Facile Synthesis of Cauliflower Leaves Biochar at Low Temperature in the Air Atmosphere for Cu(II) and Pb(II) Removal from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Chemical Reagents and Materials
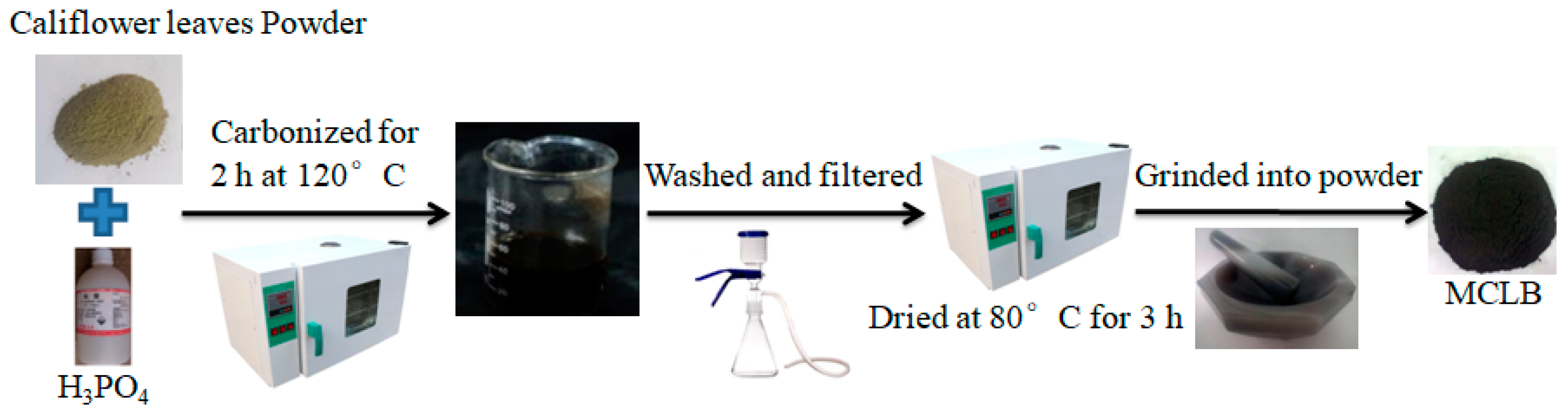
2.2. Biochar Preparation
2.3. Biochar Characterizations
2.4. Adsorption Kinetics and Isotherm Experiments
2.5. Influence of Initial pH
2.6. Competitive Adsorption
2.7. Adsorption and Desorption Experiments
2.8. Statistical Analyses
3. Results and Discussion
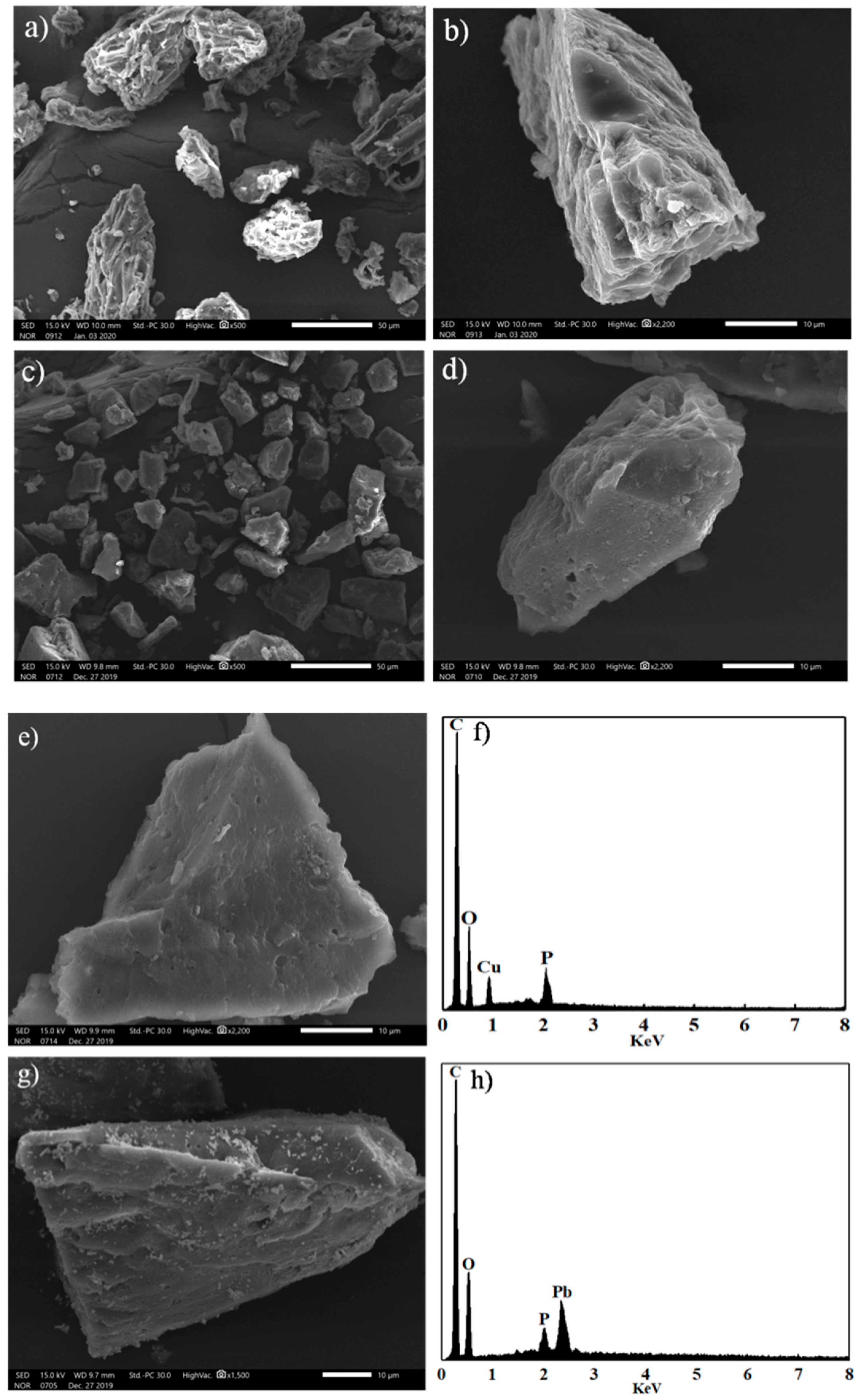
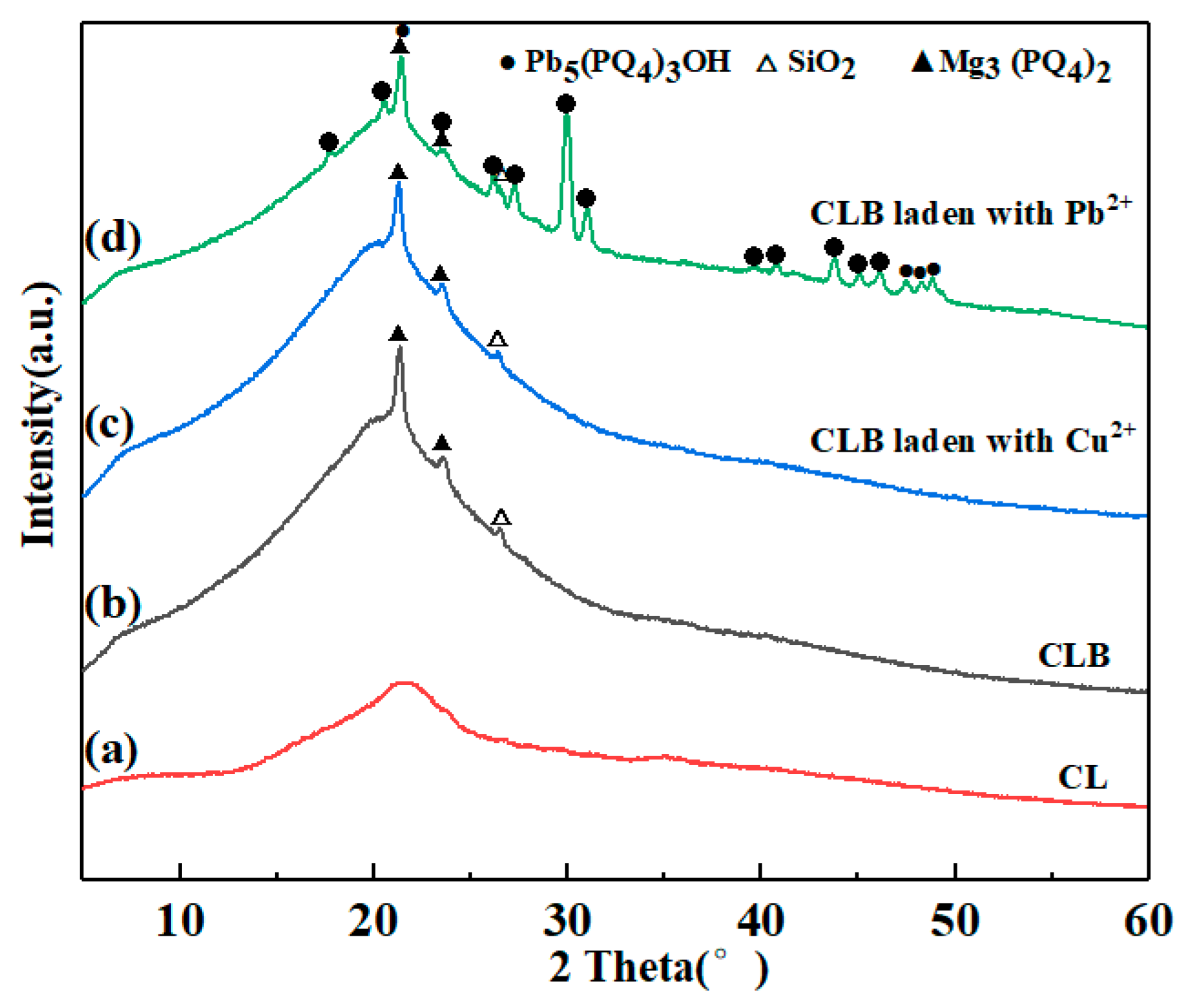
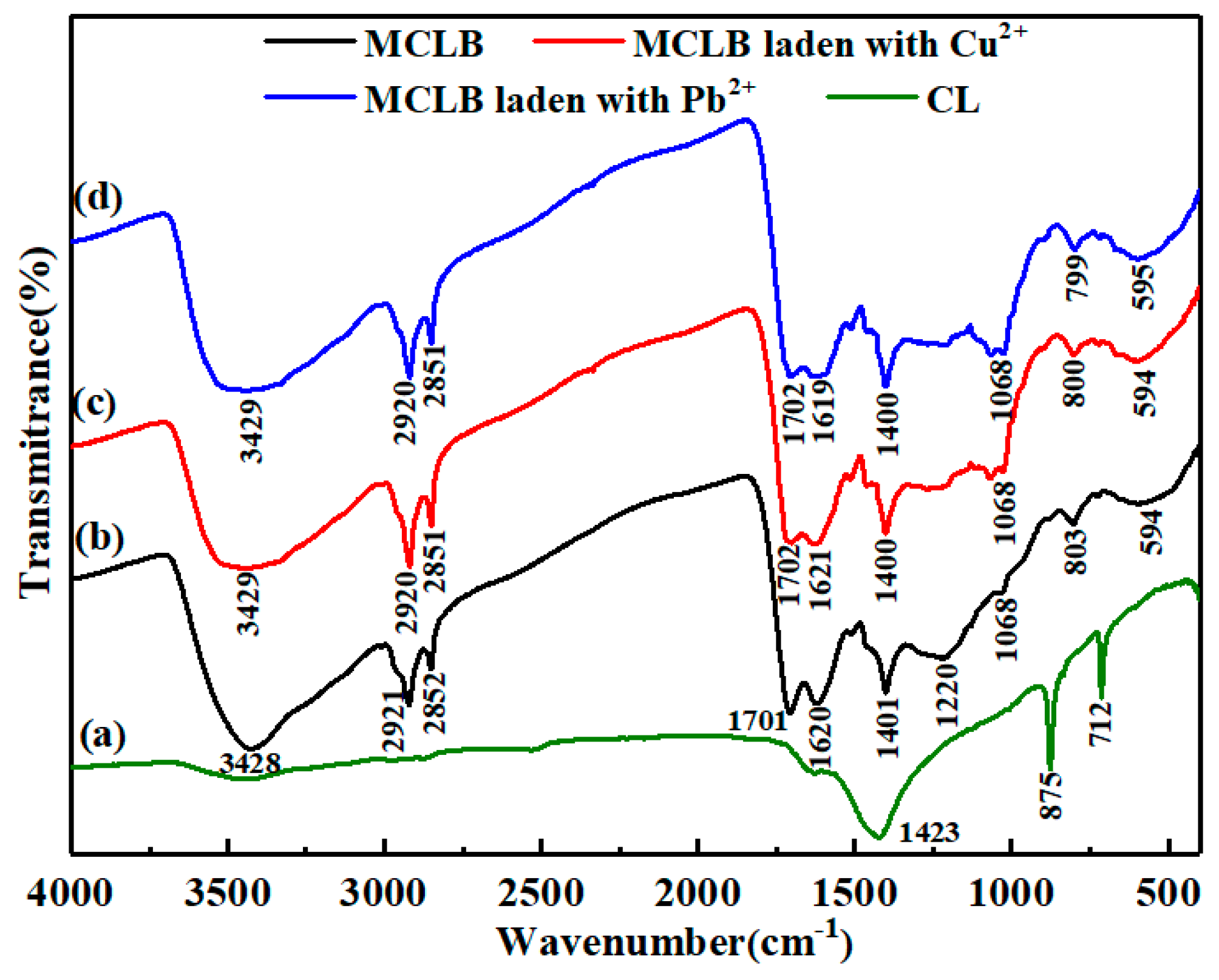
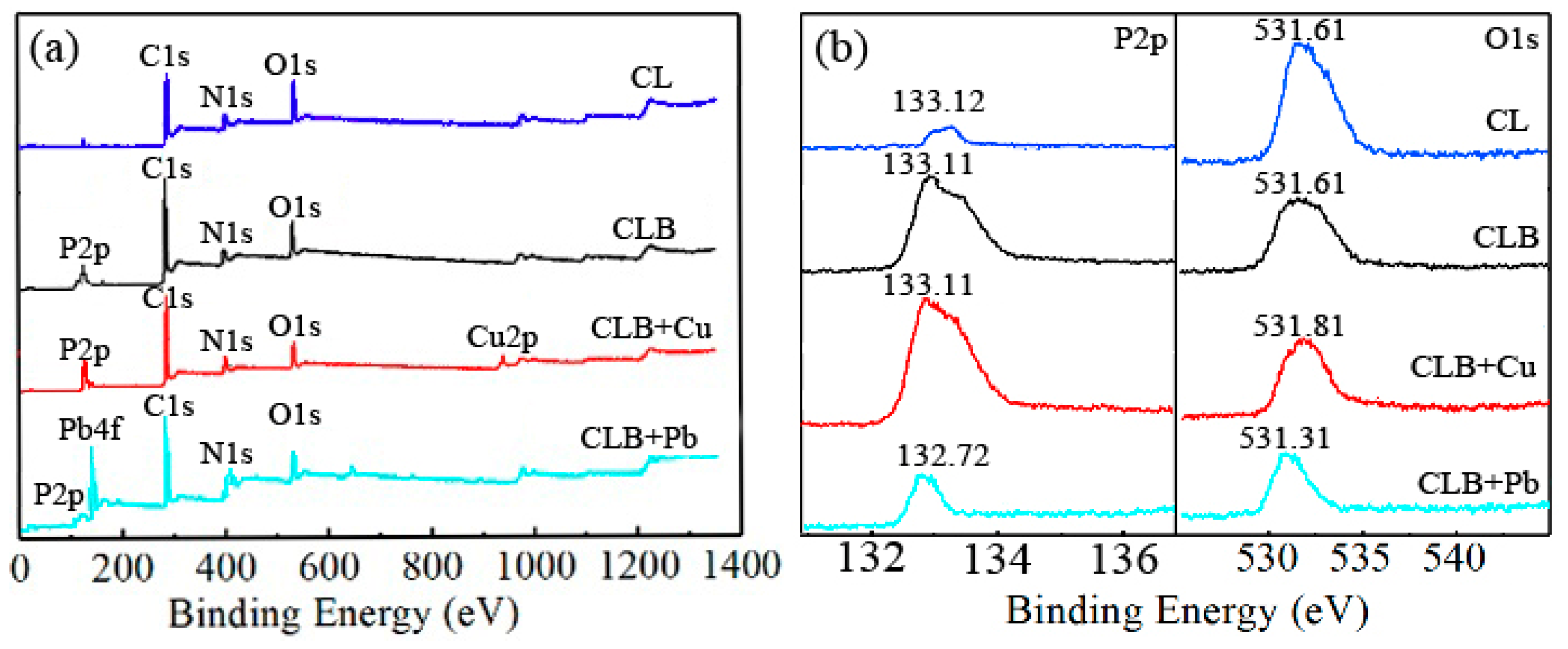
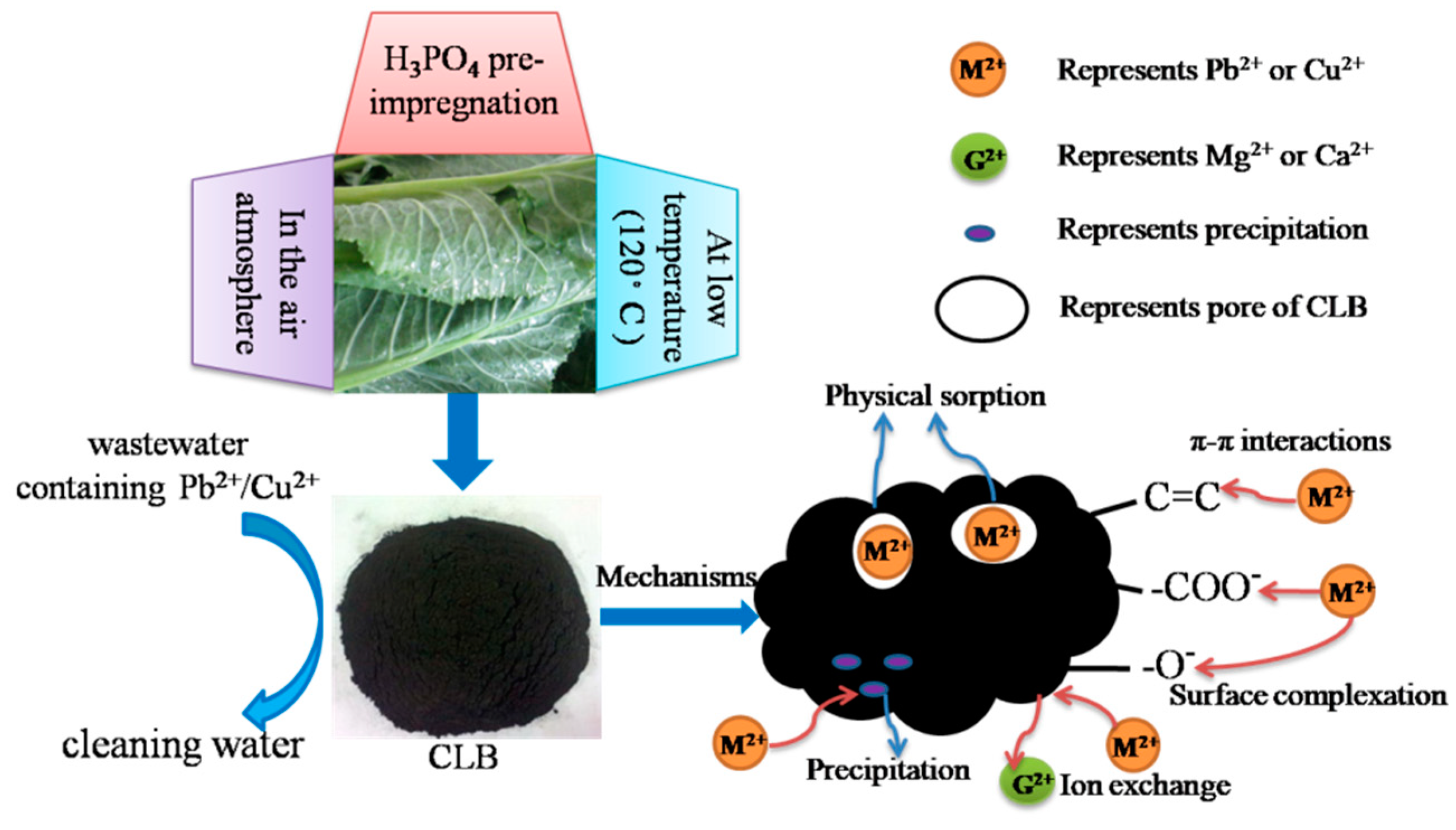
3.1. Characterization and Preparation Mechanism of CLB
3.2. Cu(II) and Pb(II) Removal Mechanisms of CLB
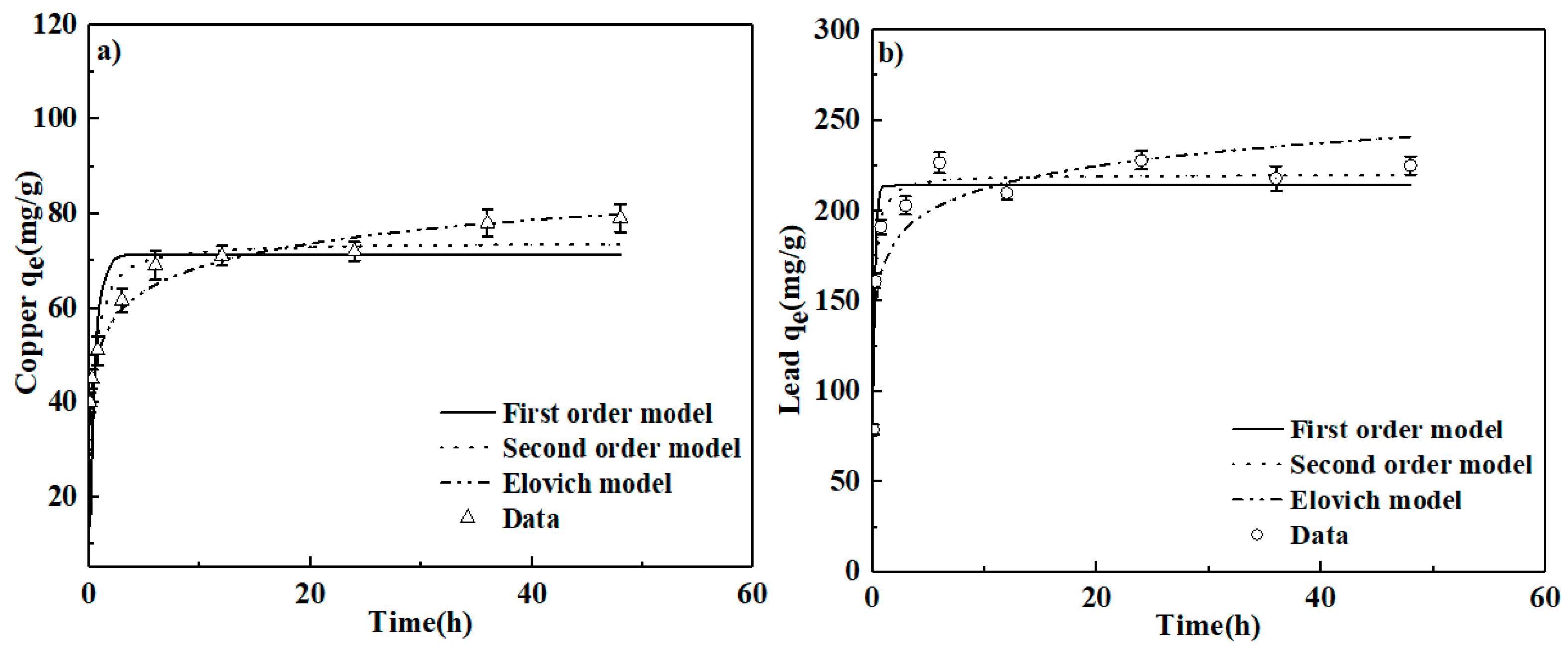
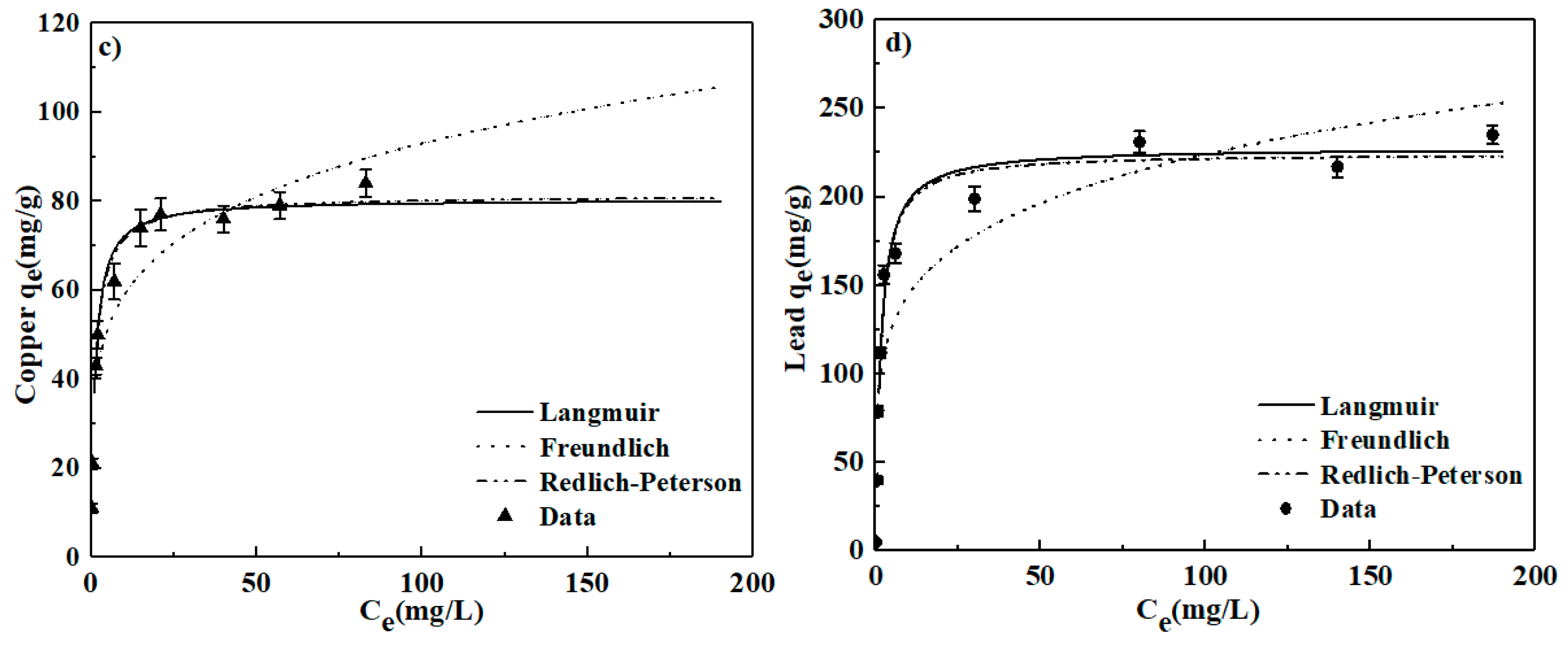
3.3. Adsorption Kinetics and Isotherms
3.4. pH Influence
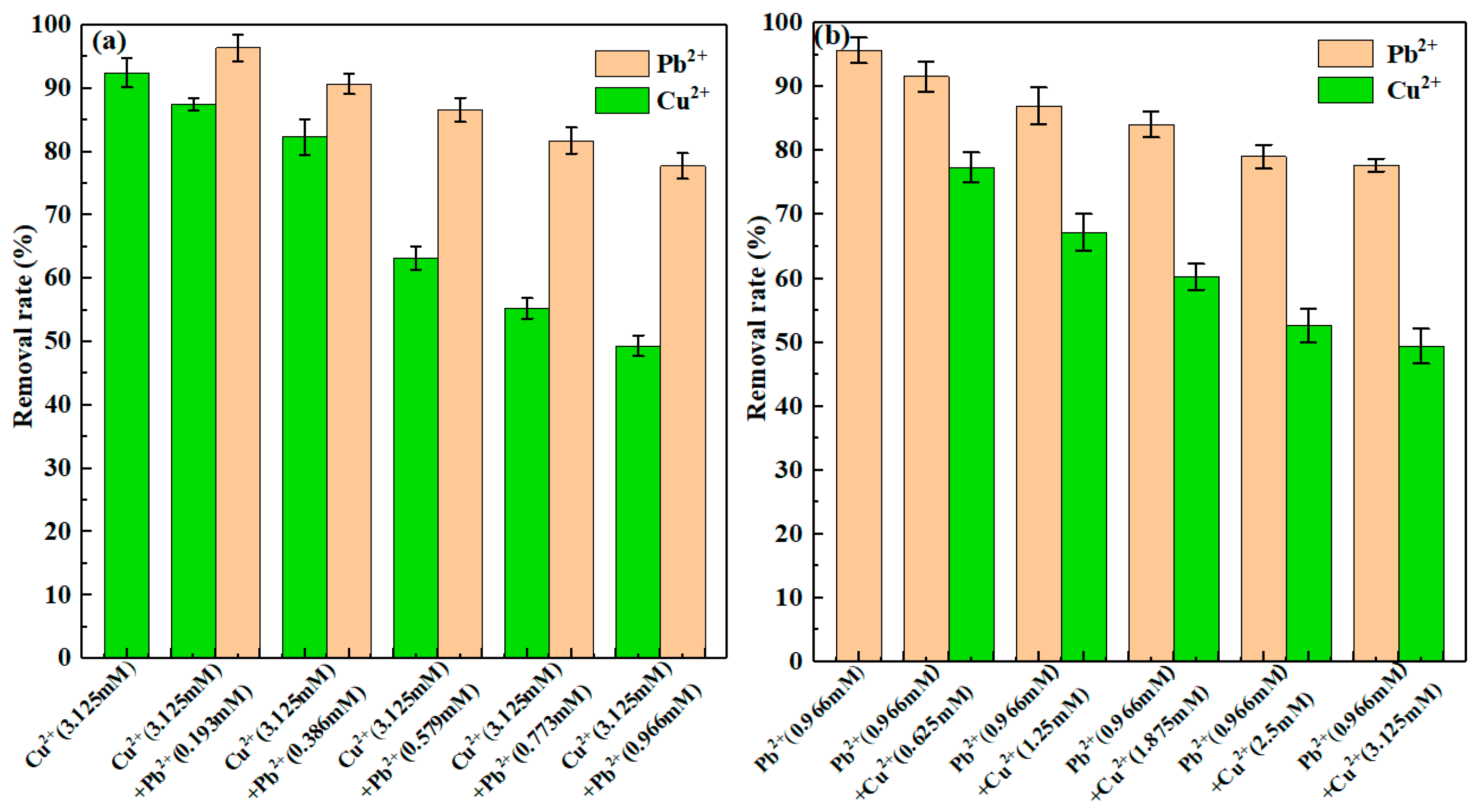
3.5. Competitive Sorption
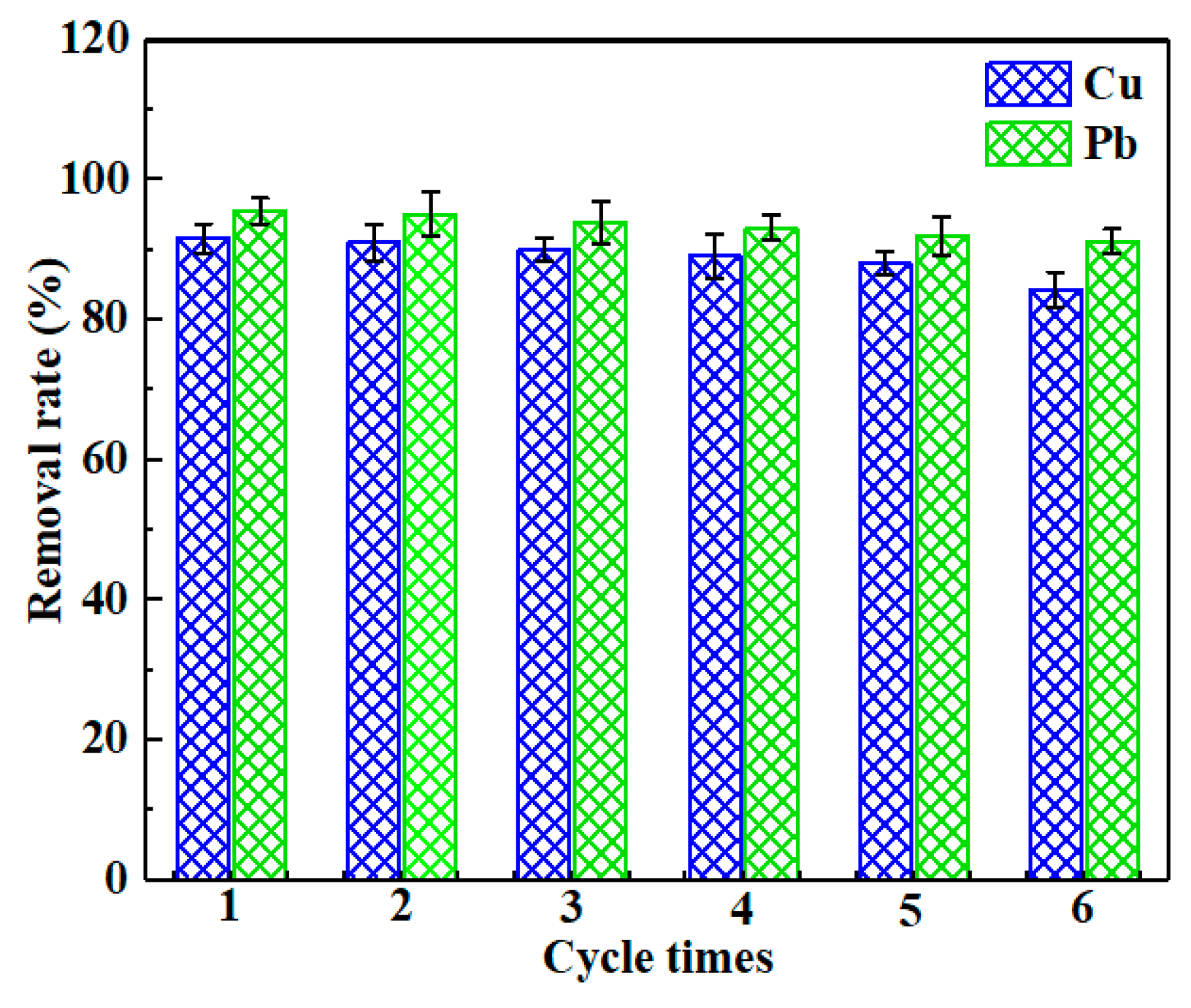
3.6. Reusability of CLB
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, M.; Qin, X.; Zeng, G.; Li, J. Impacts of human activity modes and climate on heavy metal “spread” in groundwater are biased. Chemosphere 2016, 152, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; de Mello, J.M.M.; Fiori, M.A.; Oliveira, J.V.; Dal Magro, J. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Yuan, M.; Xie, T.; Yan, G.; Chen, Q.; Wang, L. Effective removal of Pb2+ from aqueous solutions by magnetically modified zeolite. Powder Technol. 2018, 332, 234–241. [Google Scholar] [CrossRef]
- Sani, H.A.; Ahmad, M.B.; Hussein, M.Z.; Ibrahim, N.A.; Musa, A.; Saleh, T.A. Nanocomposite of ZnO with montmorillonite for removal of lead and copper ions from aqueous solutions. Process Saf. Environ. Prot. 2017, 109, 97–105. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Wang, S.; Peng, J.; Cui, W. Sulfoethyl functionalized silica nano-particle as an adsorbent to selectively adsorb silver ions from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2017, 71, 330–337. [Google Scholar] [CrossRef]
