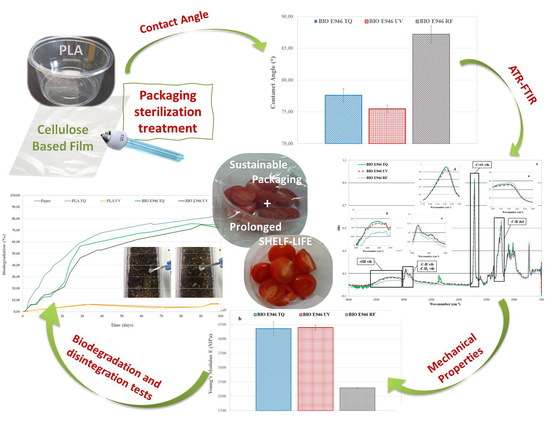
Compostable Polylactide and Cellulose Based Packaging for Fresh-Cut Cherry Tomatoes: Performance Evaluation and Influence of Sterilization Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedure for Fresh-Cut Tomato Sample Preparation
2.3. Sterilization Methods
2.4. Contact Angle Measurements
2.5. Mechanical Properties
2.6. Puncture Resistance
2.7. ATR-FTIR Analysis
2.8. Overall Migration Test
2.9. OTR and WVTR
2.10. Biodegradation Degree
2.11. Disintegration Test
2.12. Statistical Analysis
3. Results and Discussion
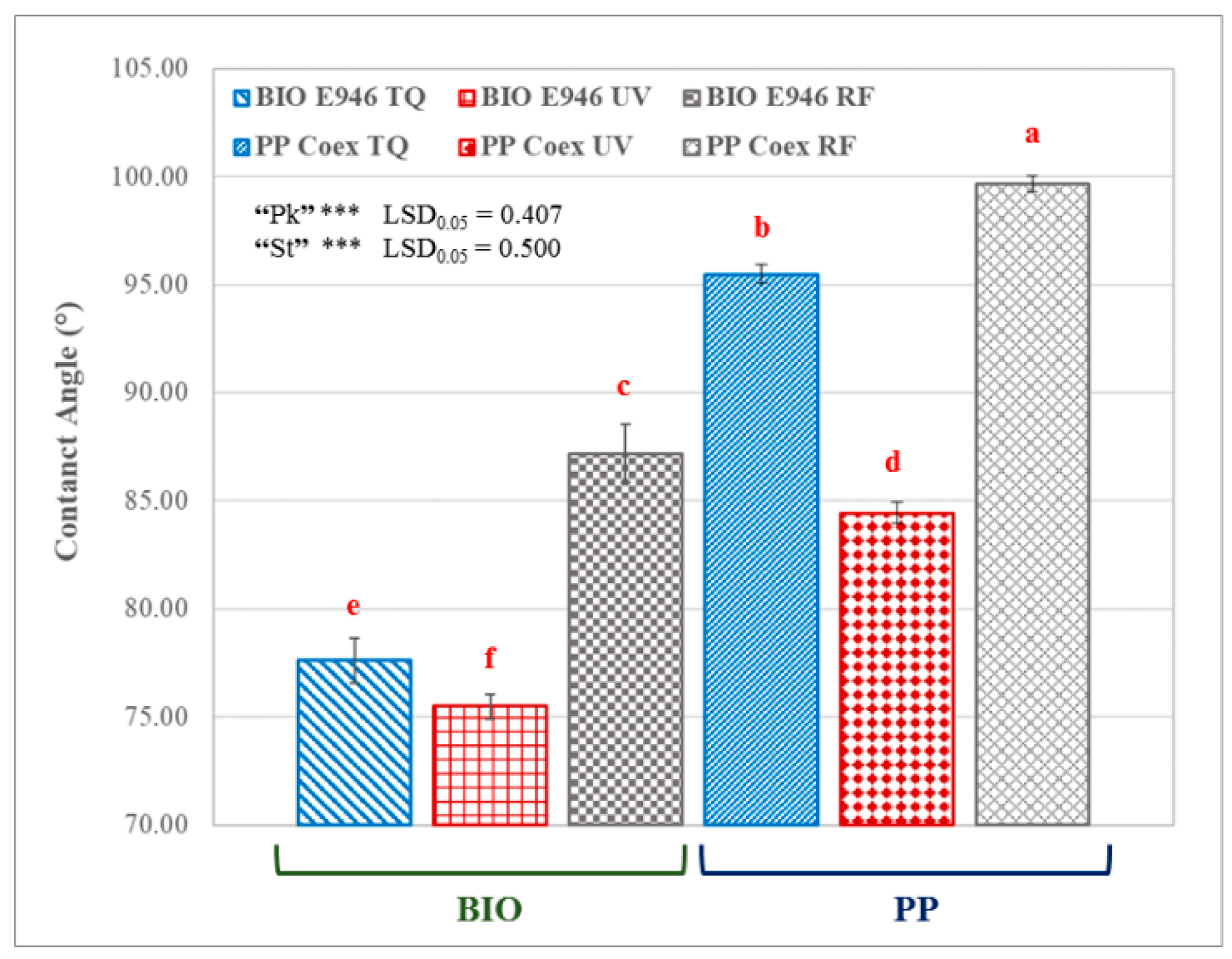
3.1. Contact Angle Measurements
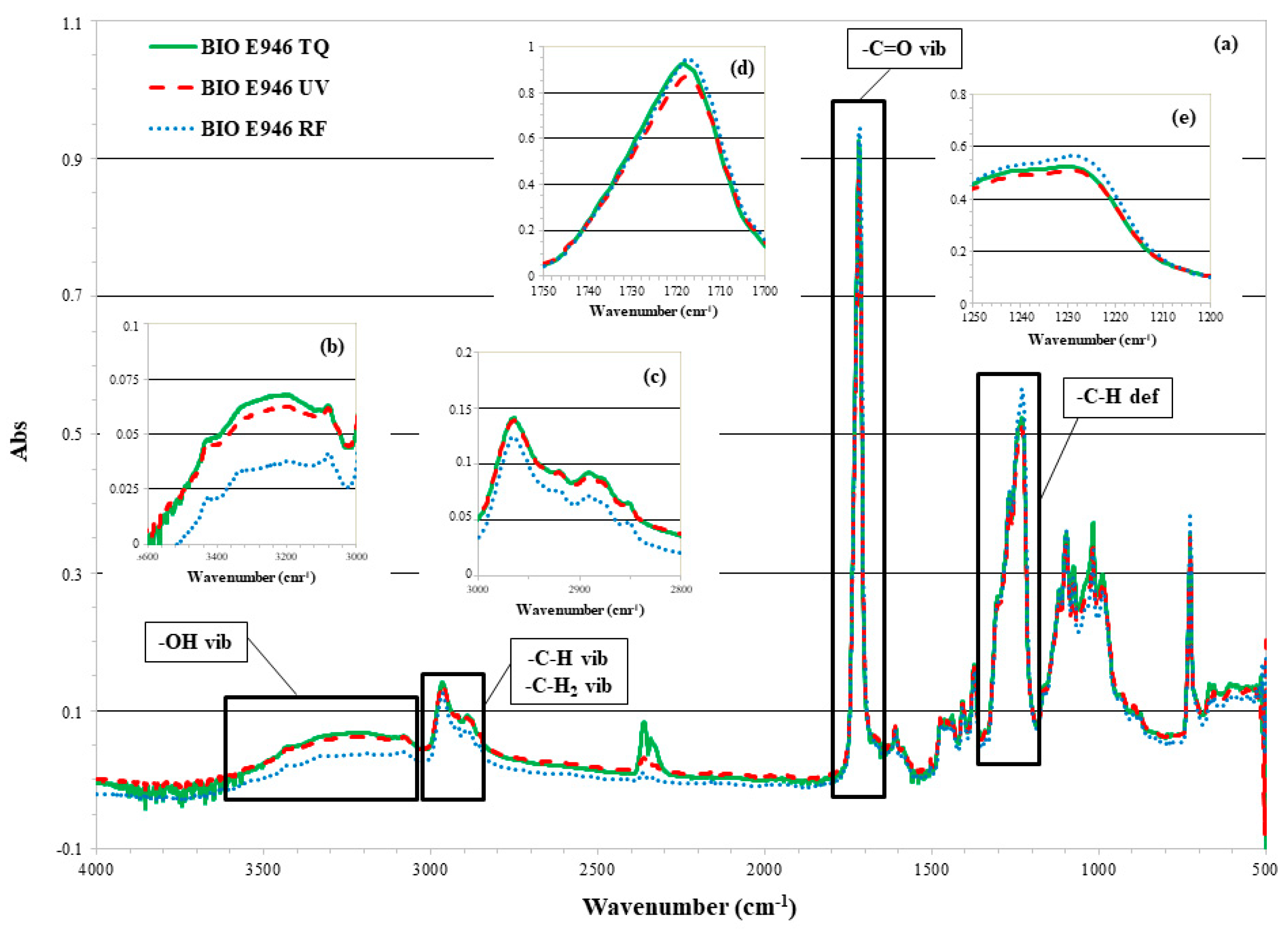
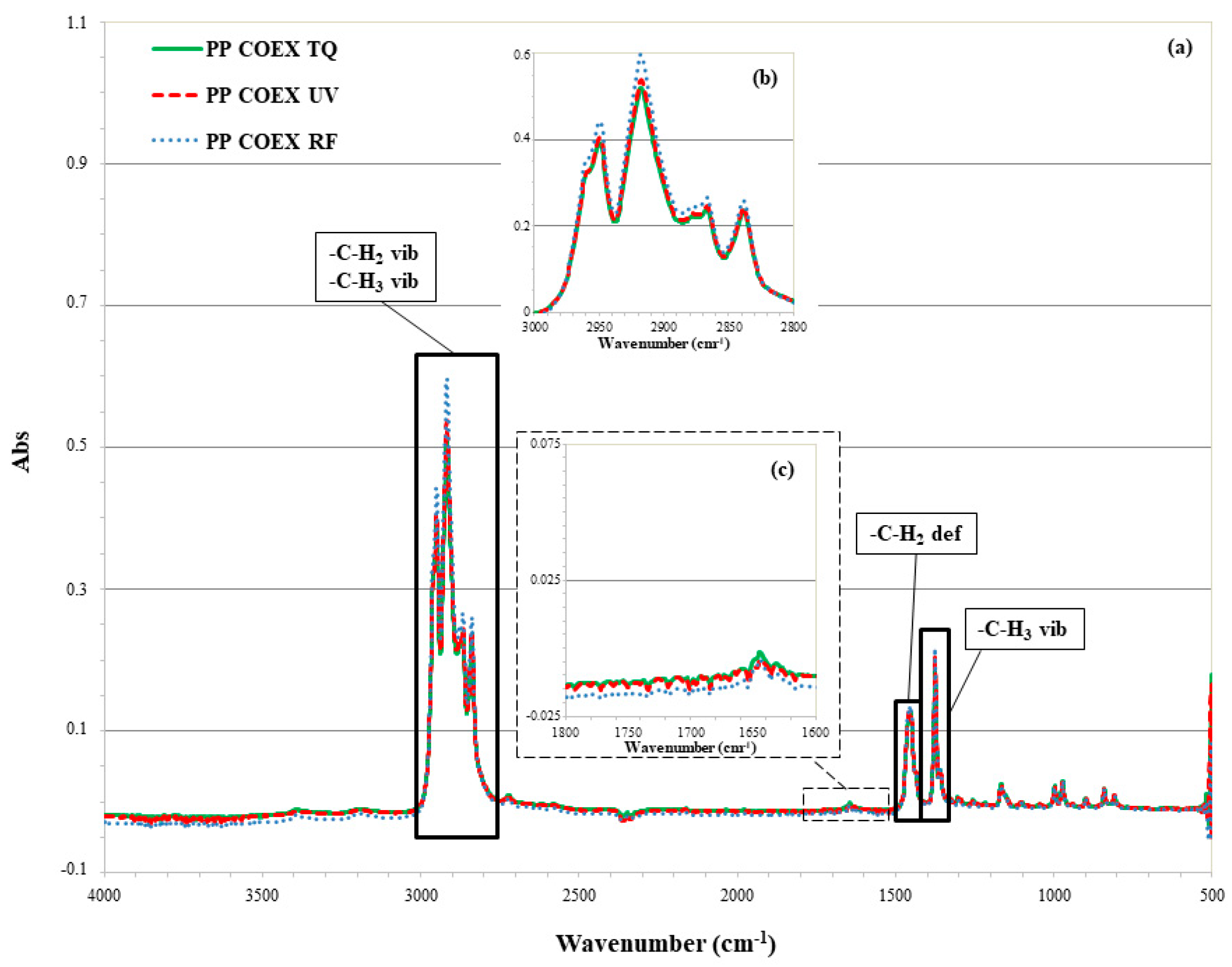
3.2. Surface Chemical Composition Analysis
3.2.1. ATR-FTIR of BIO E946
3.2.2. ATR-FTIR of PP Coex
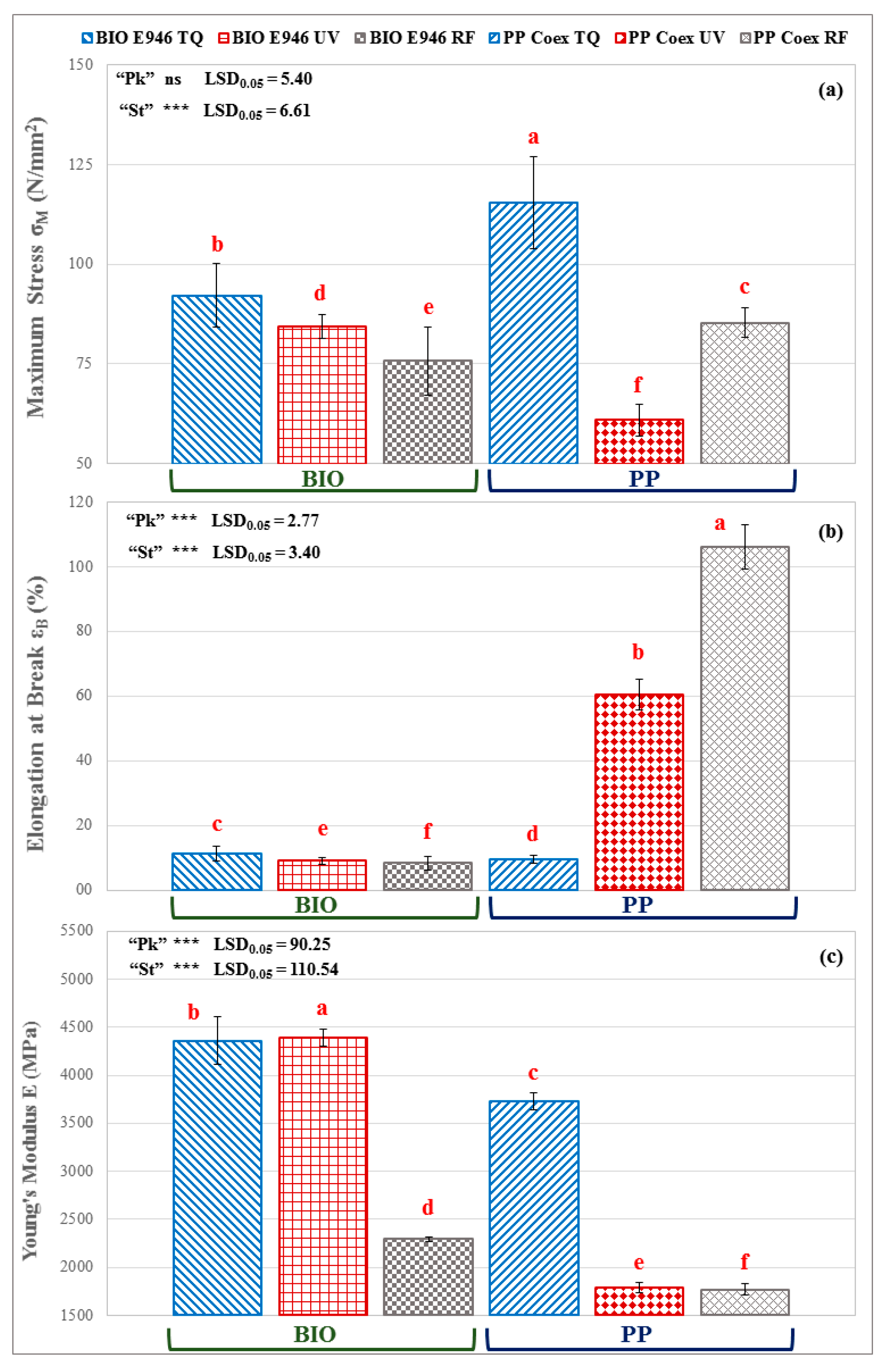
