Impact of Bi and Sn on Microstructure and Corrosion Resistance of Zinc Coatings Obtained in Zn-AlNi Bath
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Research Scope and Methodology
2.2.1. Neutral Salt Spray (NSS) Test
2.2.2. Corrosion Test in Humid Atmosphere Containing SO2
2.2.3. Potentiodynamic Test
2.2.4. Microstructure Characterization
3. Results
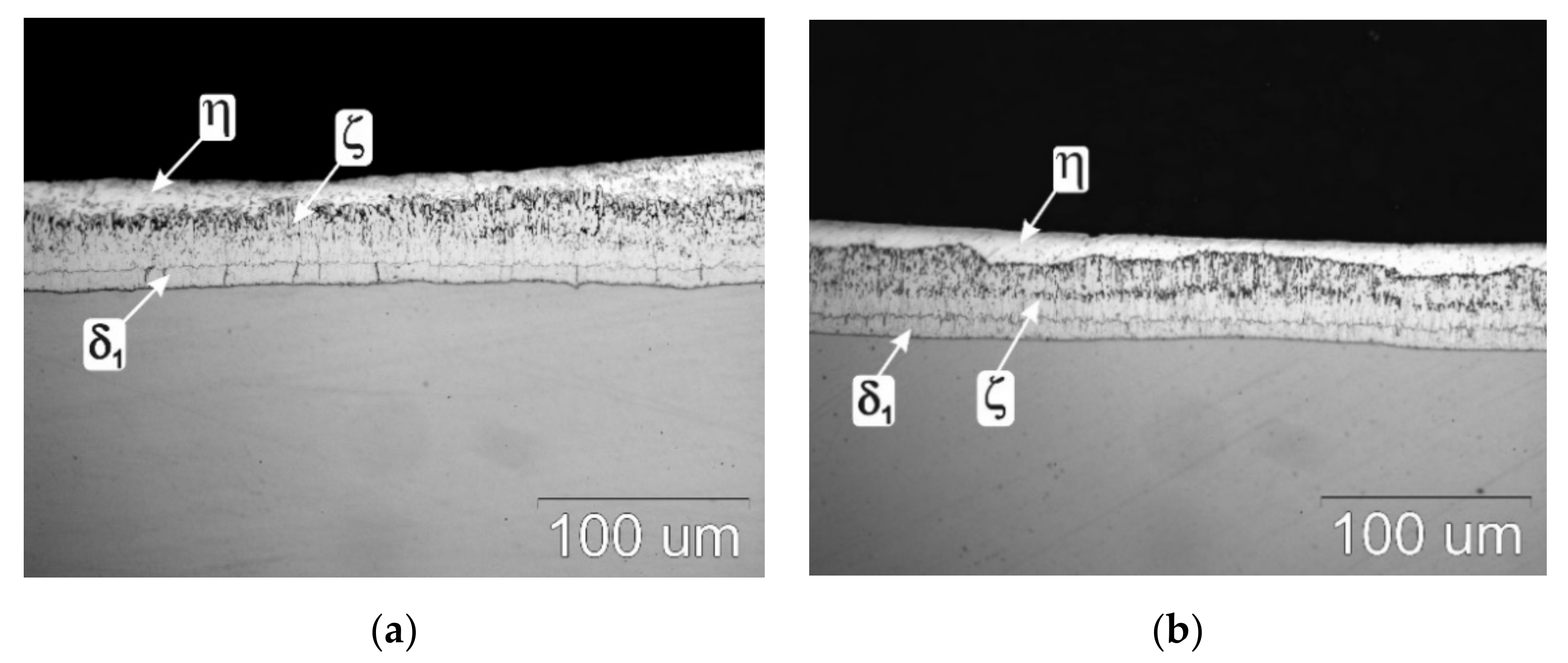
3.1. Top Surface Outlook, Cross-Section and Thickness of Coating
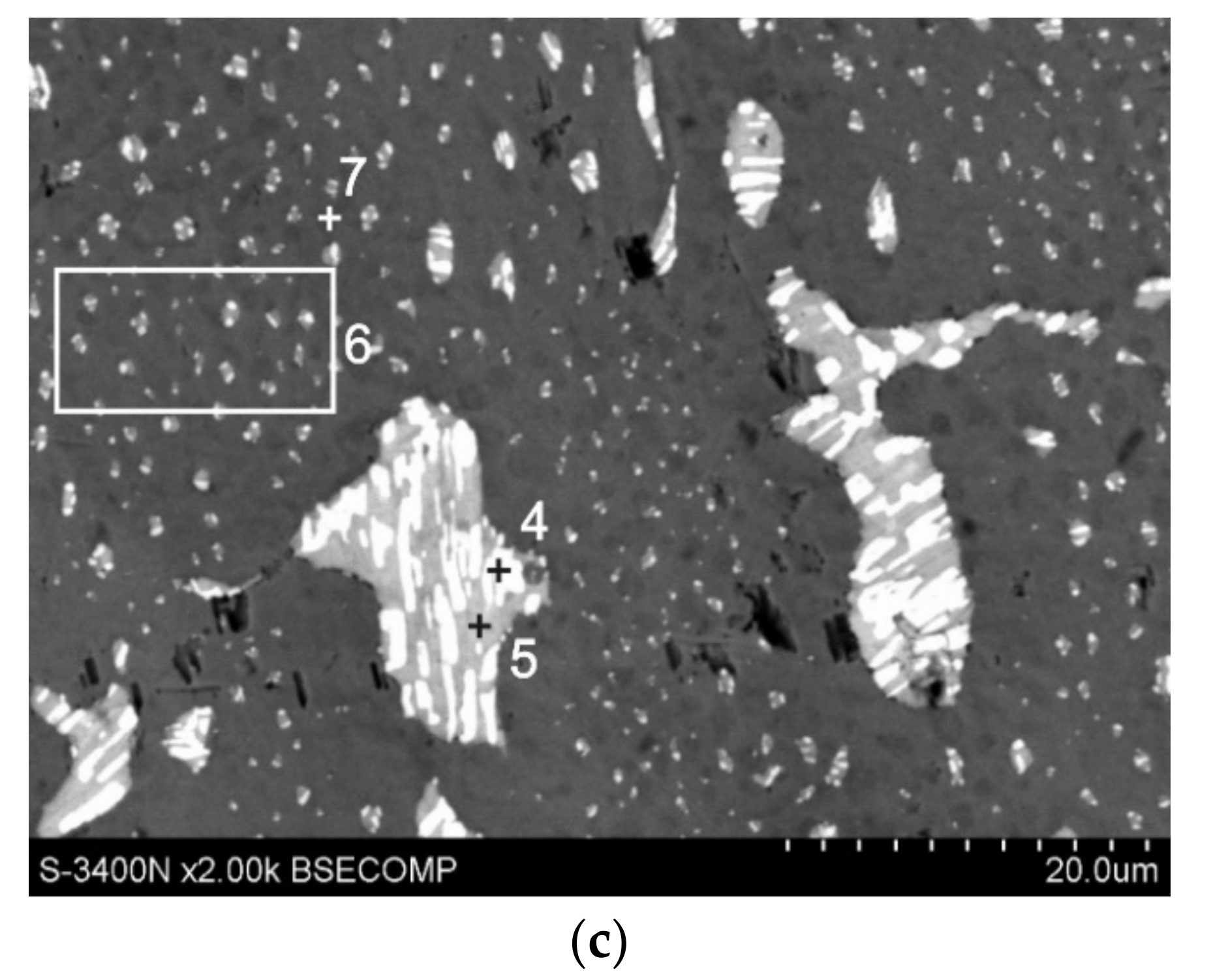
3.2. Top Surface Microstructure of Coating
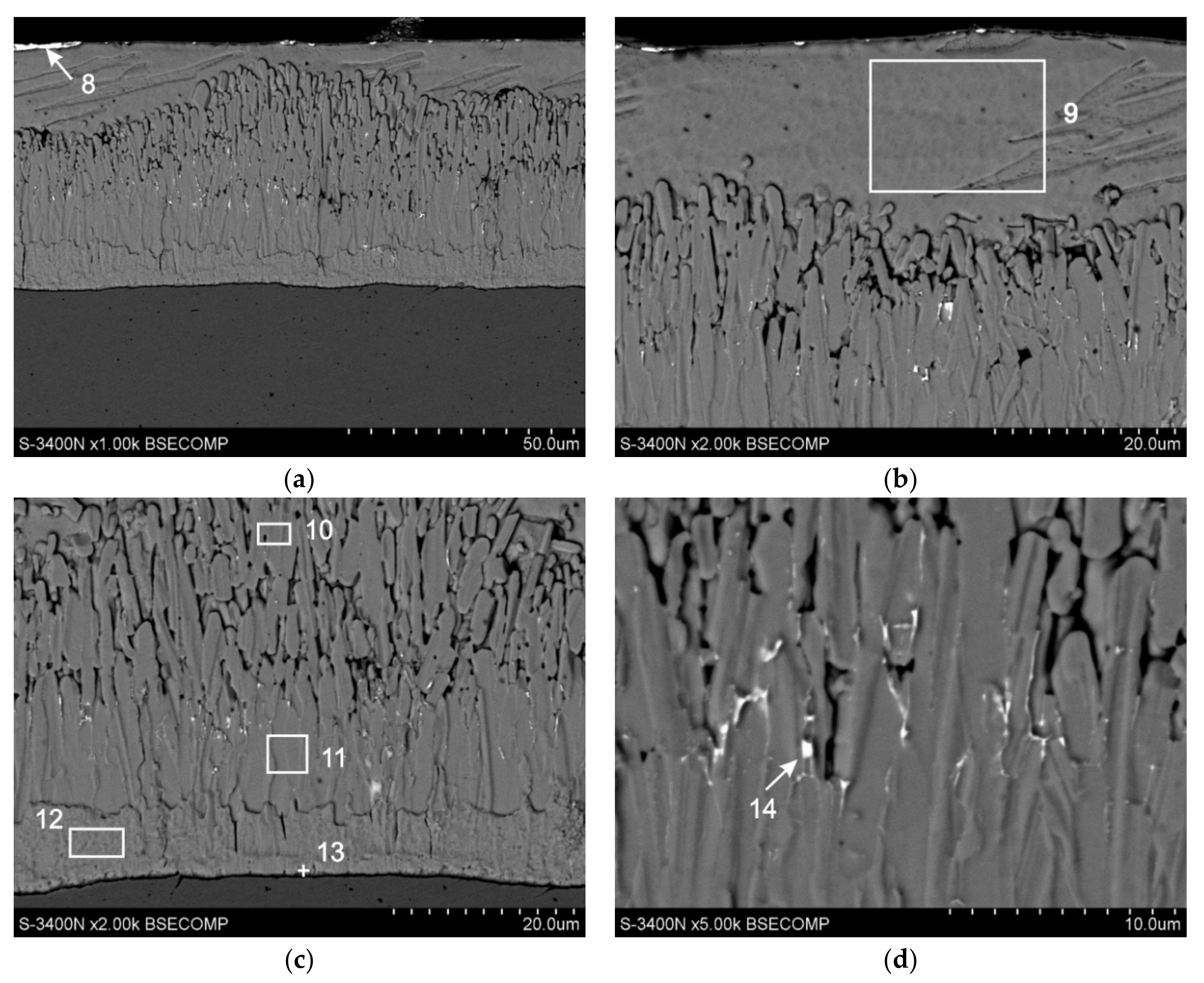
3.3. Cross-Sectional Microstructure of Coating
3.4. Results of Corrosion Resistance
