Optical and Mechanical Properties of Highly Translucent Dental Zirconia
Abstract
:1. Introduction
2. Materials and Methods
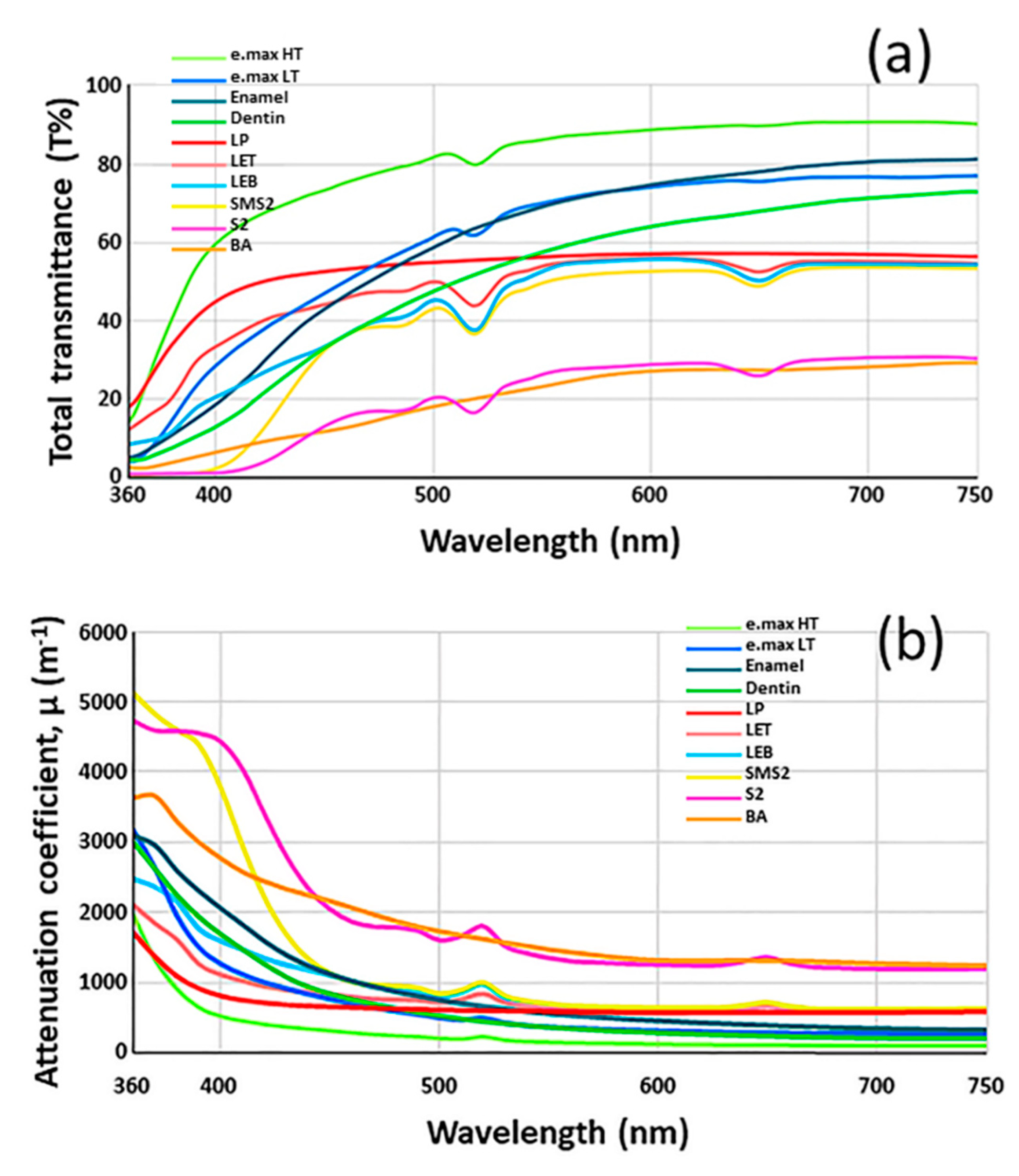
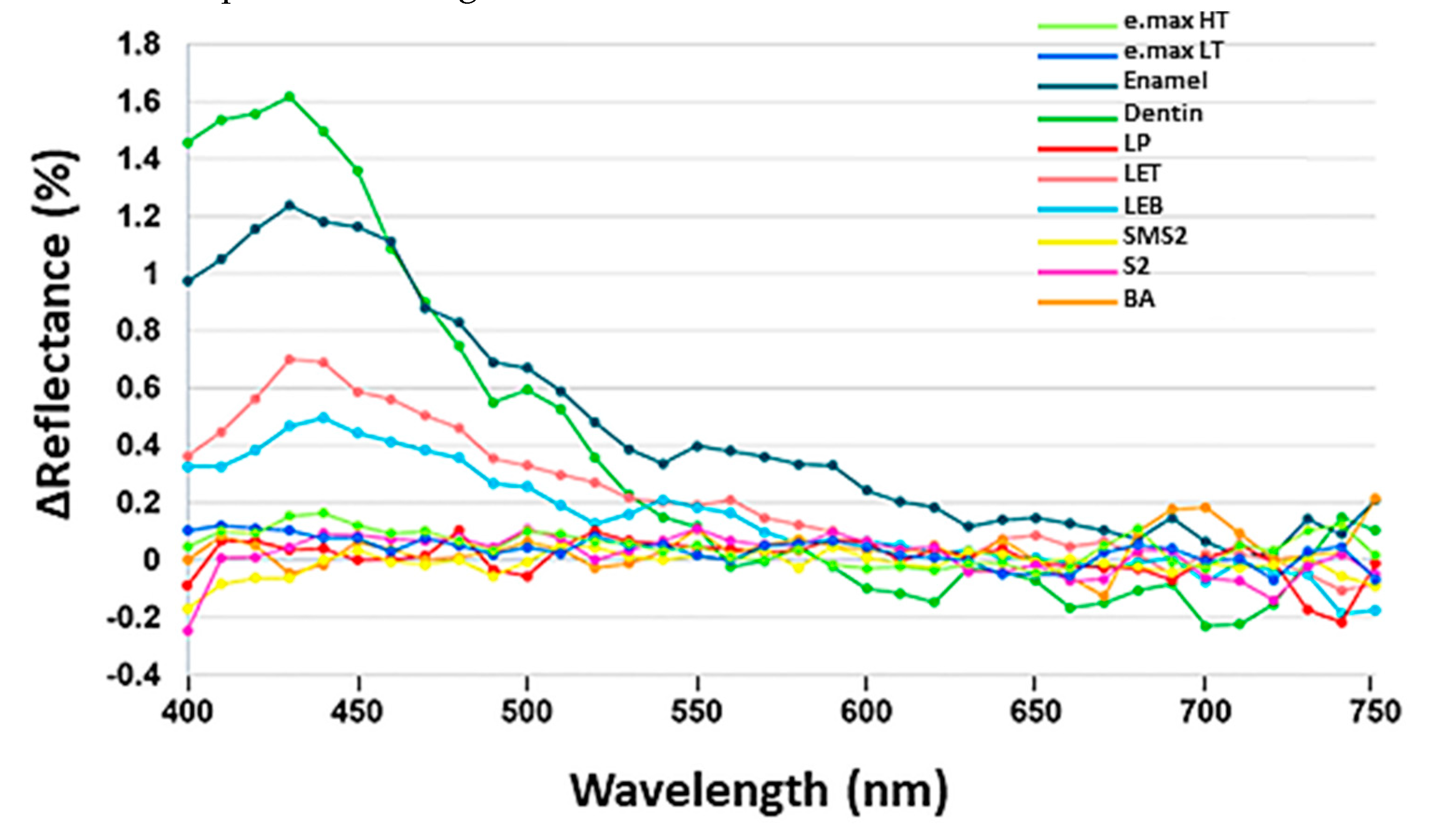
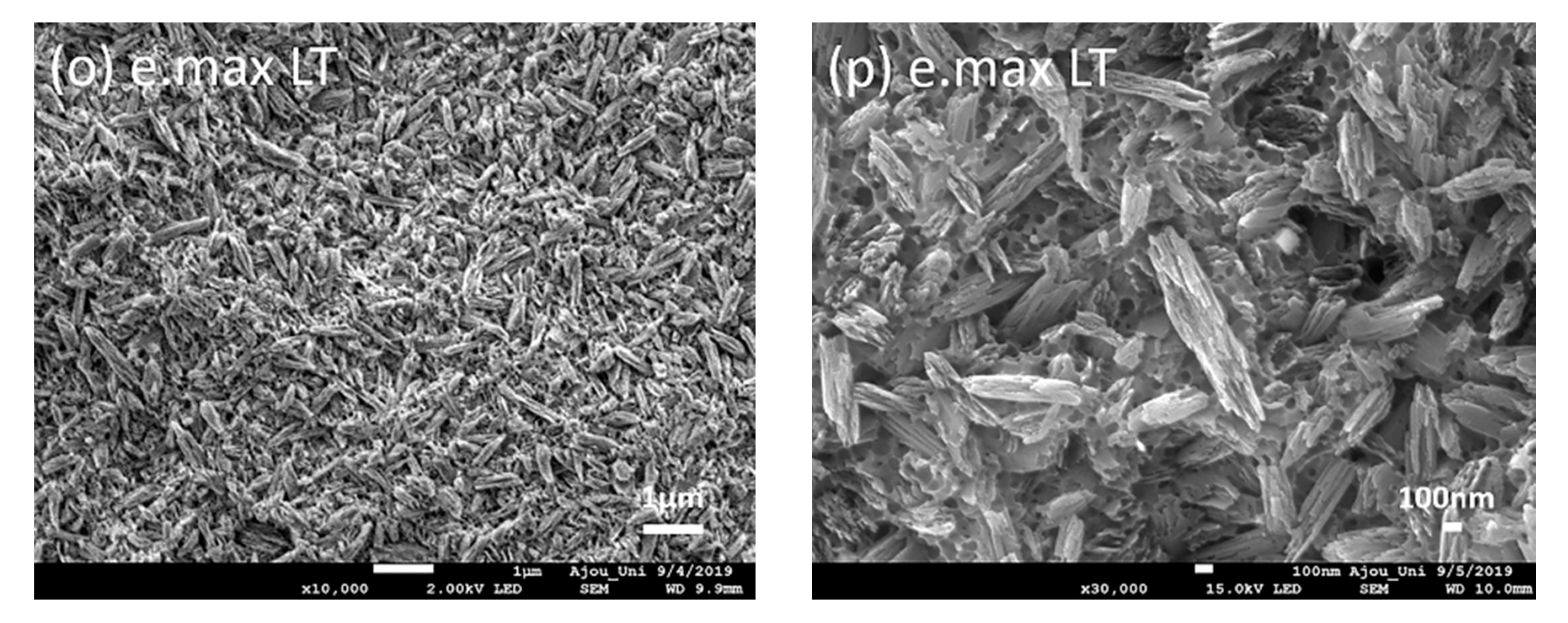
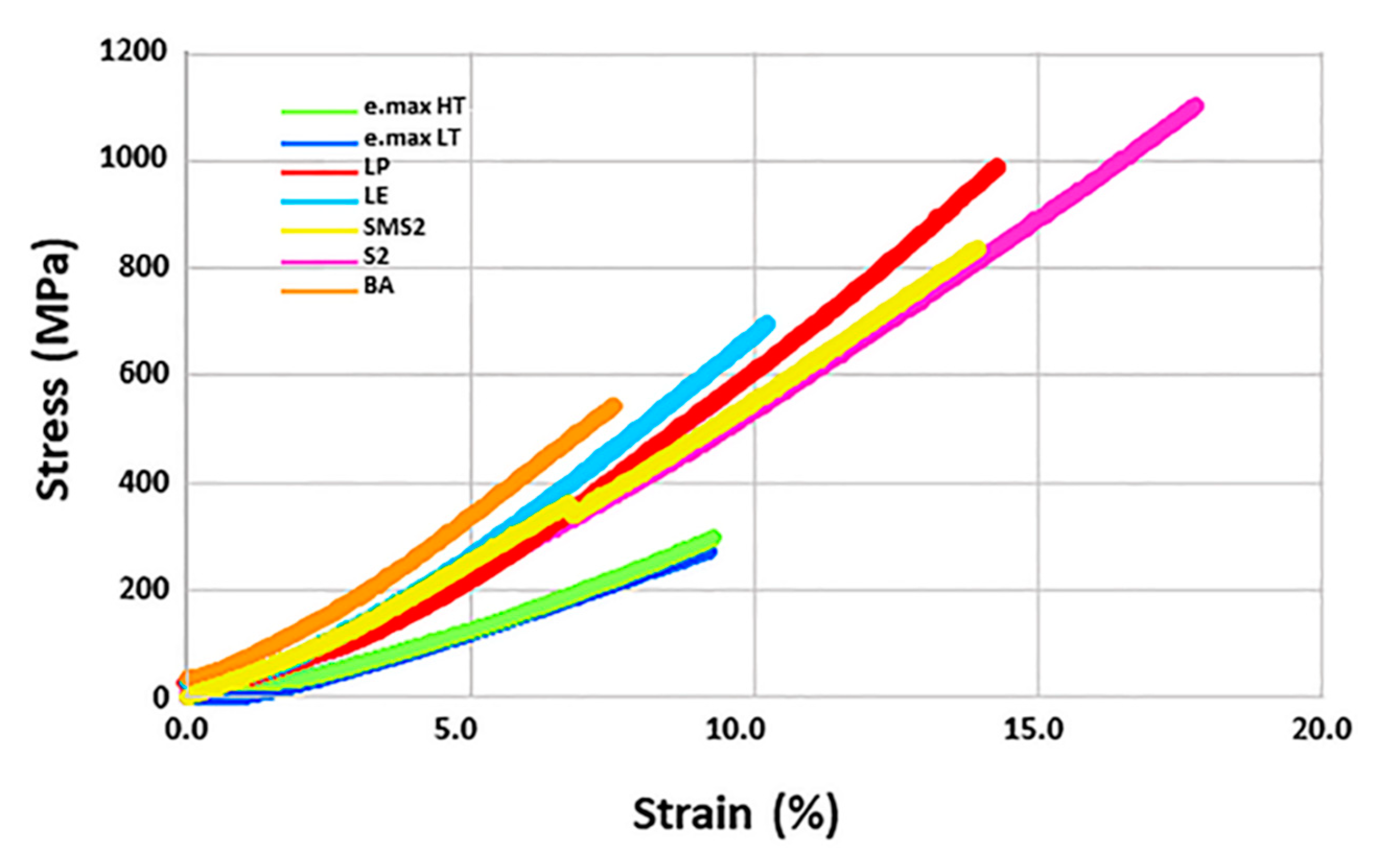
3. Results
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Zhang, Y.; Lawn, B.R. Novel zirconia materials in dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K. Effect of a rapid-cooling protocol on the optical and mechanical properties of dental monolithic zirconia containing 3–5 mol%Y2O3. Materials 2020, 13, 1923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabatabaian, F. Color Aspect of Monolithic Zirconia Restorations: A Review of the Literature. J. Prosthodont. 2019, 28, 276–287. [Google Scholar] [CrossRef]
- Zhang, F.; Inokoshi, M.; Batuk, M.; Hadermann, J.; Naert, I.; Van Meerbeek, B.; Vleugels, J. Strength, toughness and aging stability of highly-translucent Y-TZP ceramics for dental restorations. Dent. Mater. 2016, 32, e327–e337. [Google Scholar] [CrossRef] [PubMed]
- Camposilvan, E.; Leone, R.; Gremillard, L.; Sorrentino, R.; Zarone, F.; Ferrari, M.; Chevalier, J. Aging resistance, mechanical properties and translucency of different yttria-stabilized zirconia ceramics for monolithic dental crown applications. Dent. Mater. 2018, 34, 879–890. [Google Scholar] [CrossRef]
- Carrabba, M.; Keeling, A.J.; Aziz, A.; Vichi, A.; Fabian Fonzar, R.; Wood, D.; Ferrari, M. Translucent zirconia in the ceramic scenario for monolithic restorations: A flexural strength and translucency comparison test. J. Dent. 2017, 60, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Baldissara, P.; Wandscher, V.F.; Marchionatti, A.M.E.; Parisi, C.; Monaco, C.; Leonardo, C. Translucency of IPS e.max and cubic zirconia monolithic crowns. J. Prosthet. Dent. 2018, 120, 269–275. [Google Scholar] [CrossRef]
- Yan, J.; Kaizer, M.R.; Zhang, Y. Load-bearing capacity of lithium disilicate and ultra-translucent zirconias. J. Mech. Behav. Biomed. Mater. 2018, 88, 170–175. [Google Scholar] [CrossRef]
- Nassary Zadeh, P.; Lümkemann, N.; Sener, B.; Eichberger, M.; Stawarczyk, B. Flexural strength, fracture toughness, and translucency of cubic/tetragonal zirconia materials. J. Prosthet. Dent. 2018, 120, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Villarroel, M.; Fahl, N.; De Sousa, A.M.; De Oliveira, O.B., Jr. Direct esthetic restorations based on translucency and opacity of composite resins. J. Esthet. Restor. Dent. 2011, 23, 73–87. [Google Scholar] [CrossRef]
- Harczuk, I.; Vahtras, O.; Ågren, H. Modeling Rayleigh scattering of aerosol particles. J. Phys. Chem. B 2016, 120, 4296–4301. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.H.; Nahavandi, A.M.; Khorshidi, S.; Hashemikamangar, S.S. Fluorescence and opalescence of two dental composite resins. Eur. J. Dent. 2019, 13, 527–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewer, J.D.; Wee, A.; Seghi, R. Advances in color matching. Dent. Clin N. Am. 2004, 48, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Dietschi, D.; Fahl, N., Jr. Shading concepts and layering techniques to master direct anterior composite restorations: An update. Br. Dent. J. 2016, 221, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Egen, M.; Braun, L.; Zentel, R.; Tännert, K.; Frese, P.; Reis, O.; Wulf, M. Artificial opals as effect pigments in clear-coating. Macromol. Mater. Eng. 2004, 289, 158–163. [Google Scholar] [CrossRef]