3.3. Tensile Mechanical Properties
3.4. Puncture Resistance
3.5. Overall Migration into Aqueous Food Simulant
3.6. OTR and WVTR
3.7. Biodegradation Test
3.8. Disintegration Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ASTM. ASTM D5488-94d: Standard Terminology of Environmental Labeling of Packaging Materials and Packages; ASTM: West Conshohocken, PA, USA, 1994. [Google Scholar]
- CEN. CEN-EN 13432: Packaging—Requirements for Packaging Recoverable through Composting and Biodegradation—Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging; EN: Brussels, Belgium, 2000. [Google Scholar]
- Rizzarelli, P.; Degli Innocenti, F.; Valenti, G.; Rapisarda, M. Biodegradation of Green Polymer Composites: Laboratory Procedures and Standard Test Methods. In Advanced Applications of Bio-Degradable Green Composites; Al-Ahmed, A., Inamuddin, Eds.; Materials Research Forum LLC: Millersville, PA, USA, 2020; Volume 68, pp. 1–44. ISBN 978-164-490-065-9. [Google Scholar]
- OECD. OECD Guideline for the Testing of Chemicals, Terrestrial Plant Test: 208: Seedling Emergence and Seedling Growth Test, September 2003; OECD: Paris, France, 2003.
- Tharanathan, R.N. Biodegradable films and composite coatings: Past, present and future. Trends Food Sci. Technol. 2003, 14, 71–78. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S. Totally and partially biodegradable polymer blends based on natural synthetic macromolecules: Preparation, physical properties, and potential as food packaging materials. J. Macromol. Sci. 1999, 39, 205–271. [Google Scholar] [CrossRef]
- European Bioplastics. Available online: http://www.european-bioplastics.org (accessed on 23 July 2020).
