Electrospun PCL Fiber Mats Incorporating Multi-Targeted B and Co Co-Doped Bioactive Glass Nanoparticles for Angiogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of B and Co Co-Doped Bioactive Glass Nanoparticles (BCo.BGNs)
2.2. Fabrication of PCL Fiber Mats Containing BCo.BGNs (PCL-BCo.BGNs Mats)
2.3. Materials Characterization
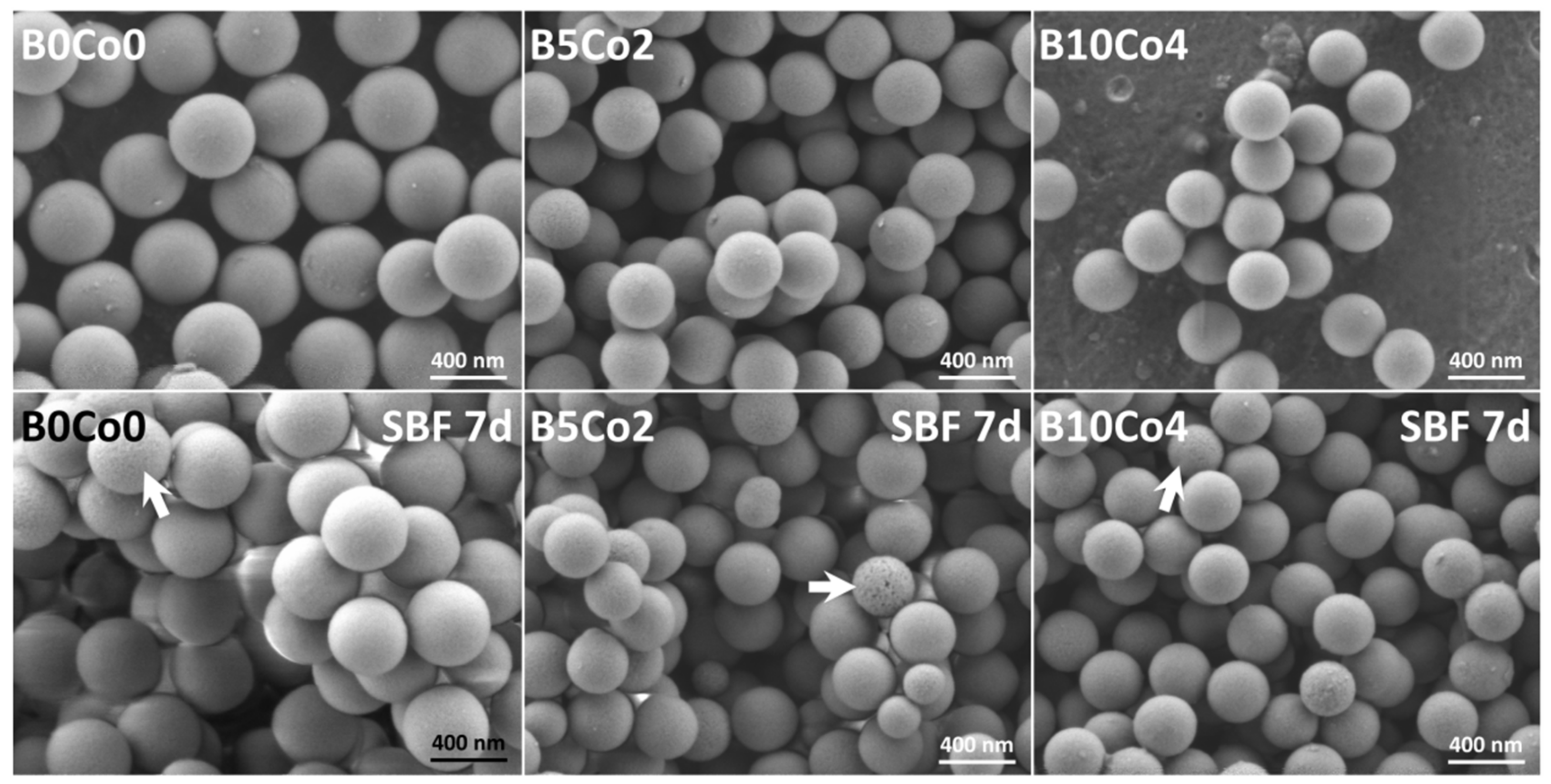
2.3.1. Ion Release and Bioactivity of BCo.BGNs in SBF
2.3.2. Degradation of PCL-BCo.BGNs Mats in SBF
2.3.3. Wettability
2.3.4. Mechanical Tests
2.4. Viability Test of ST-2 Cells
2.5. Fluorescent Staining
2.6. VEGF Measurement
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kanczler, J.M.; Wells, J.A.; Gibbs, D.M.R.; Marshall, K.M.; Tang, D.K.O.; Oreffo, R.O.C. Bone tissue engineering and bone regeneration. In Principles of Tissue Engineering, 5th ed.; Lanza, R., Langer, R., Vacanti, J.P., Atala, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 917–935. [Google Scholar]
- Coffee, M.; Biswanath, S.; Bolesani, E.; Zweigerdt, R. Heart muscle tissue engineering. In Essential Current Concepts in Stem Cell Biology, 1st ed.; Brand-Saberi, B., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 99–121. [Google Scholar]
- Blaker, J.J.; Nazhat, S.N.; Boccaccini, A.R. Development and characterisation of silver-doped bioactive glass-coated sutures for tissue engineering and wound healing applications. Biomaterials 2004, 25, 1319–1329. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, Y.; Duan, G.; Mei, C.; Greiner, A.; Agarwal, S. Electrospun nanofiber reinforced composites: A review. Polym. Chem. 2018, 9, 2685–2720. [Google Scholar] [CrossRef]
- Li, W.J.; Mauck, R.L.; Tuan, R.S. Electrospun nanofibrous scaffolds: Production, characterization, and applications for tissue engineering and drug delivery. J. Biomed. Nanotechnol. 2005, 1, 259–275. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Nerurkar, N.L.; Elliott, D.M.; Mauck, R.L. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J. Orthop. Res. 2007, 25, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, W.; Müller, T.; Schubert, D.W.; Boccaccini, A.R.; Yao, Q.; Roether, J.A. Electrospun polyhydroxybutyrate/poly (ε-caprolactone)/58S sol–gel bioactive glass hybrid scaffolds with highly improved osteogenic potential for bone tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 17098–17108. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, C.; Deng, X. Biodegradable polyester blends for biomedical application. J. Appl. Polym. Sci. 1995, 56, 103–112. [Google Scholar] [CrossRef]
- Soliman, S.; Sant, S.; Nichol, J.W.; Khabiry, M.; Traversa, E.; Khademhosseini, A. Controlling the porosity of fibrous scaffolds by modulating the fiber diameter and packing density. J. Biomed. Mater. Res. Part A 2011, 96, 566–574. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.Y.; Khil, M.S.; Ra, Y.M.; Lee, D.R. Characterization of nano-structured poly (ε-caprolactone) nonwoven mats via electrospinning. Polymer 2003, 44, 1287–1294. [Google Scholar] [CrossRef]