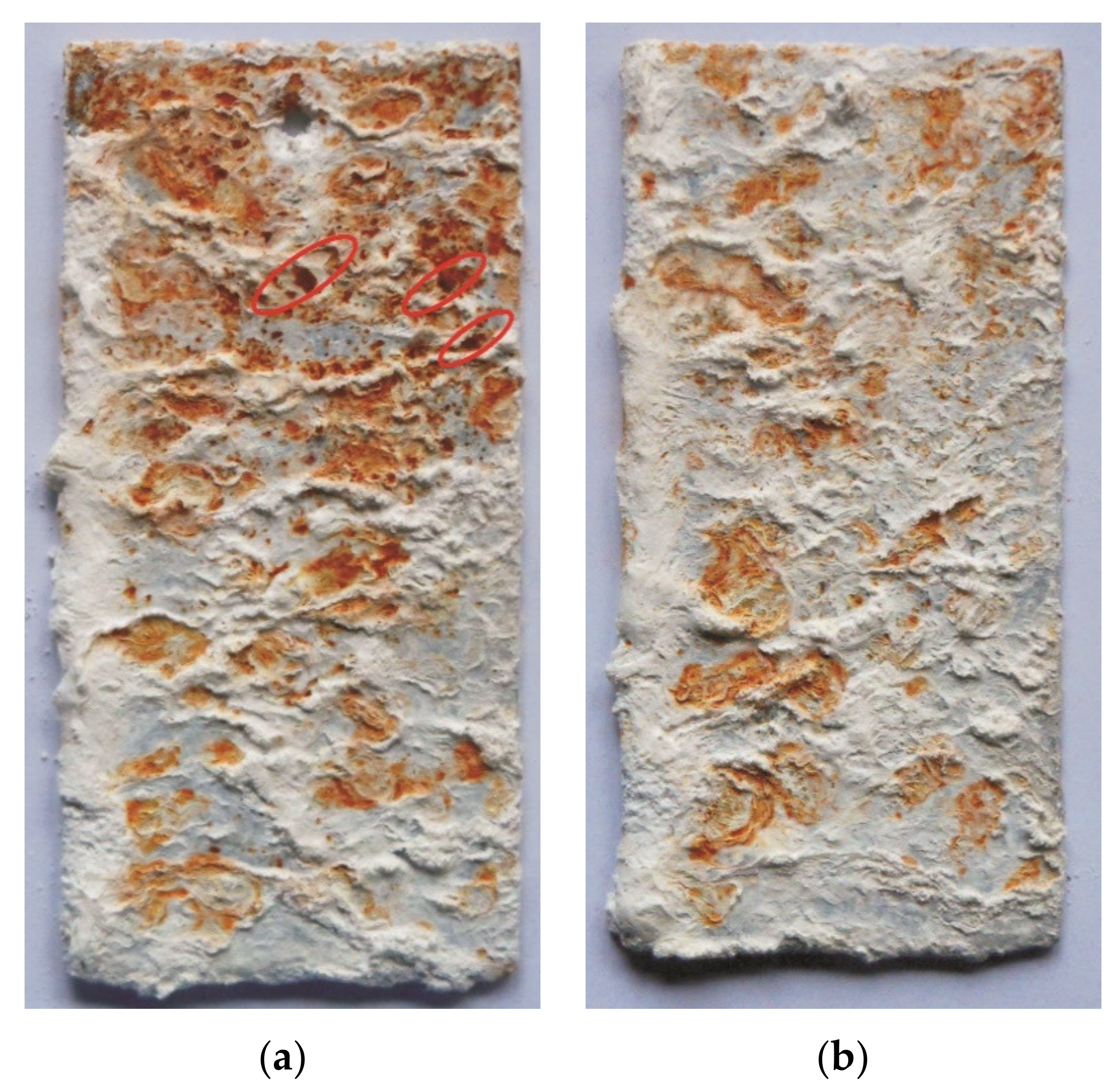
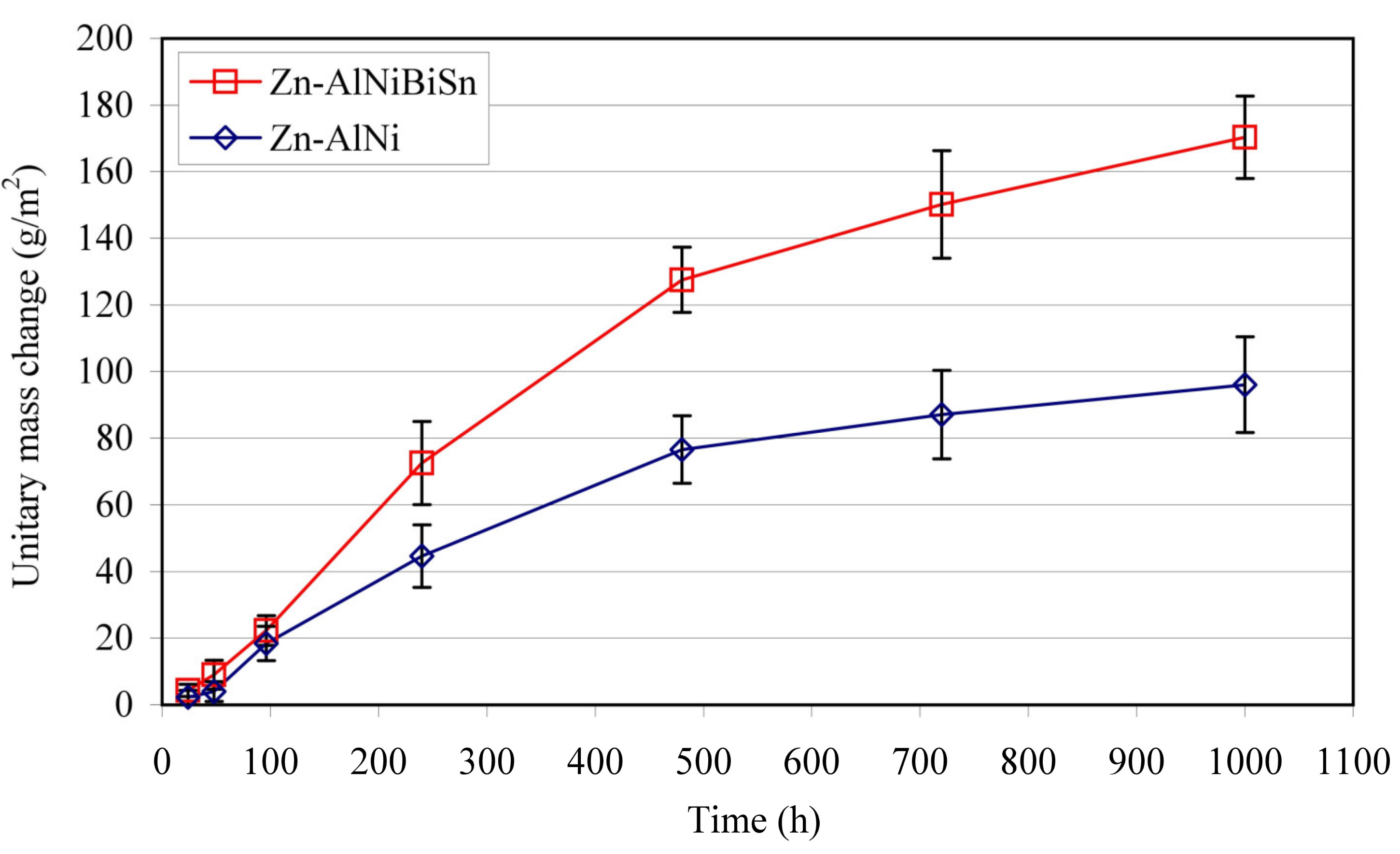
3.4.1. NSS Test
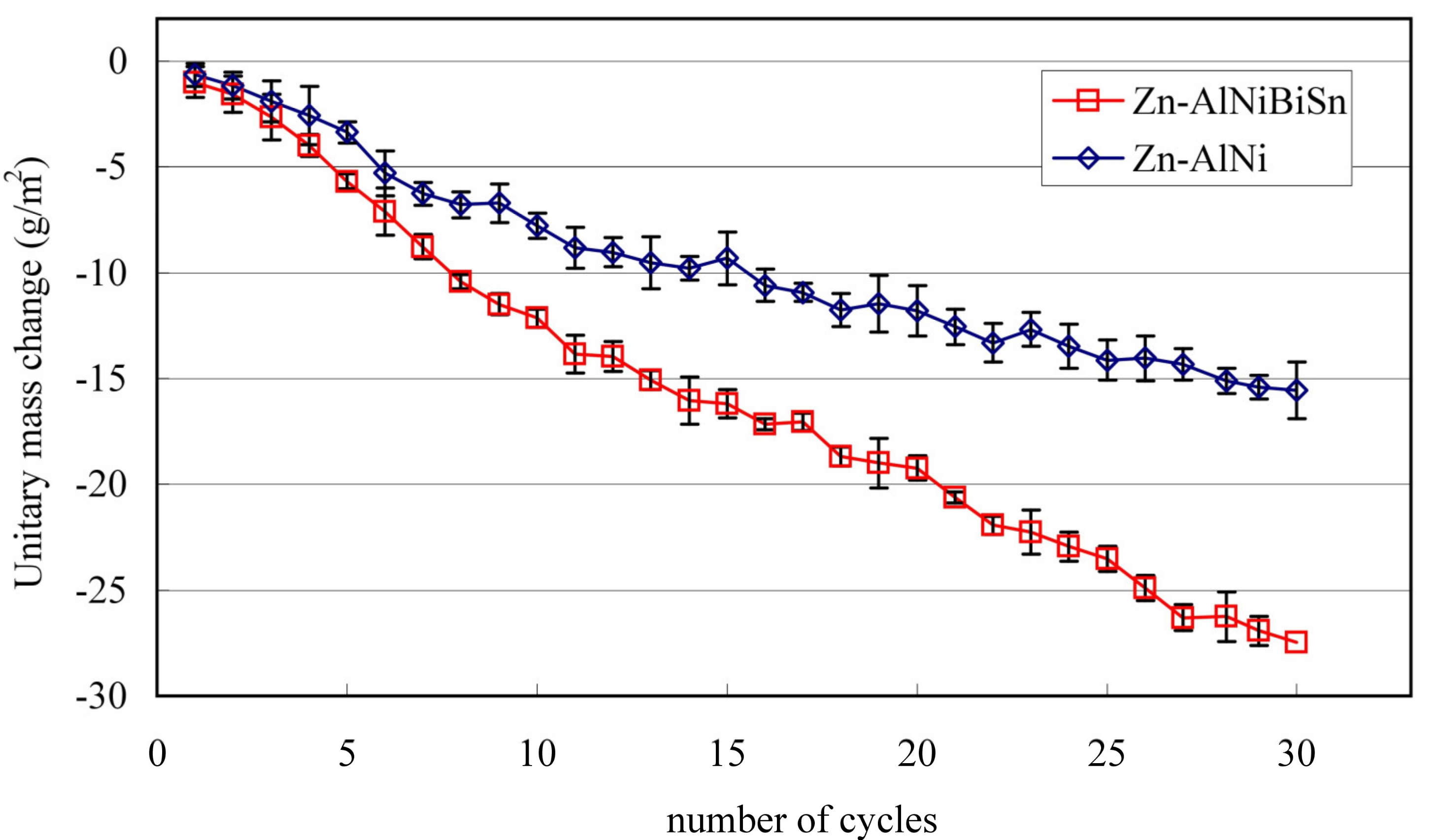
3.4.2. Test in Humid Atmosphere Containing SO2
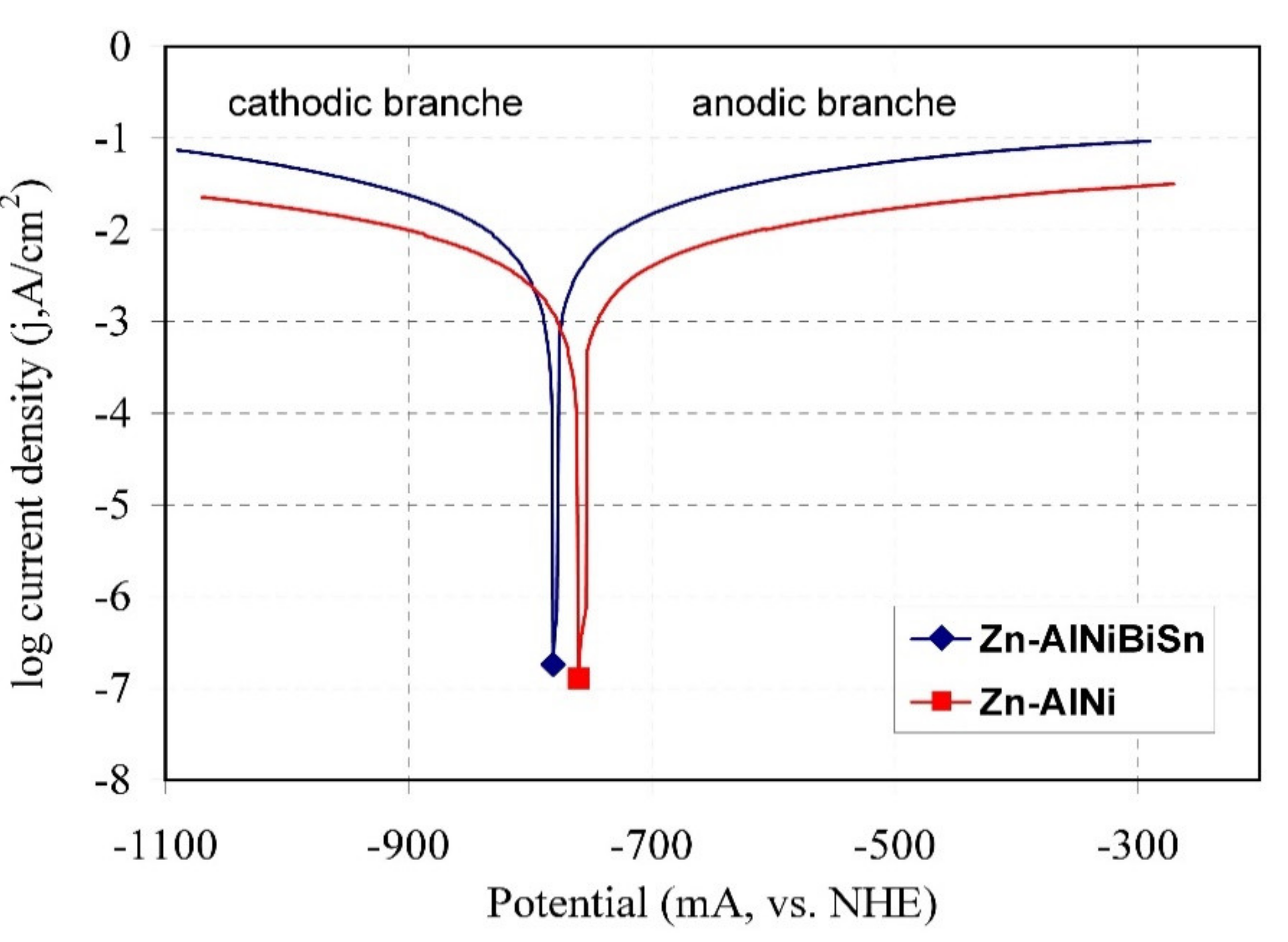
3.4.3. Potentiodynamic Test
4. Conclusions
- The coatings received in the Zn-AlNiBiSn bath showed poorer corrosion resistance compared to those received in the Zn-AlNi bath without Bi and Sn as alloying additions. In the conducted corrosion tests in a neutral salt spray and in a sulfur dioxide test in a humid atmosphere, these coatings showed higher unitary mass changes in comparison with the coatings received in the Zn-AlNi bath. After the tests were completed in the salt chamber, puncture points to the substrate were found, despite having a slightly greater thickness than the coating received in the Zn-AlNi bath. Potentiodynamic tests of Zn-AlNiBiSn coatings showed more negative values of corrosion potential (Ecorr) and a higher value of corrosion current density (jcorr).