- Abdelhafez, A.A.; Li, J. Removal of Pb(II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel. J. Taiwan Inst. Chem. Eng. 2016, 61, 367–375. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Gao, R.; Fu, Q.; Hu, H.; Wang, Q.; Liu, Y.; Zhu, J. Highly-effective removal of Pb by co-pyrolysis biochar derived from rape straw and orthophosphate. J. Hazard. Mater. 2019, 371, 191–197. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Todd, O.A.; Crowley, M.; Ricchetti, L.; Pittman, C.U.; Anderson, R.; Mohan, D.; Mlsna, T. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. J. Chem. Eng. 2018, 331, 480–491. [Google Scholar] [CrossRef]
- Wu, J.; Huang, D.; Liu, X.; Meng, J.; Tang, C.; Xu, J. Remediation of As(III) and Cd(II) co-contamination and its mechanism in aqueous systems by a novel calcium-based magnetic biochar. J. Hazard. Mater. 2018, 348, 10–19. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Simsir, H.; Eltugral, N.; Karagoz, S. Hydrothermal carbonization for the preparation of hydrochars from glucose, cellulose, chitin, chitosan and wood chips via low-temperature and their characterization. Bioresour. Technol. 2017, 246, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Xu, X.; Hu, X.; Ding, Z.; Chen, Y. Effects of copyrolysis of sludge with calcium carbonate and calcium hydrogen phosphate on chemical stability of carbon and release of toxic elements in the resultant biochars. Chemosphere 2017, 189, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, S.; Banik, C.; Rathke, S.J.; Laird, D.A. Arsenic sorption on zero-valent iron-biochar complexes. Water Res. 2018, 137, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Zhou, N.; Chen, H.; Feng, Q.; Yao, D.; Chen, H.; Wang, H.; Zhou, Z.; Li, H.; Tian, Y.; Lu, X. Effect of phosphoric acid on the surface properties and Pb(II) adsorption mechanisms of hydrochars prepared from fresh banana peels. J. Clean. Prod. 2017, 165, 221–230. [Google Scholar] [CrossRef]
