-G.; Ahn, S.-k. Effect of Isomeric Amine Chain Extenders and Crosslink Density on the Properties of Liquid Crystal Elastomers. Materials 2020, 13, 3094. https://doi.org/10.3390/ma13143094
Lee Y, Choi S, Kang B-G, Ahn S-k. Effect of Isomeric Amine Chain Extenders and Crosslink Density on the Properties of Liquid Crystal Elastomers. Materials. 2020; 13(14):3094. https://doi.org/10.3390/ma13143094
Chicago/Turabian StyleLee, Yoojin, Subi Choi, Beom-Goo Kang, and Suk-kyun Ahn. 2020. "Effect of Isomeric Amine Chain Extenders and Crosslink Density on the Properties of Liquid Crystal Elastomers" Materials 13, no. 14: 3094. https://doi.org/10.3390/ma13143094