- The structure of Zn-AlNiBiSn coatings showed the occurrence of typical intermetallic phases: Γ, δ1 and ζ and the presence of Sn-Bi alloy precipitations located on the top surface of coating and partly in the intermetallic ζ phase layer. Al and Ni were not found in the structure of the coating received in the Zn-AlNiBiSn bath. The presence of BiSn alloy precipitates may cause the formation of local corrosion cells, which causes an increase in the corrosion rate of coatings.
Author Contributions
Funding
Conflicts of Interest
References
- Kuklik, V.; Kudláček, J. Hot-Dip Galvanizing of Steel Structures; Elsevier Ltd.: Boston, MA, USA, 2016. [Google Scholar]
- Kania, H.; Sipa, J.; Saternus, M. Zanurzeniowe powłoki aluminiowe na stali zbrojeniowej B500SP. Ochr. Przed Korozją 2019, 62, 140–145. (In Polish) [Google Scholar] [CrossRef]
- Suliga, M.; Wartacz, R. The influence of the angle of working part of die on the zinc coating thickness and mechanical properties of medium carbon steel wires. Arch. Met. Mater. 2019, 64, 1295–1299. [Google Scholar] [CrossRef]
- Dallin, G.W. Continuous Hot-Dip Galvanizing—Process and Products. Galvinfo Center—A Program of the International Zinc Association, Galvanizing-2015; IZA: Durham, NC, USA, 2015. [Google Scholar]
- Liberski, P. Antykorozyjne Metalowe Powłoki Zanurzeniowe; Politechnika Śląska: Gliwice, Poland, 2013. [Google Scholar]
- Kania, H.; Liberski, P. The structure and growth kinetics of zinc coatings on link chains produced of the 23MnNiCrMo5-2 steel. Solid State Phenom. 2014, 212, 145–150. [Google Scholar] [CrossRef]
- International Lead and Zinc Study Group (ILZSG). Available online: http://www.ilzsg.org/static/enduses.aspx?from=1 (accessed on 31 January 2020).
- Kania, H.; Liberski, P. Synergistic influence of the addition of Al, Ni and Pb to a zinc bath upon growth kinetics and structure of coatings. Solid State Phenom. 2014, 212, 115–120. [Google Scholar] [CrossRef]
- Kania, H.; Liberski, P. Synergistic influence of Al, Ni, Bi and Sn addition to a zinc bath upon growth kinetics and the structure of coatings. IOP Conf. Ser. Mater. Sci. Eng. 2012, 35, 012004. [Google Scholar] [CrossRef]
- Porter, F.C. Zinc Handbook: Properties Processing and Use in Design; Marcel Dekker: New York, NY, USA, 1991. [Google Scholar]
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Reumont, G.; Perrot, P.; Foct, J. Thermodynamic study of the galvanizing process in a Zn-0.1%Ni bath. J. Mater. Sci. 1998, 33, 4759–4768. [Google Scholar] [CrossRef]
- Tang, N.Y. Alternative description of dross formation when galvanizing steels in zinc-nickel baths. J. Phase Equilibria 1995, 16, 110–112. [Google Scholar] [CrossRef]
- Lewis, G.P.; Pedersen, J.G. Optimizing the nickel-zinc process for hot dip galvanizing. In Proceedings of the 3rd Asian Pacific General Galvanizing Conference, Queensland, Australia, 8 September 1996. [Google Scholar]
- Fasoyino, F.A.; Weinberg, F. Spangle formation in galvanized sheet steel coatings. Met. Trans. B Process Met. 1990, 21, 549–558. [Google Scholar] [CrossRef]
- Strutzenberger, J.; Faderl, J. Solidification and spangle formation of hot-dip-galvanized zinc coatings. Met. Mater. Trans. A Phys. Met. Mater. Sci. 1998, 29, 631–646. [Google Scholar] [CrossRef]
- Beguin, P.; Bosschaerts, M.; Dhaussy, D.; Pankert, R.; Gilles, M. Galveco a solution for galvanizing reactive steel. In Proceedings of the 19th International Galvanizing Conference, EGGA, Berlin, Germany, 1–8 March 2000. [Google Scholar]
- Reumont, G.; Perrot, P. Fundamental study of lead additions in industrial zinc. In Proceedings of the 18th International Galvanizing Conference, EGGA, Birmingham, UK, 8–11 June 1997. [Google Scholar]
- Gagne, M. Hot-dip galvanizing with zinc-bismuth alloys. Metall 1999, 53, 269–271. [Google Scholar]
- Tatarek, A.; Saternus, M. Badanie zjawisk rozpuszczania dyfuzyjnego stali reaktywnych w kąpieli cynkowej z dodatkiem bizmutu. Ochr. Przed Korozją 2018, 7, 186–190. (In Polish) [Google Scholar]
- Mendala, J. The possibility of the LME phenomenon in elements subjected to metallization in Zn bath with Bi addition. Solid State Phenom. 2015, 226, 167–172. [Google Scholar] [CrossRef]
- DASt-Richtlinie 022—Guideline for Hot-Dip-Zinc-Coating of Prefabricated Load-Bearing Steel Components; Deutscher Ausschuß für Stahlbau: Düsseldorf, Germany, 2009.
