PEG Grafted Polymethacrylates Bearing Antioxidants as a New Class of Polymer Conjugates for Application in Cosmetology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
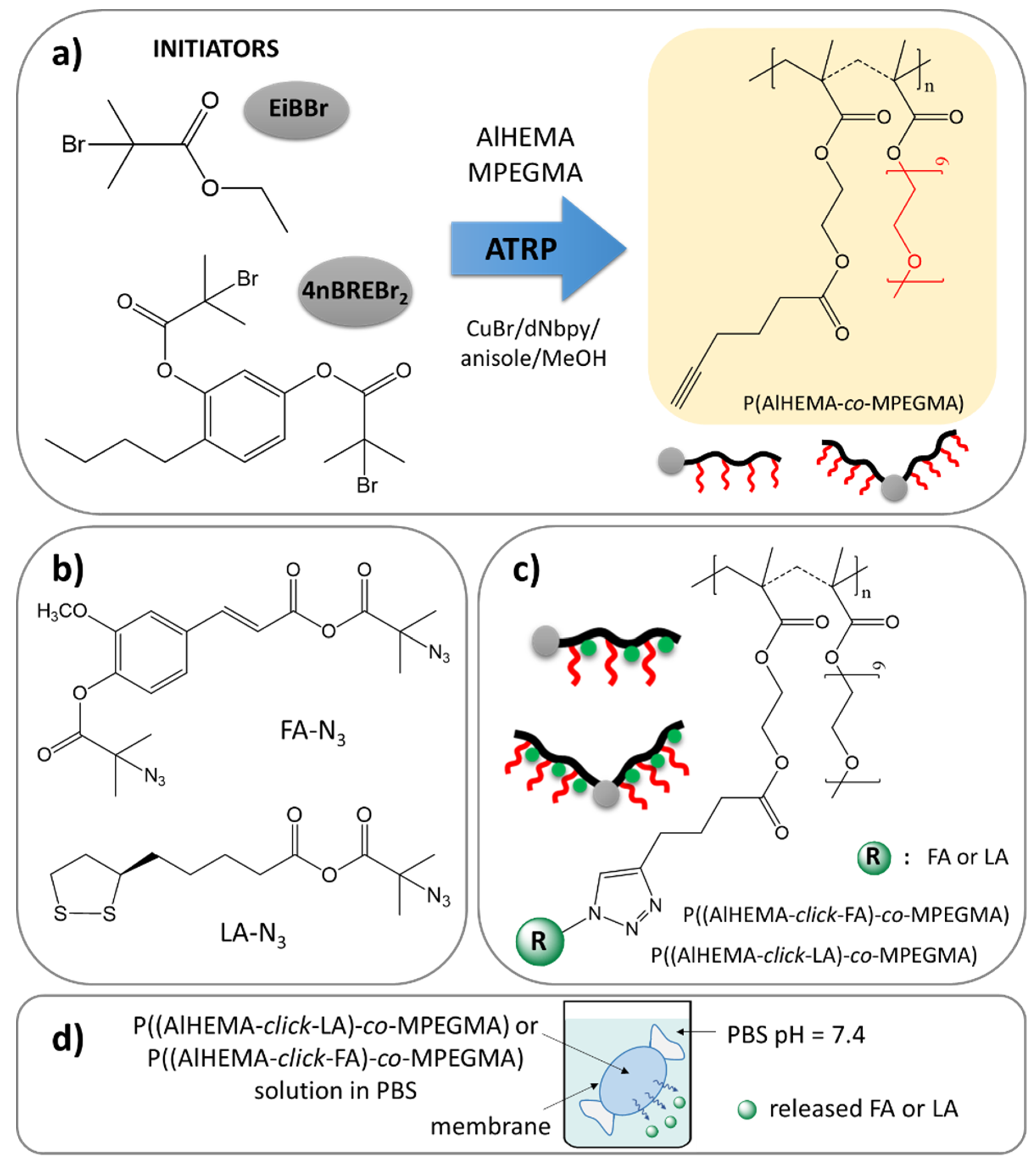
2.3. P(AlHEMA-co-MPEGMA) Synthesized in the Presence of EiBBr (Example for I)
2.4. P(AlHEMA-co-MPEGMA) Synthesized in the Presence of 4nBREBr2 (Example for III)
2.5. Modification of FA to the Azide Derivative (FA-N3)
2.6. Modification of LA to the Azide Derivative (LA-N3)
2.7. CuAAC Reaction (Example for I-FA)
2.8. Determination of Antioxidant Amount in Polymer
2.9. Antioxidant Release
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Duncan, R.; Spreafico, F. Polymer conjugates. Clin. Pharmacokinet. 1994, 27, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.; Ghandehari, H. Polymeric conjugates for drug delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Hong, Y.; Xi, Y.; Zou, Y.; Gao, J.; Du, J. Synthesis, self-assembly, and biomedical applications of antimicrobial peptide–polymer conjugates. Biomacromolecules 2018, 19, 1701–1720. [Google Scholar] [CrossRef]
- Lueckerath, T.; Strauch, T.; Koynov, K.; Barner-Kowollik, C.; Ng, D.Y.W.; Weil, T. DNA–polymer conjugates by photoinduced RAFT polymerization. Biomacromolecules 2019, 20, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haag, R.; Kratz, F. Polymer therapeutics: Concepts and applications. Angew. Chem. 2006, 45, 1198–1215. [Google Scholar] [CrossRef]
- Vasey, P.; Kaye, S.; Morrison, R.; Twelves, C.; Wilson, P.; Duncan, R.; Thomson, A.; Murray, L.; Hilditch, T.; Murray, T.; et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: First member of a new class of chemotherapeutic agents-drug-polymer conjugates. Clin. Cancer Res. 1999, 5, 83–94. [Google Scholar]
- Seymour, L.; Ferry, D.; Anderson, D.; Hesslewood, S.; Julyan, P.; Poyner, R.; Doran, J.; Young, A.; Burtles, S.; Kerr, D. Hepatic drug targeting: Phase I evaluation of polymer-bound doxorubicin. J. Clin. Oncol. 2002, 15, 1668–1676. [Google Scholar] [CrossRef]
- Meerum, T.J.; Bokkel, H.W.; Schellens, J.; Schot, M.; Mandjes, I.; Zurlo, M.; Rocchetti, M.; Rosing, H.; Koopman, F.; Beijnen, J. Phase I clinical and pharmacokinetic study of PNU166945, a novel water-soluble polymer-conjugated prodrug of paclitaxel. Anticancer Drugs 2001, 12, 315–323. [Google Scholar] [CrossRef]
- Caiolfa, V.; Zamal, M.; Fiorini, A.; Frigerio, E.; D’Argy, R.; Ghigleri, A.; Farao, M.; Angelucci, F.; Suarato, A. Polymer-bound camptothecin: Initial biodistribution and antitumour activity studies. J. Control. Release 2000, 65, 105–119. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Aher, N.; Patil, R.; Khandare, J. Poly(ethylene glycol)-prodrug conjugates: Concept, design, and applications. J. Drug Deliv. 2012, 2012, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Chipman, S.D.; Oldham, F.B.; Pezzoni, G.; Singer, J.W. Biological and clinical characterization of paclitaxel poliglumex (PPX, CT-2103), a macromolecular polymer–drug conjugate. Int. J. Nanomed. 2006, 1, 375–383. [Google Scholar] [CrossRef]