- Li, W.J.; Danielson, K.G.; Alexander, P.G.; Tuan, R.S. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly (ϵ-caprolactone) scaffolds. J. Biomed. Mater. Res. Part A 2003, 67, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, M.P.; Venugopal, J.R.; Chyan, T.T.; Hai, L.B.; Chan, C.K.; Lim, A.Y.; Ramakrishna, S. Electrospun biocomposite nanofibrous scaffolds for neural tissue engineering. Tissue Eng. Part A 2008, 14, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hou, H.; Agarwal, S.; Greiner, A. Polyimide nanofibers by “Green” electrospinning via aqueous solution for filtration applications. ACS Sustain. Chem. Eng. 2016, 4, 4797–4804. [Google Scholar] [CrossRef]
- Liverani, L.; Killian, M.S.; Boccaccini, A.R. Fibronectin functionalized electrospun fibers by using benign solvents: Best way to achieve effective functionalization. Front. Bioeng. Biotechnol. 2019, 7, 68. [Google Scholar] [CrossRef]
- Ferreira, J.L.; Gomes, S.; Henriques, C.; Borges, J.P.; Silva, J.C. Electrospinning polycaprolactone dissolved in glacial acetic acid: Fiber production, nonwoven characterization, and in vitro evaluation. J. Appl. Polym. Sci. 2014, 131, 41068. [Google Scholar] [CrossRef]
- Ghosal, K.; Thomas, S.; Kalarikkal, N.; Gnanamani, A. Collagen coated electrospun polycaprolactone (PCl) with titanium dioxide (TiO2) from an environmentally benign solvent: Preliminary physico-chemical studies for skin substitute. J. Polym. Res. 2014, 21, 410. [Google Scholar] [CrossRef]
- Schueren, L.V.; Schoenmaker, B.D.; Kalaoglu, Ö.I.; Clerck, K.D. An alternative solvent system for the steady state electrospinning of polycaprolactone. Eur. Polym. J. 2011, 47, 1256–1263. [Google Scholar] [CrossRef]
- Kanani, A.G.; Bahrami, S.H. Effect of changing solvents on poly (ε-caprolactone) nanofibrous webs morphology. J. Nanomater. 2011, 31, 524153. [Google Scholar] [CrossRef]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Liverani, L.; Boccaccini, A.R. Versatile production of poly (epsilon-caprolactone) fibers by electrospinning using benign solvents. Nanomaterials 2016, 6, 75. [Google Scholar] [CrossRef]
- Aytac, Z.; Yildiz, Z.I.; Kayaci-Senirmak, F.; Tekinay, T.; Uyar, T. Electrospinning of cyclodextrin/linalool-inclusion complex nanofibers: Fast-dissolving nanofibrous web with prolonged release and antibacterial activity. Food Chem. 2017, 231, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.J.; Kuan, C.H.; Wu, H.C.; Tsai, J.C.; Chen, T.M.; Hsieh, D.J.; Wang, T.W. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomater. 2014, 10, 4156–4166. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Gao, Q.; Li, Y.; Rahaman, M.N.; Teramoto, A.; Abe, K. In vitro evaluation of electrospun gelatin-bioactive glass hybrid scaffolds for bone regeneration. J. Appl. Polym. Sci. 2013, 127, 2588–2599. [Google Scholar] [CrossRef]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef]
