Is Biochar from the Torrefaction of Sewage Sludge Hazardous Waste?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Production from Sewage Sludge
2.2. Determination of Physical and Chemical Properties of Sewage Sludge and Produced Biochars
2.3. Determination of Heavy Metal Leachability from Sewage Sludge and Produced Biochars
2.4. Toxicity Bioassay Using Biochar
2.5. Statistical Analyses
3. Results and Discussion
3.1. The Leachability of Biochars Obtained from Sewage Sludge via Torrefaction
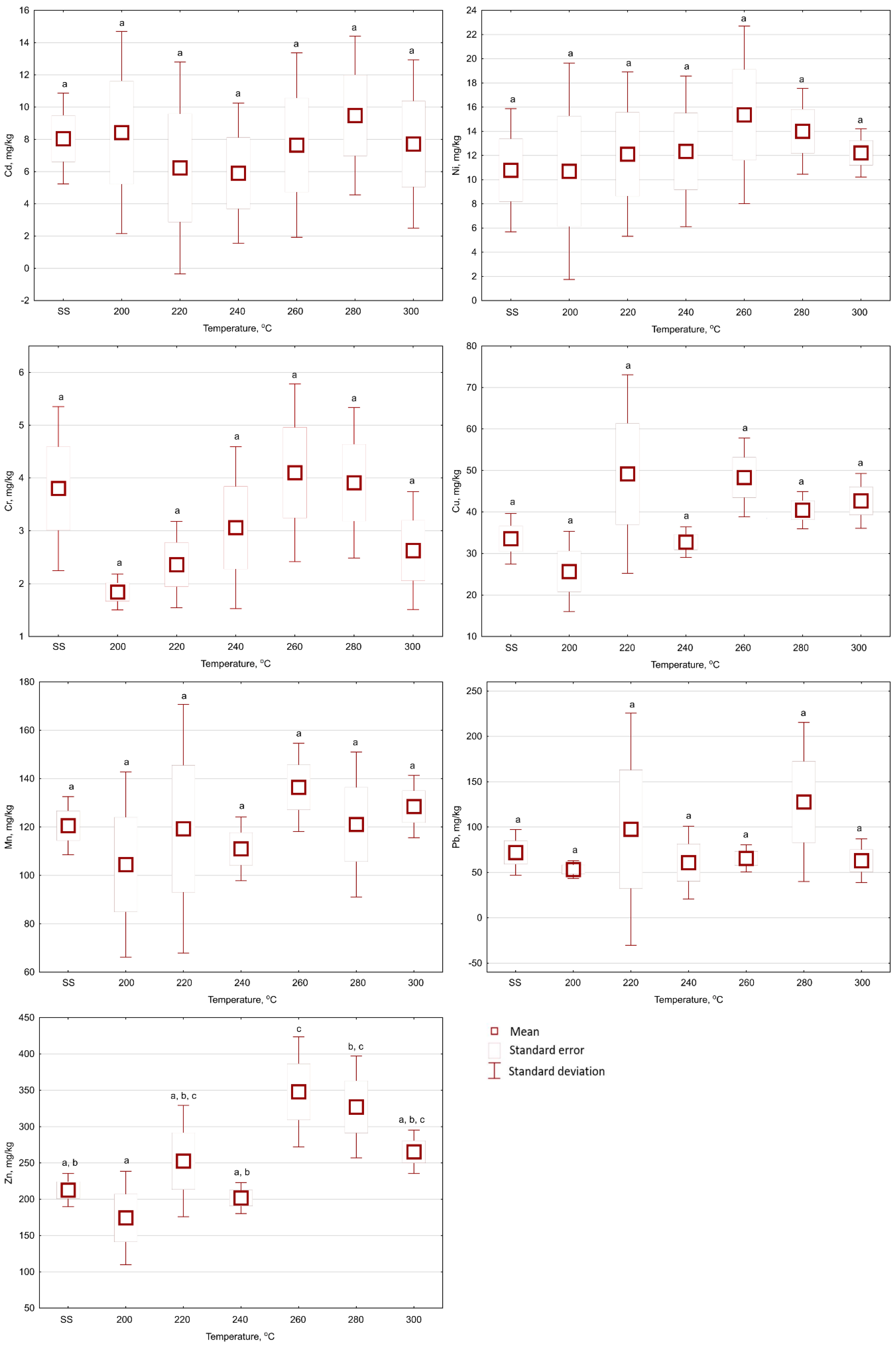
3.2. The Influences of Temperature and Time of the Torrefaction on Heavy Metal Content in Biochars from Sewage Sludge
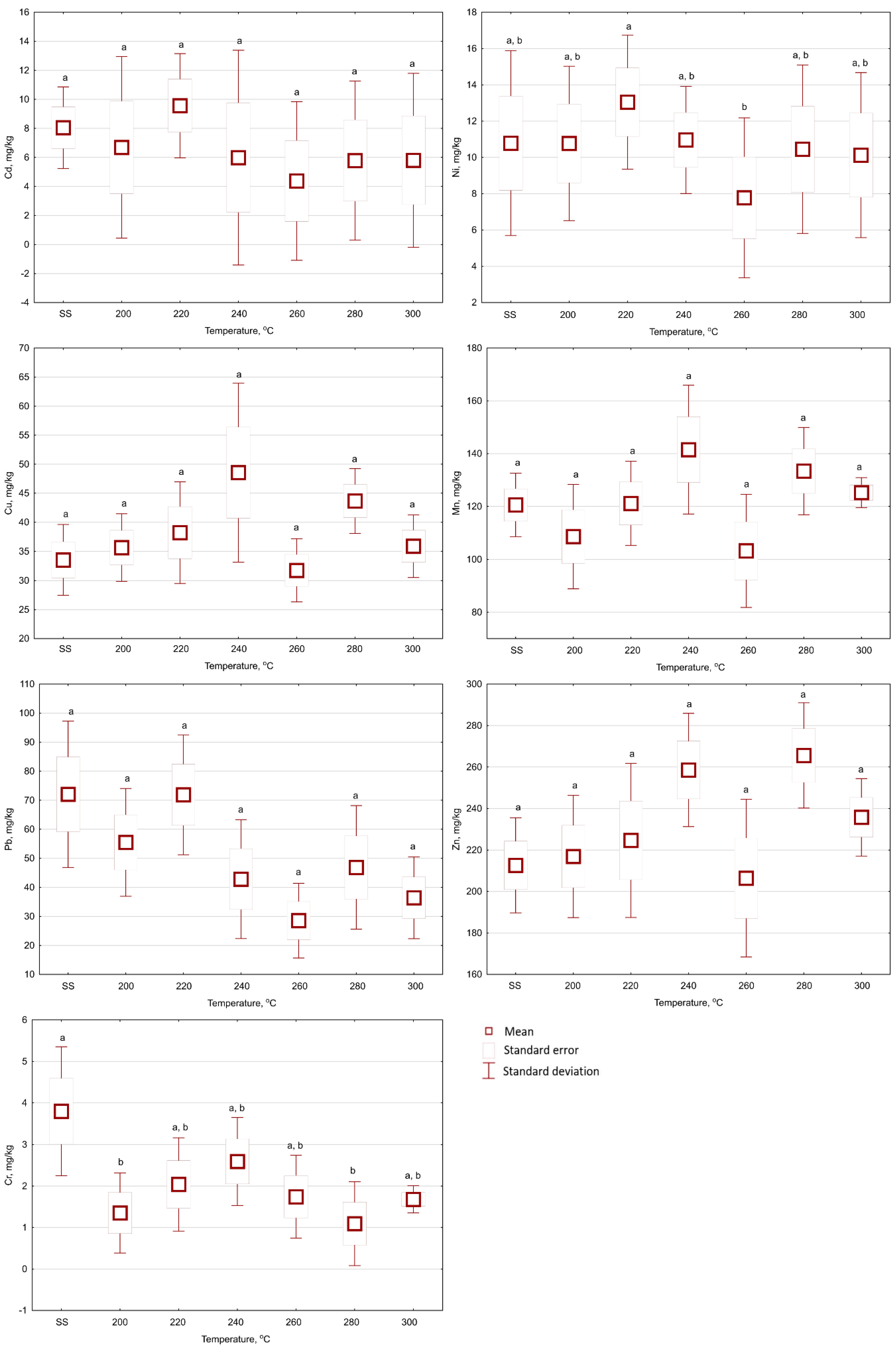
3.3. Heavy Metal Leachability from Biochars in Relation to Torrefaction Temperature and Retention Time
| Times, Min | Temperature, °C | Mass Yield, % | pH | Cu | Mn | Cd | Pb | Zn | Cr | Ni | Cu | Mn | Cd | Pb | Zn | Cr | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg·kg−1 d.m. | leachability | ||||||||||||||||
| Sewage Sludge | 7.2 | − | 0.93 | 0.49 | 1.73 | 2.68 | − | − | − | 0.8% | 6.1% | 2.4% | 1.3% | − | − | ||
| 20 | 200 | 94 | 7.4 | 0.01 | 0.84 | 0.53 | 2.08 | 1.11 | − | − | − | 0.7% | 7.0% | 2.4% | 0.5% | − | − |