- Chen, H.; Li, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, Z.; Li, Y.; Zhang, Y. Adsorption of cadmium and lead ions by phosphoric acid-modified biochar generated from chicken feather: Selective adsorption and influence of dissolved organic matter. Bioresour. Technol. 2019, 292, 121948. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced adsorption of Cu(II) and Cd(II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef]
- Pathak, U.; Jhunjhunwala, A.; Roy, A.; Das, P.; Kumar, T.; Mandal, T. Efficacy of spent tea waste as chemically impregnated adsorbent involving ortho-phosphoric and sulphuric acid for abatement of aqueous phenol—Isotherm, kinetics and artificial neural network modelling. Environ. Sci. Pollut. Res. 2020, 27, 20629–20647. [Google Scholar] [CrossRef]
- Kan, Y.; Yue, Q.; Li, D.; Wu, Y.; Gao, B. Preparation and characterization of activated carbons from waste tea by H3PO4 activation in different atmospheres for oxytetracycline removal. J. Taiwan Inst. Chem. Eng. 2017, 71, 494–500. [Google Scholar] [CrossRef]
- Pavi, S.; Kramer, L.E.; Gomes, L.P.; Miranda, L.A.S. Biogas production from co-digestion of organic fraction of municipal solid waste and fruit and vegetable waste. Bioresour. Technol. 2017, 228, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.; Lehmann, J. Comparison of wet-digestion and dry-ashing methods for total elemental analysis of biochar. Commun. Soil Sci. Plant Anal. 2012, 43, 1042–1052. [Google Scholar] [CrossRef]