- Mendala, J.; Liberski, P. Liquid metal embrittlement of steel with a coating obtained by batch hot dip method in a Zn + 2% Sn bath. Solid State Phenom. 2014, 212, 107–110. [Google Scholar] [CrossRef]
- EN ISO 1461:2009, Hot Dip Galvanized Coatings On Fabricated Iron And Steel Articles. Specifications And Test Methods; Comite Europeen de Normalisation: Brussels, Belgium, 1994.
- Kania, H.; Saternus, M.; Kudláček, J. Structural aspects of decreasing the corrosion resistance of zinc coating obtained in baths with Al, Ni, and Pb additives. Materials 2020, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- ISO 9227:2017, Corrosion Tests In Artificial Atmospheres—Salt Spray Tests; Polish Version PN EN ISO 9227:2017; Polish Committee for Standardization: Warszawa, Poland, 2017.
- PN-EN ISO 6988:2000, Metallic and Other Non-Organic Coatings—Sulfur Dioxide Test With General Condensation Of Moisture; Polish Committee for Standardization: Warszawa, Poland, 2000.
- Coccoa, V.; Iacovielloa, F.; D’Agostinoa, L.; Natali, S. Sn and Ti influence on damage of bent hot-dip galvanizing phases. Struct. Integr. Procedia 2017, 3, 224–230. [Google Scholar] [CrossRef]
- Massalski, T.B. Binary Alloy Phase Diagrams; ASM International: Novelty, OH, USA, 1990. [Google Scholar]
- Malakhov, D.V. Thermodynamic assessment of the Bi-Zn system. Calphad 2000, 24, 1–14. [Google Scholar] [CrossRef]
- Fries, S.G.; Lukas, H.L. System Sn–Zn. In Thermochemical Database for Light Metal Alloys; Ansara, I., Dinsdale, A.T., Rand, M.H., Eds.; Official Publications of the European Communitie: Luxemburg, Luxemburg, 1998; Volume 2, p. 288. [Google Scholar]
- Ohtani, H.; Miyashita, M.; Ishida, K. Thermodynamic study of phase equilibria in the Sn-Ag-Zn system. J. Jpn. Inst. Met. Mater. 1999, 63, 685–694. [Google Scholar] [CrossRef]
- Okamoto, H. Bi-Sn (Bismuth-Tin). J. Phase Equilibria Diffus. 2010, 31, 205. [Google Scholar] [CrossRef]
- Lee, B.J.; Oh, C.S.; Shim, J.H. Thermodynamic assessment of the Sn-In and Sn-Bi systems. J. Electron. Mater. 1996, 25, 983–991. [Google Scholar] [CrossRef]
- Kopyciński, D. The shaping of zinc coating on surface steels and ductile iron casting. Arch. Foundry Eng. 2010, 10, 463–468. [Google Scholar]
- Liberski, P.; Tatarek, A.; Kania, H.; Podolski, P. Coating growth on silicon-containing iron alloys in hot dip galvanizing process. In Proceedings of the 22nd International Galvanizing Conference Intergalva 2009, EGGA, Madrid, Spain, 8–12 June 2009; pp. 181–187. [Google Scholar]
- Pankert, R.; Dhaussy, D.; Beguin, P. Three years industrial experience with the Galveco alloy. In Proceedings of the 20th International Galvanizing Conference Intergalva 2003, EGGA, Amsterdam, The Netherlands, 1–4 June 2003. [Google Scholar]
- Pistofidis, N.; Vourlias, G.; Konidaris, S.; Pavlidou, E.; Stergiou, A.; Stergioudis, G. The effect of bismuth on the structure of zinc hot-dip galvanized coating. Mater. Lett. 2007, 61, 994–997. [Google Scholar] [CrossRef]
- Avettand-Fènoël, M.N.; Goodwin, F.E.; Foct, J. Effect of tin added to the zinc bath on the formation and the microstructure of hot-dip galvanized coatings. Int. J. Mater. Res. 2006, 97, 1183–1192. [Google Scholar] [CrossRef]
- Waseda, Y.; Suzuki, S. Characterization of Corrosion Products on Steel Surfaces; (Series: Advances in Materials Research); Springer: Berlin/Heidelberg, Germany, 2006; Volume 7. [Google Scholar]
- Zhang, X.G. Corrosion and Electrochemistry of Zinc. Springer-Verlag New York Inc: New York, NY, USA, 2013. [Google Scholar]
- Kania, H.; Sipa, J. Microstructure characterization and corrosion resistance of zinc coating obtained on high-strength grade 10.9 bolts using a new thermal diffusion process. Materials 2019, 12, 1400. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.G.; Scully, J.R.; Shoesmith, D.W.; Buchheit, R.G. Electrochemical Techniques in Corrosion Science and Engineering; Marcel Dekker, Inc.: New York, NY, USA; Basel, Switzerland, 2003. [Google Scholar]