| 20 | 220 | 94 | 7.3 | 0.23 | 0.81 | 0.54 | 2.60 | 0.87 | − | − | 0.7% | 0.7% | 5.6% | 3.5% | 0.4% | − | − |
| 20 | 240 | 94 | 7.2 | − | 0.87 | 0.46 | 1.59 | 0.43 | − | − | − | 0.7% | 4.1% | 2.4% | 0.2% | − | − |
| 20 | 260 | 93 | 7.5 | − | 0.75 | 0.47 | 1.54 | 1.46 | − | − | − | 0.6% | 6.2% | 2.9% | 0.5% | − | − |
| 20 | 280 | 91 | 7.3 | − | 1.76 | 1.09 | 3.53 | 1.26 | − | − | − | 1.3% | 12.2% | 6.2% | 0.5% | − | − |
| 20 | 300 | 89 | 7.1 | − | 1.07 | 0.78 | 2.83 | 0.83 | − | − | − | 0.8% | 6.4% | 1.7% | 0.3% | − | − |
| 40 | 200 | 91 | 7.1 | − | 1.27 | 0.73 | 2.27 | 0.72 | − | − | − | 1.2% | 8.6% | 4.3% | 0.4% | − | − |
| 40 | 220 | 92 | 7.1 | − | 1.53 | 1.04 | 3.26 | 1.15 | − | − | − | 1.3% | 16.7% | 3.3% | 0.5% | − | − |
| 40 | 240 | 90 | 7.1 | − | 1.30 | 0.83 | 4.34 | 0.91 | − | − | − | 1.2% | 14.0% | 7.1% | 0.5% | − | − |
| 40 | 260 | 90 | 7.0 | − | 1.20 | 1.22 | 7.77 | 1.79 | − | − | − | 0.9% | 16.0% | 11.9% | 0.5% | − | − |
| 40 | 280 | 88 | 7.1 | − | 2.08 | 0.85 | 3.87 | 0.91 | − | − | − | 1.7% | 8.9% | 3.0% | 0.3% | − | − |
| 40 | 300 | 83 | 7.0 | − | 1.33 | 1.06 | 4.67 | 1.40 | − | − | − | 1.0% | 13.8% | 7.4% | 0.5% | − | − |
| 60 | 200 | 90 | 7.1 | 0.04 | 1.36 | 1.06 | 4.24 | 1.18 | − | − | 0.1% | 1.3% | 15.9% | 7.6% | 0.5% | − | − |
| 60 | 220 | 91 | 7.1 | − | 1.07 | 0.83 | 3.09 | 0.61 | − | − | − | 0.9% | 8.6% | 4.3% | 0.3% | − | − |