- Johnson, P.R.; Sun, N.; Elimelech, M. Colloid transport in geochemically heterogeneous porous media: Modeling and measurements. Environ. Sci. Technol. 1996, 30, 3284–3293. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, W.; Masek, O.; Chen, X.; Gu, B.; Sharma, B.K. Roles of phosphoric acid in biochar formation: Synchronously improving carbon retention and sorption capacity. J. Environ. Qual. 2017, 46, 393–401. [Google Scholar] [CrossRef]
- Sun, X.; Cheng, P.; Wang, H.; Xu, H.; Dang, L.; Liu, Z.; Lei, Z. Activation of graphene aerogel with phosphoric acid for enhanced electrocapacitive performance. Carbon 2015, 92, 1–10. [Google Scholar] [CrossRef]
- Gambi, A.; Delorenzi, A.; Giorgianni, S.; Stoppa, P. High-Resolution FTIR Spectrum of Vinyl Fluoride: Rovibrational Analysis of the Torsion Mode at 712 cm−1. J. Mol. Spectrosc. 1995, 171, 504–512. [Google Scholar] [CrossRef]
- Li, F.; Cao, X.; Zhao, L.; Wang, J.; Ding, Z. Effects of mineral additives on biochar formation: Carbon retention, stability, and properties. Environ. Sci. Technol. 2014, 48, 11211–11217. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Rockstraw, D.A. Physicochemical properties of carbons prepared from pecan shell by phosphoric acid activation. Bioresour. Technol. 2007, 98, 1513–1521. [Google Scholar] [CrossRef]
- Gokel, G.W.; Barbour, L.J.; Ferdani, R.; Hu, J. Lariat ether receptor systems show experimental evidence for alkali metal cation-π interactions. Acc. Chem. Res. 2002, 35, 878–886. [Google Scholar] [CrossRef]
- Harvey, O.R.; Herbert, B.E.; Rhue, R.D.; Kuo, L.-J. Metal interactions at the biochar-water Interface: Energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ. Sci. Technol. 2011, 45, 5550–5556. [Google Scholar] [CrossRef]
- Xie, H.; Wu, M.; Gao, T.; Jing, J.; Zhou, G.; Zhang, H.; Fu, M. Influence of structure of activated carbon with superhigh specific surface area on hydrogen storage capacity. J. Mater. Res. 2013, 28, 605–610. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, J.; Zhou, X.; Guo, J.; Liu, Y. Highly efficient removal of Cu(II), Cd(II) and Pb(II) by carboxyl-modified multi-porous biochar. Sep. Sci. Technol. 2018, 53, 2860–2869. [Google Scholar] [CrossRef]