- Lee, Y.K. Opalescence of human teeth and dental esthetic restorative materials. Dent. Mater. J. 2016, 35, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.K. Influence of filler on the difference between the transmitted and reflected colors of experimental resin composites. Dent. Mater. 2008, 24, 1243–1247. [Google Scholar] [CrossRef]
- Cho, M.S.; Yu, B.; Lee, Y.K. Opalescence of all-ceramic core and veneer materials. Dent. Mater. 2009, 25, 695–702. [Google Scholar] [CrossRef]
- Eltit, F.; Ebacher, V.; Wang, R. Inelastic deformation and microcracking process in human dentin. J. Struct. Biol. 2013, 183, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Catelan, A.; Guedes, A.P.; Suzuki, T.Y.; Takahashi, M.K.; Souza, E.M.; Briso, A.L.; Santos, P.H. Fluorescence intensity of composite layering combined with surface sealant submitted to staining solutions. J. Esthet. Restor. Dent. 2015, 27, S33–S40. [Google Scholar] [CrossRef]
- Mazzoni, A.; Maravić, T.; Tezvergil-Mutluay, A.; Tjäderhane, L.; Scaffa, P.M.C.; Seseogullari-Dirihan, R.; Bavelloni, A.; Gobbi, P.; Pashley, D.H.; Tay, F.R.; et al. Biochemical and immunohistochemical identification of MMP-7 in human dentin. J. Dent. 2018, 79, 90–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahmiri, R.; Standard, O.C.; Hart, J.N.; Sorrell, C.C. Optical properties of zirconia ceramics for esthetic dental restorations: A systematic review. J. Prosthet. Dent. 2018, 119, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Cao, X.; Zhang, C.; He, T.; Hua, N.; Xian, Y. Rare earth ions enhanced near infrared fluorescence of Ag2S quantum dots for the detection of fluoride ions in living cells. Nanoscale 2017, 9, 14031–14038. [Google Scholar] [CrossRef] [PubMed]
- Kepiński, L.; Maczka, M.; Hanuza, J. Anti-Stokes Yb3+ emission--valuable structure information in spectra of rare earth compounds measured with FT-Raman spectrometers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2006, 65, 1025–1029. [Google Scholar] [CrossRef]
- Benourdja, S.; Kaynar, Ü.H.; Ayvacikli, M.; Karabulut, Y.; Guinea, J.G.; Canimoglu, A.; Chahed, L.; Can, N. Preparation and cathodoluminescence characteristics of rare earth activated BaAl2O4 phosphors. Appl. Radiat. Isot. 2018, 139, 34–39. [Google Scholar] [CrossRef]
- Rafael, C.F.; Cesar, P.F.; Fredel, M.; Magini, R.S.; Liebermann, A.; Maziero Volpato, C.A. Impact of laboratory treatment with coloring and fluorescent liquids on the optical properties of zirconia before and after accelerated aging. J. Prosthet. Dent. 2018, 120, 276–281. [Google Scholar] [CrossRef]
- Uo, M.; Okamoto, M.; Watari, F.; Tani, K.; Morita, M.; Shintani, A. Rare earth oxide-containing fluorescent glass filler for composite resin. Dent. Mater. J. 2005, 24, 49–52. [Google Scholar] [CrossRef]
- Hardin, C.L.; Kodera, Y.; Basun, S.A.; Evans, D.R.; Garay, J.E. Transparent, luminescent terbium doped zirconia: Development of optical-structural ceramics with integrated temperature measurement functionalities. Opt. Mater. Express 2013, 3, 893–903. [Google Scholar] [CrossRef]