| 60 | 240 | 89 | 7.1 | − | 1.09 | 0.67 | 2.52 | 0.66 | − | − | − | 0.8% | 11.3% | 5.9% | 0.3% | − | − |
| 60 | 260 | 88 | 7.1 | 0.05 | 0.94 | 0.57 | 2.49 | 0.59 | − | − | 0.2% | 0.9% | 13.0% | 8.7% | 0.3% | − | − |
| 60 | 280 | 87 | 6.9 | − | 1.23 | 0.87 | 3.70 | 0.71 | − | − | − | 0.9% | 15.1% | 7.9% | 0.3% | − | − |
| 60 | 300 | 81 | 7.0 | − | 1.45 | 0.92 | 4.25 | 1.16 | − | − | − | 1.2% | 15.8% | 11.7% | 0.5% | − | − |
| Hazardous waste threshold value * | − | − | 50 | − | 1 | 10 | 50 | 10 | 10 | − | − | − | − | − | − | − | |
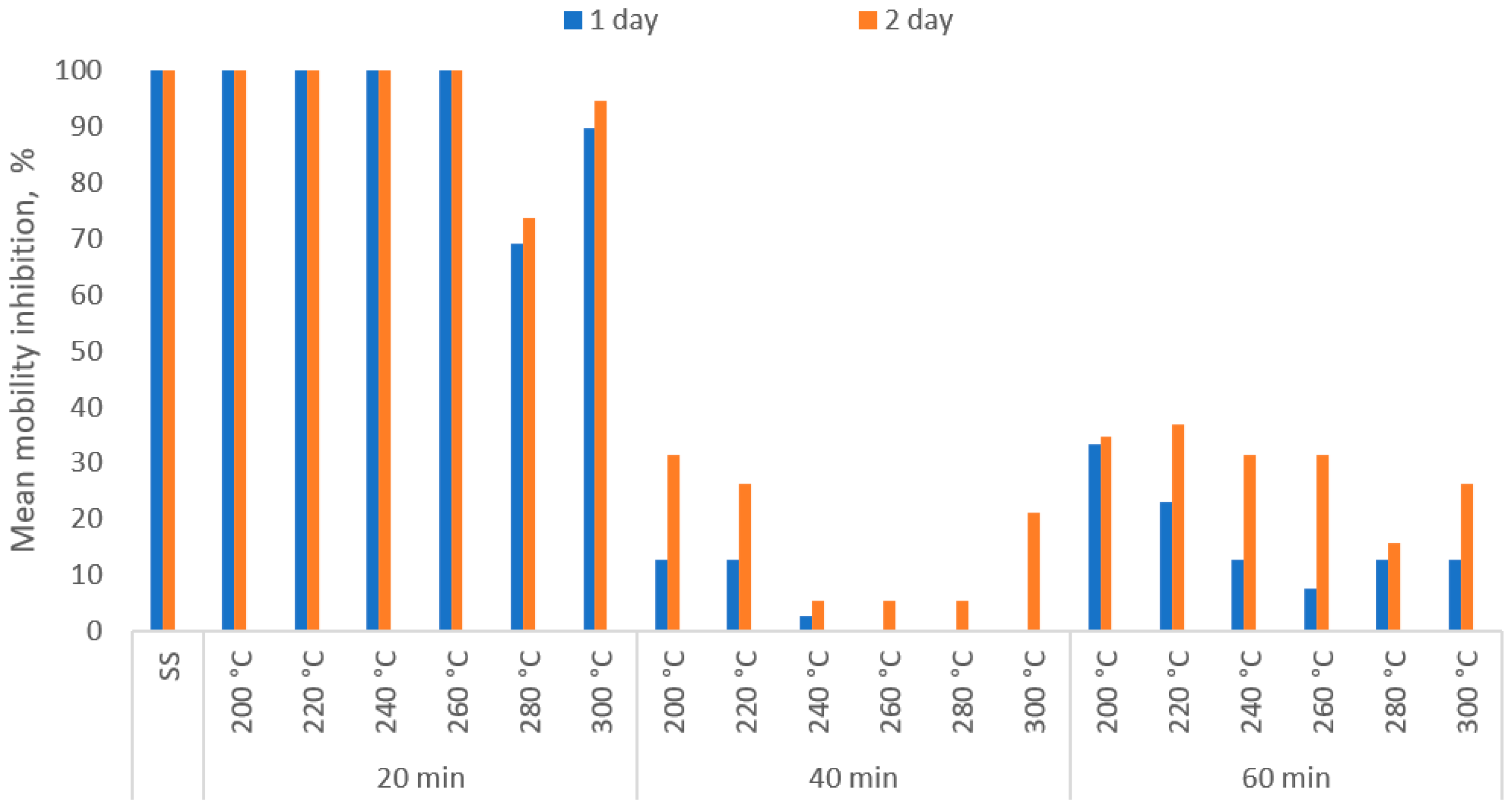
3.4. SS Biochars’ Ecotoxicity in Relation to Torrefaction Temperature and Process Retention Time
4. Conclusions
- The torrefaction process did not increase the contents of HMs in the biochars in relation to the raw SS. The individual HM concentrations in the biochars were not significantly dependent on the process temperature and retention time. The HM content in the biochar was relatively low and fulfilled the requirements of the Polish Regulation for agricultural use of SS. However, because of the high content of Cd from 4.4 to 12.3 mg Cd·kg−1 d.m. (also present in raw SS), it excluded the biochars from agricultural use as an “organic fertilizer.”