- Sim, L.C.; Leong, K.H.; Ibrahima, S.; Saravanan, P. Graphene oxide and Ag engulfed TiO2 nanotube arrays for enhanced electron mobility and visible-light-driven photocatalytic performance. J. Mater. Chem. A 2014, 2, 5315–5322. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen peroxide modification enhances the ability of biochar (hydrochar) produced from hydrothermal carbonization of peanut hull to remove aqueous heavy metals: Batch and column tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, J.; Zhang, S.; Zhang, X.; Chen, H. Effect of phosphorus-modified biochars on immobilization of Cu (II), Cd (II), and As (V) in paddy soil. J. Hazard. Mater. 2020, 390, 121349. [Google Scholar] [CrossRef]
- Wang, H.; Gao, B.; Wang, S.; Fang, J.; Xue, Y.; Yang, K. Removal of Pb(II), Cu(II), and Cd(II) from aqueous solutions by biochar derived from KMnO4 treated hickory wood. Bioresour. Technol. 2015, 197, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, Y.; Zheng, B.; Cai, X. Competitive removal of Cd(II) and Pb(II) by biochars produced from water hyacinths: Performance and mechanism. RSC Adv. 2016, 6, 5223–5232. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Wu, F.; Yao, Y.; Yuan, Y.; Bi, X.; Luo, X.; Shahbazian-Yassar, R.; Zhang, C.; Amine, K. Mesocarbon microbead carbon-supported magnesium hydroxide nanoparticles: Turning spent Li-ion battery anode into a highly efficient phosphate adsorbent for wastewater treatment. ACS Appl. Mater. Interfaces 2016, 8, 21315–21325. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, B.; Lin, L.; Qiu, W.; Song, Z. Adsorption of Cu(II) and Cd(II) from aqueous solutions by ferromanganese binary oxide–biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Mahdi, Z.; Hanandeh, A.E.; Yu, Q.J. Preparation, characterization and application of surface modified biochar from date seed for improved lead, copper, and nickel removal from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 103379. [Google Scholar] [CrossRef]