- Nakamura, T.; Okamura, S.; Nishida, H.; Usami, H.; Nakano, Y.; Wakabayashi, K.; Sekino, T.; Yatani, H. Fluorescence of thulium-doped translucent zirconia. Dent. Mater. J. 2018, 37, 1010–1016. [Google Scholar] [CrossRef] [Green Version]
- Jang, Y.S.; Oh, S.H.; Oh, W.S.; Lee, M.H.; Lee, J.J.; Bae, T.S. Effects of liner-bonding of implant-supported glass-ceramic crown to zirconia abutment on bond strength and fracture resistance. Materials 2019, 12, 2798. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Poggio, C.; Lasagna, A.; Chiesa, M.; Scribante, A. Vickers micro-hardness of new restorative CAD/CAM dental materials: Evaluation and comparison after exposure to acidic drink. Materials 2019, 12, 1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, R.; Jang, Y.S.; Lee, M.H.; Bae, T.S. Comparative evaluation of mechanical properties and wear ability of five CAD/CAM dental blocks. Materials 2019, 12, 2252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, J.L.O.; Filho, E.M.; Salgado, J.A.P.; Salgado, M.A.C.; de Moraes, L.C.; de Moraes, M.E.L.; de Melo Castilho, J.C. Comparative analysis of human and bovine teeth: Radiographic density. Braz. Oral Res. 2008, 22, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Pecho, O.E.; Ghinea, R.; Ionescu, A.M.; Cardona, J.d.L.; Paravina, R.D.; Pérez Mdel, M. Color and translucency of zirconia ceramics, human dentine and bovine dentine. J. Dent. 2012, 40, e34–e40. [Google Scholar] [CrossRef]
- Yu, B.; Ahn, J.S.; Lee, Y.K. Measurement of translucency of tooth enamel and dentin. Acta Odontol. Scand. 2009, 67, 57–64. [Google Scholar] [CrossRef]