- Consistently decreased leachability of HMs from biochar in comparison to raw SS was measured only in the case of Zn. For other metals, no such trend was visible, i.e., no significant trend for specific variants compared to raw SS.
- The leachability of Cd (12%) from torrefied SS increased compared to the untreated SS (6%), and exceeded the threshold values for hazardous waste.
- In the case of crustaceans D. magna Straus, the toxicity of water extracts from biochars was reduced (compared to the raw SS) as the temperature and residence time of torrefaction increased.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Malinska, K. Biochar as a response to current environmental problems. In Engineering and Environmental Protection; Częstochowa University of Technology, Institute of Environmental Engineering: Częstochowa, Poland, 2012; Volume 15, pp. 387–403. [Google Scholar]
- Pawlak-Kruczek, H.; Wnukowski, M.; Niedzwiecki, L.; Czerep, M.; Kowal, M.; Krochmalny, K.; Zgóra, J.; Ostrycharczyk, M.; Baranowski, M.; Tic, W.J.; et al. Torrefaction as a valorization method used prior to the gasification of sewage sludge. Energies 2019, 12, 175. [Google Scholar] [CrossRef] [Green Version]
- Pawlak-Kruczek, H.; Czerep, M.; Ostrycharczyk, M.; Wnukowski, M.; Baranowski, M.; Krochmalny, K.; Niedzwiecki, L.; Kowal, M. Sustainable utilization of the sewage sludge using combined drying, torrefaction and plasma gasification technologies. J. Phys. Conf. Ser. 2019, 1398, 012018. [Google Scholar] [CrossRef]
- Pulka, J.; Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. Is the biochar produced from sewage sludge a good quality solid fuel? Arch. Environ. Prot. 2016, 42, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Pulka, J.; Manczarski, P.; Koziel, J.A.; Białowiec, A. Torrefaction of sewage sludge: Kinetics and fuel properties of biochars. Energies 2019, 12, 565. [Google Scholar] [CrossRef] [Green Version]
- Pulka, J.; Manczarski, P.; Stępień, P.; Styczyńska, M.; Koziel, J.A.; Białowiec, A. Waste-to-Carbon: Is the torrefied sewage sludge with high ash content a better fuel or fertilizer? Materials 2020, 13, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Tang, C.; Li, C.; Yuan, J.; Tran, K.Q.; Bach, Q.V.; Qiu, R.; Yang, Y. Wet torrefaction of biomass for high quality solid fuel production: A review. Renew. Sustain. Energy Rev. 2018, 91, 259–271. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Arora, A.; Gupta, A.; Saeed, M.A.; Niedzwiecki, L.; Andrews, G.; Phylaktou, H.; Gibbs, B.; Newlaczyl, A.; Livesey, P.M. Biocoal—Quality control and assurance. Biomass Bioenergy 2020, 135, 105509. [Google Scholar] [CrossRef]
- Malińska, K.; Zabochnicka-Świątek, M. Selection of bulking agents for composting of sewage sludge. Environ. Prot. Eng. 2013, 39, 89–101. [Google Scholar]
- Mroczek-Krzyzelewska, E.; Konieczny, P.; Lewicki, A.; Janczak, D. Changes in acrylamide monomer content during composting of dairy processing sludge. Appl. Ecol. Environ. Res. 2013, 15, 39–50. [Google Scholar] [CrossRef]
- Babel, S.; Del Mundo Dacera, D. Heavy metal removal from contaminated sludge for land application: A review. Waste Manag. 2006, 26, 988–1004. [Google Scholar] [CrossRef]
- Pathak, A.; Dastidar, M.G.; Sreekrishnan, T.R. Bioleaching of heavy metals from sewage sludge: A review. J. Environ. Manag. 2009, 90, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Nowak, M.; Kacprzak, M.; Grobelak, A. Osady ściekowe jako substytut glebowy w procesach remediacji i rekultywacji terenów skażonych metalami ciężkimi (Sewage sludge as a soil substitute in remediation and reclamation processes of heavy metal contaminated areas). Inżynieria i Ochrona Środowiska (Eng. Environ. Prot.) 2010, 13, 121–131. [Google Scholar]
- Grobelak, A.; Stępień, W.; Kacprzak, M. Osady ściekowe jako składnik nawozów i substytutów gleb (Sewage sludge as a component of fertilizers and soil substitutes). Inżynieria Ekologiczna (Ecol. Eng.) 2016, 48, 52–60. [Google Scholar] [CrossRef]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.P.; Gerber, H. Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Bucheli, T.D.; Hilber, I.; Schmidt, H.-P. Polycyclic aromatic hydrocarbons and polychlorinated aromatic compounds in biochar. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2015; pp. 595–624. [Google Scholar]
- Lu, T.; Yuan, H.; Wang, Y.; Huang, H.; Chen, Y. Characteristic of heavy metals in biochar derived from sewage sludge. J. Mater. Cycles Waste Manag. 2016, 18, 725–733. [Google Scholar] [CrossRef]