- Lee, M.E.; Park, J.H.; Chung, J.W. Comparison of the lead and copper adsorption capacities of plant source materials and their biochars. J. Environ. Manag. 2019, 236, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Z.; Song, S.; Han, J.; Chen, H.; Wang, X.; Sun, R.; Cheng, J. Adsorption of copper(II) and lead(II) from seawater using hydrothermal biochar derived from Enteromorpha. Mar. Pollut. Bull. 2019, 149, 110586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, W.; Cao, H.; Hu, D.; Chen, X.; Guan, Y.; Tang, J.; Gao, H. Ultra-efficient sorption of Cu2+ and Pb2+ ions by light biochar derived from Medulla tetrapanacis. Bioresour. Technol. 2019, 291, 121818. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, W.; Yang, Y.; Huang, X.; Wang, S.; Qiu, R. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012, 46, 854–862. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Zheng, H.; Li, F.; Ngo, H.H.; Guo, W.; Liu, C.; Chen, L.; Xing, B. Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol. 2015, 177, 308–317. [Google Scholar] [CrossRef]
- Luo, X.; Yu, L.; Wang, C.; Yin, X.; Mosa, A.; Lv, J.; Sun, H. Sorption of vanadium (V) onto natural soil colloids under various solution pH and ionic strength conditions. Chemosphere 2017, 169, 609–617. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, S.; He, F.; Liu, Y.; Mao, W.; Guan, Y. Highly efficient and selective capture of heavy metals by poly(acrylic acid) grafted chitosan and biochar composite for wastewater treatment. Bioresour. Technol. 2019, 378, 122215. [Google Scholar] [CrossRef]
- Shi, T.; Jia, S.; Chen, Y.; Wen, Y.; Du, C.; Guo, H.; Wang, Z. Adsorption of Pb(II), Cr(III), Cu(II), Cd(II) and Ni(II) onto a vanadium mine tailing from aqueous solution. J. Hazard. Mater. 2009, 169, 838–846. [Google Scholar] [CrossRef]