- Wiegand, A.; Vollmer, D.; Foitzik, M.; Attin, R.; Attin, T. Efficacy of different whitening modalities on bovine enamel and dentin. Clin. Oral Investig. 2005, 9, 91–97. [Google Scholar] [CrossRef]
- Aydın, B.; Pamir, T.; Baltaci, A.; Orman, M.N.; Turk, T. Effect of storage solutions on microhardness of crown enamel and dentin. Eur. J. Dent. 2015, 9, 262–266. [Google Scholar] [CrossRef] [Green Version]
- International Commission on Illumination. CIE 15:2004, Colorimetry, 3rd ed.; CIE: Vienna, Austria, 1913. Available online: http://cie.co.at/publications/colorimetry-3rd-edition (accessed on 25 November 2019).
- Nogueira, A.D.; Della Bona, A. The effect of a coupling medium on color and translucency of CAD-CAM ceramics. J. Dent. 2013, 41, e18–e23. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Kim, S.H. Optical properties of pre-colored dental monolithic zirconia ceramics. J. Dent. 2016, 55, 75–81. [Google Scholar] [CrossRef]
- Spink, L.S.; Rungruanganut, P.; Megremis, S.; Kelly, J.R. Comparison of an absolute and surrogate measure of relative translucency in dental ceramics. Dent. Mater. 2013, 2, 702–707. [Google Scholar] [CrossRef]
- Rayyan, M.R. Marginal adaptation of monolithic high-translucency versus porcelain-veneered zirconia crowns. Int. J. Prosthodont. 2019, 32, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Lu, H.; Powers, J.M. Influence of fluorescent and opalescent properties of resin composites on the masking effect. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 76, 26–32. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. ISO 6872:2015, Dentistry-Ceramic Materials; ISO: Geneva, Switzerland, 1947. Available online: https://www.iso.org.standard/59936.html (accessed on 11 April 2018).