- Gaudio, C.D.; Baiguera, S.; Boieri, M.; Mazzanti, B.; Ribatti, D.; Bianco, A.; Macchiarini, P. Induction of angiogenesis using VEGF releasing genipin-crosslinked electrospun gelatin mats. Biomaterials 2013, 34, 7754–7765. [Google Scholar] [CrossRef] [PubMed]
- Said, S.S.; Yin, H.; Elfarnawany, M.; Nong, Z.; O’Neil, C.; Leong, H.; Lacefield, J.C.; Mequanint, K.; Pickering, J.G. Fortifying angiogenesis in ischemic muscle with FGF9-loaded electrospun poly (ester amide) fibers. Adv. Healthc. Mater. 2019, 8, 1801294. [Google Scholar] [CrossRef]
- Ren, X.; Han, Y.; Wang, J.; Jiang, Y.; Yi, Z.; Xu, H.; Ke, Q. An aligned porous electrospun fibrous membrane with controlled drug delivery–an efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018, 70, 140–153. [Google Scholar] [CrossRef]
- Phipps, M.C.; Xu, Y.; Bellis, S.I. Delivery of platelet-derived growth factor as a chemotactic factor for mesenchymal stem cells by bone-mimetic electrospun scaffolds. PLoS ONE 2012, 7, e40831. [Google Scholar] [CrossRef]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Investigation of angiogenesis and its mechanism using zinc oxide nanoparticle-loaded electrospun tissue engineering scaffolds. RSC Adv. 2014, 4, 51528–51536. [Google Scholar] [CrossRef]
- Boldbaatar, K.; Dashnyam, K.; Knowles, J.C.; Lee, H.H.; Lee, J.H.; Kim, H.W. Dual-ion delivery for synergistic angiogenesis and bactericidal capacity with silica-based microsphere. Acta Biomater. 2019, 83, 322–333. [Google Scholar] [CrossRef]
- Chen, S.; Michálek, M.; Galusková, D.; Michálková, M.; Švančárek, P.; Talimian, A.; Kaňková, H.; Kraxner, J.; Zheng, K.; Liverani, L.; et al. Multi-targeted B and Co co-doped 45S5 bioactive glass with angiogenic potential for bone regeneration. Mater. Sci. Eng. C 2020, 112, 110909. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, E.; Partap, S.; Azevedo, M.M.; Jell, G.; Stevens, M.M.; O’Brien, F.J. Hypoxia-mimicking bioactive glass/collagen glycosaminoglycan composite scaffolds to enhance angiogenesis and bone repair. Biomaterials 2015, 52, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Hupa, L.; Jokic, B.; Detsch, R.; Grünewald, A.; Boccaccini, A.R. Angiogenic potential of boron-containing bioactive glasses: In vitro study. J. Mater. Sci. 2017, 52, 8785–8792. [Google Scholar] [CrossRef]
- Zheng, K.; Boccaccini, A.R. Sol-gel processing of bioactive glass nanoparticles: A review. Adv. Colloid Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Erol, M.; Stark, W.J.; Mohn, D.; Hong, Z.; Mano, J.F. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos. Sci. Technol. 2010, 70, 1764–1776. [Google Scholar] [CrossRef]