- Pang, X.; Jiang, Y.; Xiao, Q.; Leung, A.W.; Hua, H.; Xu, C. pH-responsive polymer–drug conjugates: Design and progress. J. Control. Release 2016, 222, 116–129. [Google Scholar] [CrossRef]
- Su, Y.; Hu, Y.; Du, Y.; Huang, X.; He, J.; You, J.; Yuan, H.; Hu, F. Redox-responsive polymer–drug conjugates based on doxorubicin and chitosan oligosaccharide-g-stearic acid for cancer therapy. Mol. Pharm. 2015, 12, 1193–1202. [Google Scholar] [CrossRef]
- Etrych, T.; Kovář, L.; Strohalm, J.; Chytil, P.; Říhová, B.; Ulbrich, K. Biodegradable star HPMA polymer–drug conjugates: Biodegradability, distribution and anti-tumor efficacy. J. Control. Release 2011, 154, 241–248. [Google Scholar] [CrossRef]
- Mundlia, J.; Ahuja, M.; Kumar, P.; Pillay, V. Improved antioxidant, antimicrobial and anticancer activity of naringenin on conjugation with pectin. 3 Biotech 2019, 9, 312. [Google Scholar] [CrossRef]
- Zhang, R.; Jones, M.M.; Moussa, H.; Keskar, M.; Huo, N.; Zhang, Z.; Visser, M.B.; Sabatini, C.; Swihart, M.T.; Cheng, C. Polymer–antibiotic conjugates as antibacterial additives in dental resins. Biomater. Sci. 2019, 7, 287–295. [Google Scholar] [CrossRef]
- Dubey, R.D.; Klippstein, R.; Wang, J.T.-W.; Hodgins, N.; Mei, K.-C.; Sosabowski, J.; Hider, R.C.; Abbate, V.; Gupta, P.N.; Al-Jamal, K.T. Novel hyaluronic acid conjugates for dual nuclear imaging and therapy in CD44-expressing tumors in mice in vivo. Nanotheranostics 2017, 1, 59–79. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Liu, J.; Dong, Y.; Sima, M.; Kopečková, P.; Brandi, M.; Kopeček, J. Release of prostaglandin E(1) from N-(2-hydroxypropyl)methacrylamide copolymer conjugates by bone cells. Macromol. Biosci. 2008, 8, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Requejo-Aguilar, R.; Alastrue-Agudo, A.; Cases-Villar, M.; Lopez-Mocholi, E.; England, R.; Vicent, M.J.; Moreno-Manzano, V. Combined polymer-curcumin conjugate and ependymal progenitor/stem cell treatment enhances spinal cord injury functional recovery. Biomaterials 2017, 113, 18–30. [Google Scholar] [CrossRef]
- Mielańczyk, A.; Neugebauer, D. Designing drug conjugates based on sugar decorated V shape and star polymethacrylates: Influence of composition and architecture of polymeric aarrier. Bioconjug. Chem. 2015, 26, 2303–2310. [Google Scholar] [CrossRef]