- Peelman, N.; Ragaert, P.; De Meulenaer, B.; Adons, D.; Peeters, R.; Cardon, L.; Van Impe, F.; Devlieghere, F. Application of bioplastics for food packaging. Trends Food Sci. Technol. 2013, 32, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayon, E.; Lopez, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Jacquot, M.; Desobry, S. Poly-Lactic Acid: Production, Applications, Nanocomposites, and Release Studies. Compr. Rev. Food Sci. Saf. 2010, 9, 552–571. [Google Scholar] [CrossRef]
- Rizzo, V.; Sapienza, G.; Restuccia, C.; Mauromicale, G.; Lombardo, S.; Pesce, G.R.; Rapisarda, M.; Perna, S.; Rizzarelli, P.; Muratore, G. Shelf life evaluation of fresh-cut globe artichoke packaged in a compostable biobased film. Ital. J. Food Sci. 2016, 28, 7–12. [Google Scholar]
- Ierna, A.; Rizzarelli, P.; Malvuccio, A.; Rapisarda, M. Effect of different anti-browning agents on quality of minimally processed early potatoes packaged on a compostable film. LWT Food Sci. Technol. 2017, 85, 434–439. [Google Scholar] [CrossRef]
- Patanè, C.; Malvuccio, A.; Saita, A.; Rizzarelli, P.; Rapisarda, M.; Rizzo, V.; Muratore, G. Quality aspects of fresh-cut ‘long-storage tomato’ as affected bypackage, calcium chloride and storage time. Int. J. Food Sci. Technol. 2017, 53, 819–827. [Google Scholar] [CrossRef]
- Patanè, C.; Malvuccio, A.; Saita, A.; Rizzarelli, P.; Siracusa, L.; Rizzo, V.; Muratore, G. Nutritional changes during storage in fresh-cut long storage tomato as affected by biocompostable polylactide and cellulose based packaging. LWT Food Sci. Technol. 2019, 101, 618–624. [Google Scholar] [CrossRef]
- NatureFlex™ Packaging Solutions. Available online: https://www.natureflex.com/packaging-solutions/ (accessed on 21 July 2020).