- Dalledone, E.; Barbosa, M.A.; Wolynec, S. Zinc-55% aluminum-1.6% silicon coating compared with zinc coating. Mater. Perform. 1995, 34, 24–28. [Google Scholar]
- Kuz, M. Handbook of Environmental Degradation of Materials; William Andrew Publishing, Inc.: Norwich, UK, 2005. [Google Scholar]
- Radu, T.; Ciocan, A.; Potecasu, F.; Balint, L. Obtaining and characterizing Zn-Al-Bi coatings on steel band. In Proceedings of the 21st International Conference on Metallurgy and Materials, METAL 2012, Brno, Czech Republic, 23–25 May 2012. Article No. ZV12. [Google Scholar]
- Abdel Hamid, Z.; Abd El Rehim, S.S.; Abou Shama, A.; Ebrahim, M. Improvement the corrosion resistance for the galvanized steel by adding Sn. J. Surf. Eng. Mater. Adv. Technol. 2016, 6, 58–71. [Google Scholar] [CrossRef]
- Khireche, S.; Boughrara, D.; Kadri, A.; Hamadou, L.; Benbrahim, N. Corrosion mechanism of Al, Al-Zn and Al-Zn-Sn alloys in 3 wt.% NaCl solution. Corros. Sci. 2014, 87, 504–516. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) CRC Handbook of Chemistry and Physics, 90th ed.; Taylor and Francis Group LLC: Boca Raton, FL, USA, 2010. [Google Scholar]
- Bockris, J.; Reddy, A.K.N. Modern Electrochemistry; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1998. [Google Scholar]




| Bath | Content (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| Al | Fe | Ni | Pb | Bi | Sn | Zn and others | |
| Zn-AlNiBiSn | 0.0051 | 0.032 | 0.049 | 0.002 | 0.067 | 0.28 | residue |
| Zn-AlNi | 0.0054 | 0.034 | 0.052 | 0.002 | 0.0004 | 0.0005 | residue |
| Points of Microanalysis | Content of Elements | |||||
|---|---|---|---|---|---|---|
| Zn-K | Sn-L | Bi-M | ||||
| wt.% | at.% | wt.% | at.% | wt.% | at.% | |
| 1 | 95.8 | 98.1 | 2.4 | 1.4 | 1.8 | 0.6 |
| 2 | 18.1 | 32.0 | 54.2 | 52.7 | 27.7 | 15.3 |
| 3 | 53.4 | 70.7 | 31.7 | 23.1 | 14.9 | 6.2 |
| 4 | 16.1 | 37.3 | 3.6 | 4.6 | 80.3 | 58.1 |
| 5 | 24.4 | 38.2 | 66.2 | 57.2 | 9.4 | 4.6 |
| 6 | 97.3 | 98.8 | 1.6 | 0.9 | 1.1 | 0.3 |
| 7 | 100 | 100 | - | - | - | - |
| Points of Microanalysis | Content of Elements | |||||||
|---|---|---|---|---|---|---|---|---|
| Zn-K | Fe-K | Sn-L | Bi-M | |||||
| wt.% | at.% | wt.% | at.% | wt.% | at.% | wt.% | at.% | |
| 8 | 8.6 | 16.8 | - | - | 58.3 | 62.9 | 33.1 | 20.3 |
| 9 | 100 | 100 | - | - | - | - | - | - |
| 10 | 94.1 | 93.2 | 5.9 | 6.8 | - | - | - | - |
| 11 | 93.8 | 92.8 | 6.2 | 7.2 | - | - | - | - |
| 12 | 90.6 | 89.2 | 9.4 | 10.8 | - | - | - | - |
| 13 | 76.5 | 73.5 | 23.5 | 26.5 | - | - | - | - |
| 14 | 49.6 | 64.5 | 4.2 | 6.4 | 33.4 | 23.9 | 12.8 | 5.2 |
| Type of Coating | Ecorr (mV, vs. NHE) | jcorr (mA/cm2) |
|---|---|---|
| Zn-AlNi | −768.37 | −6.24 |
| Zn-AlNiBiSn | −781.29 | −18.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kania, H.; Saternus, M.; Kudláček, J. Impact of Bi and Sn on Microstructure and Corrosion Resistance of Zinc Coatings Obtained in Zn-AlNi Bath. Materials 2020, 13, 3788. https://doi.org/10.3390/ma13173788
Kania H, Saternus M, Kudláček J. Impact of Bi and Sn on Microstructure and Corrosion Resistance of Zinc Coatings Obtained in Zn-AlNi Bath. Materials. 2020; 13(17):3788. https://doi.org/10.3390/ma13173788
Chicago/Turabian StyleKania, Henryk, Mariola Saternus, and Jan Kudláček. 2020. "Impact of Bi and Sn on Microstructure and Corrosion Resistance of Zinc Coatings Obtained in Zn-AlNi Bath" Materials 13, no. 17: 3788. https://doi.org/10.3390/ma13173788
APA StyleKania, H., Saternus, M., & Kudláček, J. (2020). Impact of Bi and Sn on Microstructure and Corrosion Resistance of Zinc Coatings Obtained in Zn-AlNi Bath. Materials, 13(17), 3788. https://doi.org/10.3390/ma13173788