- Stępień, P.; Pulka, J.; Serowik, M.; Białowiec, A. Thermogravimetric and calorimetric characteristics of alternative fuel in terms of its use in low-temperature pyrolysis. Waste Biomass Valoriz. 2019, 10, 1669–1677. [Google Scholar] [CrossRef]
- Madanayake, B.N.; Gan, S.; Eastwick, C.; Ng, H.K. Thermo-chemical and structural changes in Jatropha curcas seed cake during torrefaction for its use as coal co-firing feedstock. Energy 2016, 100, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Polish Standard PN-EN 14774-1:2010E. Solid Biofuels—Determination of Moisture Content—Drier Method—Part 1: Total Moisture—Reference Method. 2010. Available online: https://sklep.pkn.pl/pn-en-14918-2010e.html (accessed on 5 August 2020).
- Polish Standard PN-EN 14082:2004. Foodstuffs—Determination of Trace Elements—Determination of Lead, Cadmium, Zinc, Copper, Iron and Chromium by Atomic Absorption Spectrometry (AAS) after Dry Ashing. Available online: https://sklep.pkn.pl/pn-en-14082-2004p.html (accessed on 5 August 2020).
- Water Quality—Calibration and Evaluation of Analytical Methods and Estimation of Performance Characteristics—Part 1: Statistical Evaluation of the Linear Calibration Function. 1999, ISO 8466-1. Available online: https://www.iso.org/standard/15664.html (accessed on 5 August 2020).
- OECD 211. OECD Guidelines for Testing of Chemicals Daphnia Magna Reproduction Test. 1998. Available online: http://www.oecd.org/chemicalsafety/risk-assessment/1948277.pdf (accessed on 6 January 2020).
- Water Quality—Determination of the Inhibition of the Mobility of Daphnia Magna Straus (Cladocera, Crustacea)—Acute Toxicity Test. 2012, ISO 6341. Available online: https://www.iso.org/standard/54614.html (accessed on 5 August 2020).
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from Conocarpus wastes. Biores. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K. The love-hate relationship of pyrolysis biochar and water: A perspective. Sci. Total Environ. 2015, 512, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Chen, B.; Lin, Y.; Guan, Y. Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups. Environ. Sci. Technol. 2014, 48, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Regulation of the Minister of Environment on Municipal Sewage Sludge. Item 257. Pol. J. Laws; 2015. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150000257 (accessed on 24 June 2020).
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of sewage sludge-derived biochars from different feedstocks and pyrolysis temperatures. J. Anal. Appl. Pyrol. 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Regulation of the Minister of Agriculture and Rural Development of 18 June 2008 on the Implementation of Certain Provisions of the Act on Fertilizers and Fertilization. No. 119. Item 765. Pol. J. Laws; 2008. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20081190765 (accessed on 24 June 2020).
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008L0098 (accessed on 24 June 2020).
- Commission Decision of 3 May 2000 Replacing Decision 94/3/EC Establishing a List of Wastes Pursuant to Article 1(a) of Council Directive 75/442/EEC on Waste and Council Decision 94/904/EC Establishing a List of Hazardous Waste Pursuant to Article 1(4) of Council Directive 91/689/EEC on Hazardous Waste (Notified Under Document Number C(2000) 1147). Text with EEA Relevance. (2000/532/EC). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02000D0532-20150601 (accessed on 24 June 2020).
- Mierzwa-Hersztek, M.; Gondek, K.; Klimkowicz-Pawlas, A.; Baran, A.; Bajda, T. Sewage sludge biochars management—Ecotoxicity, mobility of heavy metals, and soil microbial biomass. Environ. Toxicol. Chem. 2018, 37, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Oleszczuk, P. The conversion of sewage sludge into biochar reduces polycyclic aromatic hydrocarbon content and ecotoxicity but increases trace metal content. Biomass Bioenergy 2015, 75, 235–244. [Google Scholar] [CrossRef]
- Regulation of the Minister of the Environment of 20 January 2015 Regarding the R10 Recovery Process. Item 132. Pol. J. Laws; 2015. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150000132 (accessed on 29 February 2020).
- SWA-Tool Consortium. Methodology for the Analysis of Solid Waste. Development of a Methodological Tool to Enhance the Precision and Comparability of Solid Waste Analysis Data. 2004. Available online: http://www.swa-tool.net/reports/WP3Wnalreport-web.pdf (accessed on 6 May 2013).