- Mielańczyk, A.; Skonieczna, M.; Mielańczyk, Ł.; Neugebauer, D. In vitro evaluation of doxorubicin-conjugates based on sugar core non-linear polymethacrylates toward anticancer drug delivery. Biocojug. Chem. 2016, 27, 893–904. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Du, X.; Sun, Y.; Zhang, M.; He, J.; Ni, P. Polyphosphoester-camptothecin prodrug with reduction-response prepared via michael addition polymerization and click reaction. ACS Appl. Mater. Interfaces 2017, 9, 13939–13949. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Dai, Y. Click polymerization for the synthesis of reduction-responsive polymeric prodrug. J. Nanopart. Res. 2018, 20, 126. [Google Scholar] [CrossRef]
- Gao, M.; Deng, J.; Chu, H.; Tang, Y.; Wang, Z.; Zhao, Y.; Li, G. Stereoselective stabilization of polymeric vitamin E conjugate micelles. Biomacromolecules 2017, 18, 4349–4356. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Vanaparthi, A.; Rompicharla, S.V.K.; Kumari, P.; Ghosh, B.; Biswas, S. Cholesterol and vitamin E-conjugated PEGylated polymeric micelles for efficient delivery and enhanced anticancer activity of curcumin: Evaluation in 2D monolayers and 3D spheroids. Artif. Cell. Nanomed. Biotechnol. 2018, 46, S773–S786. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Wu, M.; Fan, L.; He, C.; Wan, J.-B.; Li, P.; Chen, M.; Li, H. Vitamin E succinate-conjugated F68 micelles for mitoxantrone delivery in enhancing anticancer activity. Int. J. Nanomed. 2016, 11, 3167–3178. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Deng, L.; Chen, Z.; Chen, Z.; Yu, J.; Liu, H.; Li, T.; Lin, T.; Chen, H.; Zhao, M.; et al. Vitamin B12-conjugated sericin micelles for targeting CD320-overexpressed gastric cancer and reversing drug resistance. Nanomedicine 2018, 14, 1. [Google Scholar] [CrossRef]
- Kim, K.S.; Song, C.J.; Jaung, J.Y.; Na, K. Two arms hydrophilic photosensitizer conjugates with vitamin B for cancer-selective photodynamic therapy. Polym. Adv. Technol. 2017, 28, 443–448. [Google Scholar] [CrossRef]
- Chakraborty, A.; Jana, N.R. Vitamin C-conjugated nanoparticle protects cells from oxidative stress at low doses but induces oxidative stress and cell death at high doses. ACS Appl. Mater. Inter. 2017, 9, 41807–41817. [Google Scholar] [CrossRef]
- Castleberry, S.A.; Quadir, M.A.; Sharkh, M.A.; Shopsowitz, K.E.; Hammond, P.T. Polymer conjugated retinoids for controlled transdermal delivery. J. Control. Release 2017, 262, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yim, H.; Jo, E.A.; Na, K. Development of a new micellar anticancer drug: Cationic polymer/vitamin A conjugate covered with hyaluronic acid. Macromol. Res. 2010, 18, 913–918. [Google Scholar] [CrossRef]