- Cao, X.D.; Ma, L.Q.; Gao, B.; Harris, W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009, 43, 3285–3291. [Google Scholar] [CrossRef]
- Petrov, N.; Budinova, T.; Khavesov, I. Adsorption of the ions of zinc, cadmium, copper, and lead on oxidized anthracite. Carbon 1992, 30, 135–139. [Google Scholar] [CrossRef]

| Header | PCL | CL | CLB |
|---|---|---|---|
| C (%) | 9.65 | 59.71 | 63.39 |
| O (%) | 78.17 | 23.57 | 14.43 |
| H (%) | 10.74 | 7.02 | 5.42 |
| S (%) | 0.29 | 0.27 | 0.31 |
| N (%) | 0.32 | 1.79 | 1.92 |
| P (%) | 0.025 | 0.21 | 1.77 |
| O/C | 8.101 | 0.395 | 0.228 |
| H/C | 1.113 | 0.118 | 0.086 |
| (O + H)/C | 9.213 | 0.425 | 0.258 |
| pH | — | 6.47 | 4.56 |
| SSA(m2/g) | — | <5 | 55.42 |
| pHzpc | — | 5.84 | 3.27 |
| Ash (%) | — | 7.24 | 9.97 |
| Element | Model | CLB Model Parameters | ||
|---|---|---|---|---|
| Parameter 1 | Parameter 2 | R2 | ||
| Kinetic models | ||||
| First-order | qe = 71.26 | Kf = 2.177 | 0.690 | |
| Cu | Second-order | qe = 73.91 | K2 = 0.047 | 0.847 |
| Elovich | α = 10007.19 | β = 0.139 | 0.945 | |
| First-order | qe = 214.12 | Kf = 6.579 | 0.914 | |
| Pb | Second-order | qe = 220.11 | K2 = 0.0486 | 0.978 |
| Elovich | α = 225981.54 | β = 0.0553 | 0.806 | |
| Isotherm models | ||||
| Langmuir | qmax = 80.41 | KL = 0.863 | 0.972 | |
| Cu | Freundlich | n = 5.034 | Kf = 37.24 | 0.866 |
| Redlich-Peterson | qmax = 81.43 | K =0.840 r = 0.928 | 0.980 | |
| Langmuir | qmax = 227.80 | KL = 0.658 | 0.971 | |
| Pb | Freundlich | n = 5.247 | Kf = 93.42 | 0.847 |
| Redlich-Peterson | qmax = 224.60 | K = 0.660 r = 1.02 | 0.981 | |
| Biosorbents | Preparation Method and Condition | Cu(II) Sorption Capacity (mg/g) | Pb(II) Sorption Capacity (mg/g) | Reference |
|---|---|---|---|---|
| H3PO4-modified banana peel biochar | Hydrothermal carbonization, anaerobism, 230 °C for 2 h | — a | 241 | [17] |