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef]
- Zornoza, R.; Moreno-Barriga, F.; Acosta, J.A.; Munoz, M.A.; Faz, A. Stability, nutrient availability and hydrophobicity of biochars derived from manure, crop residues, and municipal solid waste for their use as soil amendments. Chemosphere 2016, 144, 122–130. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Wang, Y.; Chen, Y.; Lei, T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Song, X.D.; Xue, X.Y.; Chen, D.Z.; He, P.J.; Dai, X.H. Application of biochar from sewage sludge to plant cultivation: Influence of pyrolysis temperature and biochar-to-soil ratio on yield and heavy metal accumulation. Chemosphere 2014, 109, 213–220. [Google Scholar] [CrossRef]
- Edo, M.; Skoglund, N.; Gao, Q.; Persson, P.E.; Jansson, S. Fate of metals and emissions of organic pollutants from torrefaction of waste wood, MSW, and RDF. Waste Manag. 2017, 68, 646–652. [Google Scholar] [CrossRef]
- Regulation of the Polish Minister of Economy of 16 July 2015 on the Acceptance of Waste for Landfilling. Item 1277. Pol. J. Laws; 2015. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20150001277 (accessed on 29 February 2020).
- Bernacka, J.; Pawłowska, L. Substancje Potencjalnie Toksyczne w Osadach z Komunalnych Oczyszczalni Scieków (Potentially Toxic Substances in Sludges from Municipal Wastewater Treatment Plants); Instytut Ochrony Środowiska (Institute of Environmental Protection): Warszawa, Poland, 2000; ISBN 838580563X. [Google Scholar]
- Waqas, M.; Khan, S.; Qing, H.; Reid, B.J.; Chao, C. The effects of sewage sludge and sewage sludge biochar on PAHs and potentially toxic element bioaccumulation in Cucumis sativa L. Chemosphere 2014, 105, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.; Li, K.; Fang, M.; Su, D.C. Toxicity evaluation of sewage sludges in Hong Kong. Environ. Int. 2001, 27, 373–380. [Google Scholar] [CrossRef]
- Abrego, J.; Atienza-Martínez, M.; Gimeno, J.R.; Aibar, J.; Quílez, D.; Gea, G. Phytotoxicity of sewage sludge biochars prepared at different pyrolysis conditions. Eur. Biomass Conf. Exhib. Proc. 2015, 948–953. [Google Scholar] [CrossRef]
- Cameron, R.D.; Koch, F.A. Toxicity of landfill leachates. J. Water Pollut. Control Fed. 1980, 52, 760–769. [Google Scholar]
- Clément, B.; Janssen, R.C.; Dû-Delepierr, E.A. Estimation of the hazard of landfills through toxicity testing of leachates. Comparison of physico-chemical characteristics of landfill leachates with their toxicity determined with a battery of tests. Chemosphere 1997, 35, 2783–2796. [Google Scholar] [CrossRef]
- Freddo, A.; Cai, C.; Reid, B.J. Environmental contextualization of potential toxic elements and polycyclic aromatic hydrocarbons in biochar. Environ. Pollut. 2012, 171, 18–24. [Google Scholar] [CrossRef]
- Hale, S.E.; Elmquist, M.; Brändli, R.; Hartnik, T.; Jakob, L.; Henriksen, T.; Werner, D.; Cornelissen, G. Activated carbon amendment to sequester PAHs in contaminated soil: A lysimeter field trial. Chemosphere 2012, 87, 177–184. [Google Scholar] [CrossRef]
- Blikra, E.V.; Sanchez, M.V.; Thomsen, M. A Review of Waste Management Decision Support Tools and Their Ability to Assess Circular Biowaste Management Systems. Sustainability 2018, 10, 3720. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Lin, Y.-L.; Chiueh, P.-T.; Den, W. Environmental and energy assessment of biomass residues to biochar as fuel: A brief review with recommendations for future bioenergy systems. J. Clean. Prod. 2020, 251, 119714. [Google Scholar] [CrossRef]