- Yamauchi, S.; Miura, S.; Kasahara, S.; Sun, J.; Egusa, H. A thick frame decreases the fracture toughness of veneering ceramics used for zirconia-based all-ceramic restorations. J. Prosthodont. Res. 2019, 63, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Han, J.; Lin, H.; An, L. Comparative study of flexural strength test methods on CAD/CAM Y-TZP dental ceramics. Regen. Biomater. 2015, 2, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Baitenov, A.; Li, Y.; Vasileva, E.; Popov, S.; Sychugov, I.; Yan, M.; Berglund, L. Thickness Dependence of Optical Transmittance of Transparent Wood: Chemical Modification Effects. ACS Appl. Mater. Interfaces 2019, 11, 35451–35457. [Google Scholar] [CrossRef]
- He, L.H.; Swain, M.V. Nanoindentation derived stress-strain properties of dental materials. Dent. Mater. 2007, 23, 814–821. [Google Scholar] [CrossRef]
- El-Meliegy, E.; van Noort, R. Lithium disilicate glass ceramics. In Glasses and Glass Ceramics for Medical Applications; Springer Publishing: New York, NY, USA, 2012; pp. 209–215. [Google Scholar]
- Holz, L.; Macias, J.; Vitorino, N.; Fernandes, A.J.S.; Costa, F.M.; Almeida, M.M. Effect of Fe2O3 doping on colour and mechanical properties of Y-TZP ceramics. Ceram. Int. 2018, 44, 17962–17971. [Google Scholar] [CrossRef]
- Zhang, F.; Vanmeensel, K.; Inokoshi, M.; Batuk, M.; Hadermann, J.; Van Meerbeek, B.; Naert, I.; Vleugels, J. Critical influence of alumina content on the low temperature degradation of 2-3mol% yttria-stabilized TPZ for dental restorations. J. Eur. Ceram. Soc. 2015, 35, 741–750. [Google Scholar] [CrossRef]
- Banda, R.; Lee, H.Y.; Lee, M.S. Separation of Zr and Hf from strong hydrochloric acid solution by solvent extraction with TEHA. J. Radioanal. Nucl. Chem. 2013, 295, 537–543. [Google Scholar] [CrossRef]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [Green Version]
- Dahl, G.T.; Döring, S.; Krekeler, T.; Janssen, R.; Ritter, M.; Weller, H.; Vossmeyer, T. Alumina-doped zirconia submicro-particles: Synthesis, thermal stability, and microstructural characterization. Materials 2019, 12, 2856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samodurova, A.; Kocjan, A.; Swain, M.V.; Kosmač, T. The combined effect of alumina and silica co-doping on the ageing resistance of 3Y-TZP bioceramics. Acta Biomater. 2015, 11, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, T.; Wood, D.J.; Shinozaki, N.; van Noort, R. Optical properties of base dentin ceramics for all-ceramic restorations. Dent. Mater. 2011, 27, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höland, W.; Ritzberger, C.; Apel, E.; Rothbrust, F.; Chevalier, J.; Brodkin, D. Shaded Zirconia Ceramics. US Patent 8,796,166 B2, 5 August 2014. [Google Scholar]
- Wang, Z.; Meijerink, A. Concentration quenching in upconversion nanocrystals. J. Phys. Chem. C Nanomater. Interfaces 2018, 122, 26298–26306. [Google Scholar] [CrossRef] [Green Version]
- Alessandretti, R.; Borba, M.; Benetti, P.; Corazza, P.H.; Ribeiro, R.; Della Bona, A. Reliability and mode of 9ailure of bonded monolithic and multilayer ceramics. Dent. Mater. 2017, 33, 191–197. [Google Scholar] [CrossRef]