- Tremarin, A.; Brandão, T.R.S.; Silva, L.M. Application of ultraviolet radiation and ultrasound treatments for Alicyclobacillus acidoterrestris spores inactivation in apple juice. LWT—Food Sci. Technol. 2017, 78, 138–142. [Google Scholar] [CrossRef]
- Hakguder Taze, B.; Unluturk, S.; Buzrul, S.; Alpas, H. The impact of UV-C irradiation on spoilage microorganisms and colour of orange juice. J. Food Sci. Technol. 2015, 52, 1000–1007. [Google Scholar] [CrossRef] [Green Version]
- Birmpa, A.; Sfika, V.; Vantarakis, A. Ultraviolet light and ultrasound as non-thermal treatments for the inactivation of microorganisms in fresh ready-to-eat foods. Int. J. Food Microbiol. 2013, 167, 96–102. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Ascione, L.; Mistretta, M.C.; Rapisarda, M.; Rizzarelli, P. Comparative Investigation on the Soil Burial Degradation Behaviour of Polymer Films for Agriculture before and after Photo-Oxidation. Polymers 2020, 12, 753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO. ISO 527-1: Determination of Tensile Properties—Part 1: General Principles; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- ISO. ISO 527-3: Determination of Tensile Properties—Part 3: Test Conditions for Film and Sheets; ISO: Geneva, Switzerland, 1996. [Google Scholar]
- UNI EN. UNI EN 14477: Packaging—Flexible Packaging Material—Determination of Puncture Resistance—Test Methods; UNI EN: Geneva, Switzerland, 2004. [Google Scholar]
- EU. Commission Regulation (EU) N. 10/2011, Plastic Materials and Articles Intended to Come into Contact with Food; EU: Brussels, Belgium, 2011.
- ISO. ISO 14851: Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in An Aqueous Medium—Method by Measuring the Oxygen Demand in A Closed Respirometer; ISO: Geneva, Switzerland, 1999. [Google Scholar]
- ISO. ISO 20200: Determination of the Degree of Disintegration of A Plastic Materials under Simulated Composting Conditions in A Laboratory-Scale Test; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- ISO. ISO 3310-1: Test Sieves—Technical Requirements and Testing—Part 1: Test Sieves of Metal wire Cloth; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Li, Y.; Bai, Y.; Huang, J.; Yuan, C.; Ding, T.; Liu, D.; Hu, Y. Airglow discharge plasma treatment affects the surface structure and physical properties of zein films. J. Food Eng. 2020, 273, 109813–109820. [Google Scholar] [CrossRef]
- Kasalkova, N.S.; Slepicka, P.; Kolska, Z.; Svorcik, V. Wettability and Other Surface Properties of Modified Polymers. In Wetting and Wettability, 1st ed.; Aliofkhazraei, M., Ed.; IntechOpen: London, UK, 2015; Volume 12, pp. 323–356. [Google Scholar] [CrossRef] [Green Version]
- Briggs, D. X-ray photoelectron spectroscopy studies of polymer surfaces. Part 1 Chromic acid etching of polyolefins. J. Mat. Sci. 1976, 11, 1270–1277. [Google Scholar] [CrossRef]