| The Property | Values ± SD | Variation Coefficient, % |
|---|---|---|
| dry mass (d.m.), % | 20.3 ± 0.3 | 1.48 |
| Zn, mg·kg−1, d.m. | 212.6 ± 23.4 | 11.00 |
| Cu, mg·kg−1, d.m. | 33.5 ± 6.2 | 18.51 |
| Cr, mg·kg−1, d.m. | 3.8 ± 1.6 | 42.11 |
| Mn, mg·kg−1, d.m. | 120.6 ± 12.3 | 10.20 |
| Pb, mg·kg−1, d.m. | 72.0 ± 25.8 | 35.42 |
| Cd, mg·kg−1, d.m. | 8.0 ± 1.3 | 16.25 |
| Ni, mg·kg−1, d.m. | 10.8 ± 2.3 | 21.30 |
| Process Time, Min | Temperature, °C | WDPT ± SD, s | Hydrophobicity Level |
|---|---|---|---|
| Dry sludge | 83 ± 14 | Slightly hydrophobic | |
| 20 | 200 | 222 ± 24 | Moderately hydrophobic |
| 220 | 340 ± 28 | Hydrophobic | |
| 240 | 499 ± 28 | Hydrophobic | |
| 260 | 559 ± 43 | Hydrophobic | |
| 280 | 745 ± 48 | Strongly hydrophobic | |
| 300 | <3600 | Extremely hydrophobic | |
| 40 | 200 | 340 ± 25 | Hydrophobic |
| 220 | 434 ± 35 | Hydrophobic | |
| 240 | 535 ± 32 | Hydrophobic | |
| 260 | 1482 ± 240 | Severely hydrophobic | |
| 280 | <3600 | Extremely hydrophobic | |
| 300 | <3600 | Extremely hydrophobic | |
| 60 | 200 | 454 ± 47 | Hydrophobic |
| 220 | 750 ± 37 | Strongly hydrophobic | |
| 240 | 2520 ± 183 | Severely hydrophobic | |
| 260 | <3600 | Extremely hydrophobic | |
| 280 | <3600 | Extremely hydrophobic | |
| 300 | <3600 | Extremely hydrophobic |
| Time. Min | Temperature. °C | TOC | Ntot | N-NH4 | N-NO2 | N-NO3 | SO4 | Cl | Ptot (P-PO4) | PO4-P | pH | Mn | Cd | Pb | Zn | Cu | Cr | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg·dm−3 | mg·dm−3 | |||||||||||||||||
| Sewage Sludge | 24,040.8 | 6660.0 | 2341.8 | 5.8 | 75.6 | 7254.0 | 1998.0 | 1378.8 | 741.6 | 7.2 | 0.97 | 0.51 | 1.80 | 2.78 | - | - | - | |
| 20 | 200 | 17,379.0 | 5526.0 | 1904.4 | 5.2 | 61.2 | 8784.0 | 1794.6 | 1000.8 | 759.6 | 7.4 | 0.87 | 0.55 | 2.13 | 1.14 | 0.01 | - | - |
| 20 | 220 | 14,275.8 | 5508.0 | 2134.8 | 5.4 | 88.2 | 6300.0 | 1992.6 | 986.4 | 725.4 | 7.3 | 0.84 | 0.55 | 2.69 | 0.90 | 0.24 | - | - |
| 20 | 240 | 16,250.4 | 4752.0 | 1594.8 | 6.5 | 95.4 | 7218.0 | 1834.2 | 865.8 | 469.8 | 7.2 | 0.90 | 0.48 | 1.64 | 0.44 | - | - | - |
| 20 | 260 | 17,226.0 | 4158.0 | 943.2 | 5.4 | 61.2 | 12,258.0 | 1116.0 | 860.4 | 457.2 | 7.5 | 0.77 | 0.48 | 1.58 | 1.50 | - | - | - |
| 20 | 280 | 18,630.0 | 4716.0 | 1099.8 | 6.1 | 64.8 | 16,470.0 | 988.2 | 995.4 | 495.0 | 7.3 | 1.80 | 1.11 | 3.60 | 1.29 | - | - | - |
| 20 | 300 | 16,273.8 | 4284.0 | 799.2 | 4.9 | 59.4 | 15,822.0 | 1463.4 | 792.0 | 478.8 | 7.1 | 1.08 | 0.79 | 2.87 | 0.85 | - | - | - |
| 40 | 200 | 18,410.4 | 3798.0 | 1530.0 | 5.2 | 55.8 | 17,010.0 | 1261.8 | 777.6 | 529.2 | 7.1 | 1.32 | 0.76 | 2.37 | 0.75 | - | - | - |
| 40 | 220 | 20,435.4 | 3150.0 | 1297.8 | 5.2 | 61.2 | 12,654.0 | 1627.2 | 687.6 | 543.6 | 7.1 | 1.55 | 1.05 | 3.30 | 1.16 | - | - | - |
| 40 | 240 | 19,184.4 | 3168.0 | 826.2 | 4.3 | 73.8 | 12,330.0 | 1265.4 | 646.2 | 486.0 | 7.1 | 1.33 | 0.84 | 4.42 | 0.92 | - | - | - |
| 40 | 260 | 18,930.6 | 3636.0 | 579.6 | 4.9 | 70.2 | 11,988.0 | 1366.2 | 822.6 | 550.8 | 7.0 | 1.24 | 1.27 | 8.07 | 1.86 | - | - | - |
| 40 | 280 | 9686.7 | 1548.0 | 367.2 | 2.4 | 55.8 | 8478.0 | 453.6 | 394.2 | 302.4 | 7.1 | 2.13 | 0.87 | 3.96 | 0.93 | - | - | - |
| 40 | 300 | 14,715.0 | 1674.0 | 284.4 | 2.2 | 45.0 | 8730.0 | 309.6 | 482.4 | 356.4 | 7.0 | 1.36 | 1.09 | 4.79 | 1.44 | - | - | - |