- Ardeshirylajimi, A.; Farhadian, S.; Adegani, F.J.; Mirzaei, S.; Zomorrod, M.S.; Langroudi, L.; Doostmohammadi, A.; Seyedjafari, E.; Soleimani, M. Enhanced osteoconductivity of polyethersulphone nanofibres loaded with bioactive glass nanoparticles in in vitro and in vivo models. Cell Prolif. 2015, 48, 455–464. [Google Scholar] [CrossRef]
- Lepry, W.C.; Smith, S.; Liverani, L.; Boccaccini, A.R.; Nazhat, S.N. Acellular bioactivity of sol-gel derived borate glass-polycaprolactone electrospun scaffolds. Biomed. Glasses 2016, 2, 88–98. [Google Scholar] [CrossRef]
- Moura, D.; Souza, M.T.; Liverani, L.; Rella, G.; Luz, G.M.; Mano, J.F.; Boccaccini, A.R. Development of a bioactive glass-polymer composite for wound healing applications. Mater. Sci. Eng. C 2017, 76, 224–232. [Google Scholar] [CrossRef]
- Zheng, K.; Taccardi, N.; Beltrán, A.M.; Sui, B.; Zhou, T.; Marthala, V.R.R.; Hartmann, M.; Boccaccini, A.R. Timing of calcium nitrate addition affects morphology, dispersity and composition of bioactive glass nanoparticles. RSC Adv. 2016, 6, 95101–95111. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Tsigkou, O.; Labbaf, S.; Stevens, M.M.; Porter, A.E.; Jones, J.R. Monodispersed bioactive glass submicron particles and their effect on bone marrow and adipose tissue-derived stem cells. Adv. Healthc. Mater. 2014, 3, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Lukowiak, A.; Lao, J.; Lacroix, J.; Nedelec, J.M. Bioactive glass nanoparticles obtained through sol–gel chemistry. Chem. Commun. 2013, 49, 6620–6622. [Google Scholar] [CrossRef] [PubMed]
- Himanshu, T.; Sp, S.; Ka, S.; Prerna, M.; Ashish, J. Studies on preparation and characterization of 45S5 bioactive glassdoped with (TiO2 + ZrO2) as bioactive ceramic material. Bioceram. Dev. Appl. 2016, 6, 2. [Google Scholar] [CrossRef]
- Naseri, S.; Lepry, W.C.; Li, W.; Waters, K.E.; Boccaccini, A.R.; Nazhat, S.N. 45S5 bioactive glass reactivity by dynamic vapour sorption. J. Non-Cryst. Solids 2016, 432, 47–52. [Google Scholar] [CrossRef]
- Hristov, H.; Nedyalkova, M.; Madurga, S.; Simeonov, V. Boron oxide glasses and nanocomposites: Synthetic, structural and statistical approach. J. Mater. Sci. Technol. 2017, 33, 535–540. [Google Scholar] [CrossRef]
- Rad, R.M.; Alshemary, A.Z.; Evis, Z.; Keskin, D.; Altunbaş, K.; Tezcaner, A. Structural and biological assessment of boron doped bioactive glass nanoparticles for dental tissue applications. Ceram. Int. 2018, 44, 9854–9864. [Google Scholar] [CrossRef]