- Rossegger, E.; Hennen, D.; Griesser, T.; Roppolo, I.; Schlög, S. Directed motion of water droplets on multi-gradient photopolymer surfaces. Polym. Chem. 2019, 10, 1882–1893. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose—structure and characterization. Cell. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Schwanninger, M.; Rodriguesc, J.C.; Pereirac, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Garside, P.; Wyeth, P. Identification of Cellulosic Fibres by FTIR Spectroscopy—Thread and Single Fibre Analysis by Attenuated Total Reflectance. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Leys, C.; Gengembre, L.; Payen, E. Comparison between XPS- and FTIR-analysis of plasma-treated polypropylene film surfaces. Surf. Interface Anal. 2008, 40, 597–600. [Google Scholar] [CrossRef]
- Mitchell, G.; France, F.; Nordon, A.; Tang, P.L.; Gibson, L.T. Assessment of historical polymers using attenuated total reflectance-Fourier transform infra-red spectroscopy with principal component analysis. Herit. Sci. 2013, 1, 28. [Google Scholar] [CrossRef] [Green Version]
- Sinha, D.; Swu, T.; Tripathy, S.P.; Mishra, R.; Dwivedi, K.K.; Fink, D. Spectroscopic and thermal studies of gamma irradiated polypropylene polymer. Radiat. Effects Defects Solids 2013, 158, 531–537. [Google Scholar] [CrossRef]
- Barbeş, L.; Radulescu, C.; Stihi, C. ATR-FTIR Spectrometry characterisation of polymeric materials. Rom. Rep. Phys. 2014, 66, 765–777. [Google Scholar]
- Kaczmarek, H.; Ołdak, D.; Malanowski, P.; Chaberska, H. Effect of short wavelength UV-irradiation on ageing of polypropylene/cellulose compositions. Polym. Degrad. Stab. 2004, 88, 189–198. [Google Scholar] [CrossRef]
- Yu, J.G.; Wang, N.; Ma, X.F. The effects of citric acid on the properties of thermoplastic starch plasticized by glycerol. Starch 2005, 57, 494–504. [Google Scholar] [CrossRef]
- Shimamura, H.; Nakamura, T. Investigation of degradation mechanisms in mechanical properties of polyimide films exposed to a low earth orbit environment. Polym. Degrad. Stab. 2010, 95, 21–33. [Google Scholar] [CrossRef]
- Rapisarda, M.; La Mantia, F.P.; Ceraulo, M.; Mistretta, M.C.; Giuffrè, C.; Pellegrino, R.; Valenti, G.; Rizzarelli, P. Photo-Oxidative and Soil Burial Degradation of Irrigation Tubes Based on Biodegradable Polymer Blends. Polymers 2019, 11, 1489. [Google Scholar] [CrossRef] [Green Version]
- Kijchavengkul, T.; Auras, R. Compostability of polymers. Polym. Inter. 2008, 57, 793–804. [Google Scholar] [CrossRef]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Kossentini Kallel, T.; Houichi, H.; Sayari, A.; Kammoun, M. Assessment of biodegradation of PLA/PCL and PLA/PEG biopolymers under aerobic and anaerobic conditions. J. Mater. Environ. Sci. 2015, 6, 2542–2549. [Google Scholar]