| 60 | 200 | 20,901.6 | 5148.0 | 982.8 | 8.8 | 111.6 | 9936.0 | 1279.8 | 444.6 | 394.2 | 7.1 | 1.40 | 1.09 | 4.38 | 1.22 | 0.04 | - | - |
| 60 | 220 | 21,378.6 | 3798.0 | 687.6 | 5.4 | 84.6 | 11,592.0 | 1252.8 | 574.2 | 369.0 | 7.1 | 1.11 | 0.85 | 3.20 | 0.63 | - | - | - |
| 60 | 240 | 15,427.8 | 3024.0 | 421.2 | 8.6 | 95.4 | 10,962.0 | 1323.0 | 415.8 | 221.4 | 7.1 | 1.12 | 0.69 | 2.59 | 0.68 | - | - | - |
| 60 | 260 | 14,554.8 | 2574.0 | 430.2 | 7.4 | 90.0 | 11,304.0 | 1476.0 | 401.4 | 207.0 | 7.1 | 0.96 | 0.58 | 2.55 | 0.60 | 0.05 | - | - |
| 60 | 280 | 12,049.9 | 1260.0 | 450.0 | 8.8 | 100.8 | 7992.0 | 1548.0 | 464.4 | 181.8 | 6.9 | 1.26 | 0.89 | 3.80 | 0.72 | - | - | - |
| 60 | 300 | 12,398.4 | 1854.0 | 655.2 | 4.9 | 86.4 | 8676.0 | 1807.2 | 379.8 | 194.4 | 7.0 | 1.48 | 0.94 | 4.35 | 1.18 | - | - | - |
| 1st Day Mobility Inhibitions | |||
| Pollutant | Function Parameters | p | r2 |
| TOC, mg·dm−3 | y = 16140.9314 + 19.5944·x | 0.3373 | 0.0542 |
| Ntot, mg·dm−3 | y = 2538.7216 + 27.8626·x | 0.00006 | 0.6226 |
| N-NH4, mg·dm−3 | y = 575.3784 + 10.6358·x | 0.0004 | 0.5278 |
| N-NO2, mg·dm−3 | y = 5.4842 + 0.004·x | 0.7049 | 0.0086 |
| N-NO3, mg·dm−3 | y = 75.6653 − 0.0016·x | 0.9882 | 0.0000 |
| SO4, mg·dm−3 | y = 11288.2594 − 11.0422·x | 0.5426 | 0.0222 |
| Cl, mg·dm−3 | y = 1160.6702 + 5.1987·x | 0.0344 | 0.2373 |
| Ptot (P-PO4), mg·dm−3 | y = 497.8575 + 4.9533·x | 0.0001 | 0.5889 |
| PO4-P, mg·dm−3 | y = 334.7252 + 2.6644·x | 0.0036 | 0.4019 |
| pH | y = 7.0289 + 0.0027·x | 0.00004 | 0.6404 |
| Mn, mg·dm−3 | y = 1.4295 − 0.0046·x | 0.0110 | 0.3240 |
| Cd, mg·dm−3 | y = 0.9612 − 0.0036·x | 0.0037 | 0.3993 |
| Pb, mg·dm−3 | y = 4.2830 − 0.0219·x | 0.0049 | 0.3805 |
| Zn, mg·dm−3 | y = 0.9938 + 0.0027·x | 0.3808 | 0.0455 |
| 2nd Day Mobility Inhibitions | |||
| Function parameters | p | r2 | |
| TOC, mg·dm−3 | y = 15891.8326 + 21.4943·x | 0.3509 | 0.0513 |
| Ntot, mg·dm−3 | y = 2180.3293 + 30.6487·x | 0.0001 | 0.5927 |
| N-NH4, mg·dm−3 | y = 436.2804 + 11.7456·x | 0.0006 | 0.5064 |
| N-NO2, mg·dm−3 | y = 5.4323 + 0.0044·x | 0.7111 | 0.0083 |
| N-NO3, mg·dm−3 | y = 76.4184 − 0.0165·x | 0.8898 | 0.0012 |
| SO4, mg·dm−3 | y = 11272.3512 − 8.9539·x | 0.6623 | 0.0115 |
| Cl, mg·dm−3 | y = 1092.2603 + 5.7496·x | 0.0385 | 0.2283 |
| Ptot (P-PO4), mg·dm−3 | y = 436.1408 + 5.4082·x | 0.0003 | 0.5523 |
| PO4-P, mg·dm−3 | y = 304.7134 + 2.8447·x | 0.0066 | 0.3604 |
| pH | y = 6.9924 + 0.003·x | 0.00005 | 0.6286 |
| Mn, mg·dm−3 | y = 1.5122 − 0.0056·x | 0.0059 | 0.3676 |
| Cd, mg·dm−3 | y = 1.0236 − 0.0043·x | 0.0018 | 0.4443 |
| Pb, mg·dm−3 | y = 4.7142 − 0.0271·x | 0.0014 | 0.4590 |
| Zn, mg·dm−3 | y = 0.9809 + 0.0025·x | 0.4683 | 0.0314 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Białowiec, A.; Pulka, J.; Styczyńska, M.; Koziel, J.A.; Kalka, J.; Jureczko, M.; Felis, E.; Manczarski, P. Is Biochar from the Torrefaction of Sewage Sludge Hazardous Waste? Materials 2020, 13, 3544. https://doi.org/10.3390/ma13163544
Białowiec A, Pulka J, Styczyńska M, Koziel JA, Kalka J, Jureczko M, Felis E, Manczarski P. Is Biochar from the Torrefaction of Sewage Sludge Hazardous Waste? Materials. 2020; 13(16):3544. https://doi.org/10.3390/ma13163544
Chicago/Turabian StyleBiałowiec, Andrzej, Jakub Pulka, Marzena Styczyńska, Jacek A. Koziel, Joanna Kalka, Marcelina Jureczko, Ewa Felis, and Piotr Manczarski. 2020. "Is Biochar from the Torrefaction of Sewage Sludge Hazardous Waste?" Materials 13, no. 16: 3544. https://doi.org/10.3390/ma13163544