- Wang, D.; Mao, L.; Dai, L.; Yuan, F.; Gao, Y. Characterization of chitosan-ferulic acid conjugates and their application in the design of β-carotene bilayer emulsions with propylene glycol alginate. Food Hydrocoll. 2018, 80, 281–291. [Google Scholar] [CrossRef]
- Li, C.; Li, J.-B. Preparation of chitosan-ferulic acid conjugate: Structure characterization and in the application of pharmaceuticals. Int. J. Biol. Macromol. 2017, 105, 1539–1543. [Google Scholar] [CrossRef]
- Luo, Q.; Han, Q.; Chen, L.; Fan, X.; Wang, Y.; Fei, Z.; Zhang, H.; Wang, Y. Redox response, antibacterial and drug package capacities of chitosan-α-lipoic acid conjugates. Int. J. Biol. Macromol. 2020, 154, 1166–1174. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, J.; Feng, X.; Li, W.; Wang, Y.; Jin, H.; Huang, H.; Liu, Y.; Fan, D. Reduction-responsive core-crosslinked micelles based on a glycol chitosan–lipoic acid conjugate for triggered release of doxorubicin. RSC Adv. 2016, 6, 31391–31400. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, M.; Zhang, J.; Sun, H.; Deng, C.; Zhong, Z. Integrated multifunctional micelles co-self-assembled from polypeptides conjugated with natural ferulic acid and lipoic acid for doxorubicin delivery. Chem. Phys. Chem. 2018, 19, 2070–2077. [Google Scholar] [CrossRef]
- Han, X.; Zhang, Z.; Shen, H.; Zheng, J.; Zhang, G. Comparison of structures, physicochemical properties and in vitro bioactivity between ferulic acid-β-cyclodextrin conjugate and the corresponding inclusion complex. Food Res. Int. 2019, 125, 108619. [Google Scholar] [CrossRef]
- Lahiani, A.; Hidmi, A.; Katzhendler, J.; Yavin, E.; Lazarovici, P. Novel synthetic PEGylated conjugate of α-lipoic acid and tempol reduces cell death in a neuronal PC12 clonal line subjected to ischemia. ACS Chem. Neurosci. 2016, 7, 1452–1462. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Farhat, D.; Lincet, H. Lipoic acid a multi-level molecular inhibitor of tumorigenesis. BBA-Rev. Cancer 2020, 1873, 188317. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Witt, E.H.; Tritschler, H.J. Alpha-lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Odrobińska, J.; Neugebauer, D. Retinol derivative as bioinitiator in the synthesis of hydroxyl-functionalized polymethacrylates for micellar delivery systems. eXPRESS Polym. Lett. 2019, 13, 806–817. [Google Scholar] [CrossRef]
- Odrobińska, J.; Niesyto, K.; Erfurt, K.; Siewniak, A.; Mielańczyk, A.; Neugebauer, D. Retinol-containing graft copolymers for delivery of skin-curing agents. Pharmaceutics 2019, 11, 378. [Google Scholar] [CrossRef] [Green Version]
- Odrobińska, J.; Mielańczyk, Ł.; Neugebauer, D. 4-n-Butylresorcinol-based linear and graft polymethacrylates for arbutin and vitamins delivery by micellar systems. Polymers 2020, 12, 330. [Google Scholar] [CrossRef] [Green Version]