- Höglund, A.; Odelius, K.; Albertsson, A.C. Crucial differences in the hydrolytic degradation between industrial polylactide and laboratory-scale poly(L-lactide). ACS Appl. Mater. Interfaces 2012, 4, 2788–2793. [Google Scholar] [CrossRef]


| Package | Sterilization Treatment | Maximum Stress σM [N mm−2] | Elongation at Break εB [%] | Young’s Modulus E [MPa] |
|---|---|---|---|---|
| BIO E946 | TQ | 92.1 ± 7.9 | 11.2 ± 2.2 | 4360 ± 252 |
| UV | 84.4 ± 3.1 | 9.1 ± 1.0 | 4396 ± 89 | |
| RF | 75.7 ± 8.5 | 8.3 ± 2.1 | 2294 ± 27 | |
| PP Coex | TQ | 115.5 ± 11.5 | 9.5 ± 1.4 | 3732 ± 88 |
| UV | 60.9 ± 4.0 | 60.5 ± 4.7 | 1788 ± 54 | |
| RF | 85.3 ± 3.8 | 106.2 ± 6.8 | 1770 ± 58 | |
| Package effect (Pk) | BIO E946 | 84.1 a | 9.5 b | 3683 a |
| PP Coex | 87.3 a | 58.7 a | 2430 b | |
| Treatment effect (St) | TQ | 103.8 a | 10.4 c | 4046 a |
| UV | 80.5 b | 34.8 b | 3092 b | |
| RF | 72.7 c | 57.3 a | 2032 c | |
| LSDPk × St (p ≤ 0.05) | - | 9.35 | 4.80 | 15.633 |
| Package | Treatment | Maximum Load F [N] | Elongation at Break SMax [mm] |
| BIO E946 | TQ | 2.49 ± 0.12 | 1.18 ± 0.05 |
| UV | 2.49 ± 0.15 | 1.13 ± 0.07 | |
| RF | 2.21 ± 0.14 | 1.19 ± 0.04 | |
| PP Coex | TQ | 3.58 ± 0.07 | 1.68 ± 0.07 |
| UV | 3.60 ± 0.05 | 1.65 ± 0.05 | |
| RF | 3.79 ± 0.09 | 1.83 ± 0.05 |
| Sample Containers | Maverage (mg dm−2) | ||
|---|---|---|---|
| PLA (26 μm) |  | TQ | nd* |
| UV | nd* | ||
| PET (23 μm) |  | TQ | 0.17 |
| UV | 0.62 | ||
| Package | Treatment | OTR (cm3 m−2 24h−1) | P O2 (cm3 mil m−2 24h−1) | WVTR (g m−2 24h−1) | P WV (g μm m−2 24h−1) |
|---|---|---|---|---|---|
| BIO E946 | TQ | 33.5 | 42.2 | 9.6 | 307.2 |
| UV | 27.0 | 34.0 | 9.0 | 289.0 | |
| PP Coex | TQ | 2292.8 | 1805.4 | 0.7 | 16.6 |
| UV | 2359.6 | 1858.0 | 0.5 | 10.6 |
| Sample | Treatment | Degree of Disintegration (%) |
|---|---|---|
| Trays in PLA | TQ | 100.0 |
| UV | 100.0 | |
| BIO E946 | TQ | 99.0 |
| UV | 97.2 | |
| Paper | TQ | 100.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapisarda, M.; Patanè, C.; Pellegrino, A.; Malvuccio, A.; Rizzo, V.; Muratore, G.; Rizzarelli, P. Compostable Polylactide and Cellulose Based Packaging for Fresh-Cut Cherry Tomatoes: Performance Evaluation and Influence of Sterilization Treatment. Materials 2020, 13, 3432. https://doi.org/10.3390/ma13153432
Rapisarda M, Patanè C, Pellegrino A, Malvuccio A, Rizzo V, Muratore G, Rizzarelli P. Compostable Polylactide and Cellulose Based Packaging for Fresh-Cut Cherry Tomatoes: Performance Evaluation and Influence of Sterilization Treatment. Materials. 2020; 13(15):3432. https://doi.org/10.3390/ma13153432
Chicago/Turabian StyleRapisarda, Marco, Cristina Patanè, Alessandra Pellegrino, Angelo Malvuccio, Valeria Rizzo, Giuseppe Muratore, and Paola Rizzarelli. 2020. "Compostable Polylactide and Cellulose Based Packaging for Fresh-Cut Cherry Tomatoes: Performance Evaluation and Influence of Sterilization Treatment" Materials 13, no. 15: 3432. https://doi.org/10.3390/ma13153432