| H3PO4-modified chicken feather biochar | Pyrolysis, anaerobism, 450 °C, 1 h | — a | 77.46 | [18] |
| H3PO4-modified pine sawdust biochar | Pyrolysis, anaerobism, 350 °C, 2 h | about 30 | — a | [19] |
| ferromanganese binary oxide–corn straw biochar | Pyrolysis, anaerobism, 600 °C, 2 h | 64.9 | — a | [40] |
| HCl modified Phoenix Dactylifera biochar | Pyrolysis, anaerobism, 550 °C, 3 h | 45.12 | 188.58 | [41] |
| Gingko leaf biochar | Pyrolysis anaerobism, 800 °C, 1.5 h | 59.9 | 138.9 | [42] |
| Enteromorpha biochar | Hydrothermal carbonization anaerobism, 250 °C, 40 min | 98 | 254 | [43] |
| Medulla tetrapanacis biochar | Pyrolysis anaerobism, 400 °C, 1 h | 168.35 | 458.72 | [44] |
| carboxyl-modified Palm fiber biochar | Pyrolysis anaerobism, 400 °C, 2 h | 132.1 | 218.2 | [33] |
| H3PO4 impregnation cauliflower leaves biochar | Hydrothermal carbonization, In air atmosphere, 120 °C, 2 h | 81.43 | 224.6 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Q.; Tian, Q.; Moeen, M.; Wang, S. Facile Synthesis of Cauliflower Leaves Biochar at Low Temperature in the Air Atmosphere for Cu(II) and Pb(II) Removal from Water. Materials 2020, 13, 3163. https://doi.org/10.3390/ma13143163
Ge Q, Tian Q, Moeen M, Wang S. Facile Synthesis of Cauliflower Leaves Biochar at Low Temperature in the Air Atmosphere for Cu(II) and Pb(II) Removal from Water. Materials. 2020; 13(14):3163. https://doi.org/10.3390/ma13143163
Chicago/Turabian StyleGe, Qilong, Qi Tian, Muhammad Moeen, and Sufang Wang. 2020. "Facile Synthesis of Cauliflower Leaves Biochar at Low Temperature in the Air Atmosphere for Cu(II) and Pb(II) Removal from Water" Materials 13, no. 14: 3163. https://doi.org/10.3390/ma13143163
APA StyleGe, Q., Tian, Q., Moeen, M., & Wang, S. (2020). Facile Synthesis of Cauliflower Leaves Biochar at Low Temperature in the Air Atmosphere for Cu(II) and Pb(II) Removal from Water. Materials, 13(14), 3163. https://doi.org/10.3390/ma13143163