- Sfondrini, M.F.; Gandini, P.; Malfatto, M.; Di Corato, F.; Trovati, F.; Scribante, A. Computerized casts for orthodontic purpose using powder-free intraoral scanners: Accuracy, execution time, and patient feedback. Biomed. Res. Int. 2018, 2018, 4103232. [Google Scholar] [CrossRef] [Green Version]



| Materials | Manufacturer | Shade | Batch No. | Sintering | |
|---|---|---|---|---|---|
| Zirconia | |||||
| 3Y-TZP | Lava Plus | 3M ESPE, USA | White | 669344 | 1500 °C for 2 h |
| Luxen Zr | DENTALMAX, Korea | S2 (A2) | 160523-S2-2 | ||
| 5Y-PSZ | Lava Esthetic | 3M ESPE, USA | A2 | 4638594 | |
| BruxZir Anterior | Glidewell, USA | 250 (A2) | BZ0019752 | ||
| Luxen Smile | DENTALMAX, Korea | SMS2 (A2) | 190222-10SMS2-1 | ||
| Glass-Ceramic | |||||
| e.max CAD | High translucent (HT) | Ivoclar Vivadent, Liechtenstein | A2 | T02466 V35858 X17428 | 820 °C for 2 min + 840 °C for 7 min |
| Low translucent (LT) | A2 | U36939 | |||
| Materials | Component (wt.%) | |
|---|---|---|
| Zirconia | ||
| 3Y-TZP | LP | Al (0.0651), Y (5.5603), Zr (91.6982), Hf (2.6765) |
| S2 | Al (0.0516), Fe (0.0535), Y (5.4426), Zr (91.6896), Hf (2.7627) | |
| 5Y-PSZ | LET | Al (0.0346), Y (9.1951), Zr (88.8130), Er (0.1741), Hf (1.7832) |
| LEB | Al (0.0391), Y (8.8127), Zr (88.8855), Er (0.4899), Hf (1.7727) | |
| BA | Ca (0.0217), Y (9.7067), Zr (87.5350), Hf (2.7367) | |
| SMS2 | Al (0.0226), Fe (0.0554), Y (7.8563), Zr (88.3084), Er (1.0339), Hf (2.7235) | |
| Glass-Ceramic | ||
| e.max CAD | HT | Al (1.2961), Si (59.7200), P (1.9325), S (0.0211), Li (13.6079), Ca (0.1034), V (0.4735), Zn (3.9805), Sr (0.0604), Zr (3.9142), Ce (5.7366), Tb (2.6394), K (6.5144) |
| LT | Al (2.7798), Si (62.7669), P (2.1251), S (0.1007), Li (13.7656), Ca (0.1091), V (0.4351), Zr (2.0843), Ce (7.6236), Tb (2.3141), K (5.8957) | |
| Materials | Density (g/cm3) | Modulus (GPa) | Hardness (GPa) | Strength (MPa) | Toughness (MPa·m1/2) | |
|---|---|---|---|---|---|---|
| Zirconia | ||||||
| 3Y-TZP | LP | 6.070 a | 231 | 14.09 (0.47) | 982.5 (47.1) | 5.18 (0.79) |
| S2 | 6.096 a | 208 | 12.74 (0.05) | 1054.4 (68.1) | 4.34 (0.09) | |
| 5Y-PSZ | LE | 6.044 a | 233 | 15.47 (0.39) | 691.3 (65.4) | 3.58 (0.75) |
| BA | 5.819 | 221 | 12.51 (0.12) | 538.7 (48.1) | 3.34 (0.17) | |
| SMS2 | 6.100 a | 214 | 13.16 (0.15) | 801.7 (64.5) | 3.18 (0.13) | |
| Glass-ceramic | ||||||
| e.max CAD | HT | 2.502 b | 102 a | 5.72 (0.08) | 288.5 (31.0) a | 2.34 (0.32) |
| LT | 2.423 b | 102 a | 6.89 (0.33) | 290.1 (27.9) a | 2.12 (0.29) | |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-K. Optical and Mechanical Properties of Highly Translucent Dental Zirconia. Materials 2020, 13, 3395. https://doi.org/10.3390/ma13153395
Kim H-K. Optical and Mechanical Properties of Highly Translucent Dental Zirconia. Materials. 2020; 13(15):3395. https://doi.org/10.3390/ma13153395
Chicago/Turabian StyleKim, Hee-Kyung. 2020. "Optical and Mechanical Properties of Highly Translucent Dental Zirconia" Materials 13, no. 15: 3395. https://doi.org/10.3390/ma13153395