- Bernaczek, K.; Mielańczyk, A.; Mielańczyk, Ł.; Neugebauer, D.; Grzywna, Z.J. Self-assembling water-soluble polymethacrylate–MTX conjugates: The significance of macromolecules architecture on drug conjugation efficiency, the final shape of particles, and drug release. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2476–2487. [Google Scholar] [CrossRef]






| M1/M2 | Time (h) | Conversion (%) | DG a (%) | DPn a | Mn,GC a (g/mol) | Mn,GPC b (g/mol) | D b | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| XNMR | XGC | ||||||||||
| M1 | M2 | M1 | M2 | ||||||||
| I | 25/75 | 2 | 59 | 58 | 58 | 60 | 76 | 238 | 102 500 | 54 400 | 1.29 |
| II | 75/25 | 2 | 44 | 44 | 40 | 45 | 28 | 163 | 49 000 | 18 500 | 1.35 |
| III | 25/75 | 2 | 54 | 17 | 41 | 24 | 64 | 114 | 45 800 | 28 300 | 1.35 |
| IV | 25/75 | 5 | 68 | 28 | 71 | 37 | 61 | 183 | 71 800 | 26 200 | 1.28 |
| V | 75/25 | 2 | 35 | 39 | 54 | 34 | 17 | 198 | 54 100 | 16 100 | 1.36 |
| DPAlHEMA | FAlHEMA (mol.%) | Eclick a (%) | Eclick b (%) | ntria.a | DC (mol.%) | Dh (nm) | PDI | RAS (mol.%) | Time (h) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Int. | Vol. | ||||||||||
| I-FA | 58 | 24 | 69 | 52 | 30 | 13 | 248 ± 36 | 250 ± 46 | 0.548 | 30 | 2.0 |
| II-FA | 119 | 73 | 18 | 17 | 20 | 13 | 106 ± 2 | 106 ± 11 | 1.000 | 12 | 3.0 |
| II-LA | 119 | 73 | 48 | 74 | 62 | 38 | c 82 ± 20 | c 71 ± 18 | 0.297 | 96 | 0.5 |
| III-FA | 41 | 36 | 38 | 38 | 16 | 14 | c 566 ± 46 | c 572 ± 75 | 1.000 | 32 | 2.5 |
| IV-FA | 71 | 39 | 34 | 33 | 23 | 20 | 323 ± 44 | 329 ± 57 | 0.836 | 8 | 2.5 |
| IV-LA | 71 | 39 | 57 | 70 | 50 | 27 | 202 ± 24 | 201 ± 32 | 0.694 | 87 | 1.0 |
| V-FA | 163 | 82 | 39 | 39 | 64 | 32 | 204 ± 15 | 204 ± 25 | 1.000 | 49 | 4.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odrobińska, J.; Neugebauer, D. PEG Grafted Polymethacrylates Bearing Antioxidants as a New Class of Polymer Conjugates for Application in Cosmetology. Materials 2020, 13, 3455. https://doi.org/10.3390/ma13163455
Odrobińska J, Neugebauer D. PEG Grafted Polymethacrylates Bearing Antioxidants as a New Class of Polymer Conjugates for Application in Cosmetology. Materials. 2020; 13(16):3455. https://doi.org/10.3390/ma13163455
Chicago/Turabian StyleOdrobińska, Justyna, and Dorota Neugebauer. 2020. "PEG Grafted Polymethacrylates Bearing Antioxidants as a New Class of Polymer Conjugates for Application in Cosmetology" Materials 13, no. 16: 3455. https://doi.org/10.3390/ma13163455
APA StyleOdrobińska, J., & Neugebauer, D. (2020). PEG Grafted Polymethacrylates Bearing Antioxidants as a New Class of Polymer Conjugates for Application in Cosmetology. Materials, 13(16), 3455. https://doi.org/10.3390/ma13163455