- Zheng, K.; Fan, Y.; Torre, E.; Balasubramanian, P.; Taccardi, N.; Cassinelli, C.; Morra, M.; Iviglia, G.; Boccaccini, A.R. Incorporation of boron in mesoporous bioactive glass nanoparticles reduces inflammatory response and delays osteogenic differentiation. Part. Part. Syst. Charact. 2020, 37, 2000054. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing, 1st ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Liverani, L.; Boccardi, E.; Beltrán, A.M.; Boccaccini, A.R. Incorporation of calcium containing mesoporous (MCM-41-type) particles in electrospun PCL fibers by using benign solvents. Polymers 2017, 9, 487. [Google Scholar] [CrossRef]
- Vrieze, S.D.; Camp, T.V.; Nelvig, A.; Hagström, B.; Westbroek, P.; Clerck, K.D. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Ghorbani, F.M.; Kaffashi, B.; Shokrollahi, P.; Seyedjafari, E.; Ardeshirylajimi, A. PCL/chitosan/Zn-doped nHA electrospun nanocomposite scaffold promotes adipose derived stem cells adhesion and proliferation. Carbohydr. Polym. 2015, 118, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Bui, N.N.; Manickam, S.S.; McCutcheon, J.R. Controlling electrospun nanofiber morphology and mechanical properties using humidity. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1734–1744. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kristl, J.; Janković, B.; Baumgartner, S.; Kocbek, P. The impact of relative humidity during electrospinning on the morphology and mechanical properties of nanofibers. Int. J. Pharm. 2013, 456, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.T.; Lee, H.Y.; Shin, U.S.; Kim, H.W. Composite nanofiber of bioactive glass nanofiller incorporated poly (lactic acid) for bone regeneration. Mater. Lett. 2010, 64, 802–805. [Google Scholar] [CrossRef]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Ceradini, D.J.; Gurtner, G.C. Homing to hypoxia: HIF-1 as a mediator of progenitor cell recruitment to injured tissue. Trends Cardiovasc. Med. 2005, 15, 57–63. [Google Scholar] [CrossRef]







| Samples | B2O3 (H3BO3) | CaO (Ca(NO3)2·4H2O) | CoO (Co(NO3)2·6H2O) | SiO2 ((C2H5O)4Si) |
|---|---|---|---|---|
| B0Co0 | 0 | 20 | 0 | 80 |
| B5Co2 | 5 | 20 | 2 | 73 |
| B10Co4 | 10 | 20 | 4 | 66 |
| Samples | B | Ca | Co | Si |
|---|---|---|---|---|
| B0Co0 | 0 | 4.44 ± 0.02 | 0 | 96 ± 2 |
| B5Co2 | 0.61 ± 0.04 | 4.28 ± 0.09 | 0.106 ± 0.008 | 95 ± 2 |
| B10Co4 | 4.03 ± 0.04 | 1.7 ± 0.1 | 0.195 ± 0.008 | 94 ± 1 |
| Samples | Average Fiber Diameter (nm) | Minimum Fiber Diameter (nm) | Maximum Fiber Diameter (nm) | Young’s Modulus (MPa) | Water Contact Angle (°) |
|---|---|---|---|---|---|
| PCL | 916 ± 603 | 105 | 2912 | 10 ± 2 | 139 ± 3 |
| PCL-B0Co0 | 770 ± 555 * | 105 | 2932 | 7 ± 1 * | 137 ± 1 |
| PCL-B5Co2 | 924 ± 561 (P > 0.05) | 149 | 2640 | 7 ± 3 (P > 0.05) | 137.8 ± 0.8 |
| PCL-B10Co4 | 1022 ± 484 (P > 0.05) | 158 | 2797 | 11 ± 3 (P > 0.05) | 137 ± 3 |
| Samples | Si (mg/L) | B (μg/L) | Co (μg/L) |
|---|---|---|---|
| PCL-B0Co0 | 15 ± 3 | 0 | 0 |
| PCL-B5Co2 | 6 ± 2 | 0 | 28 ± 4 |
| PCL-B10Co4 | 4 ± 2 | 70 ± 11 | 32 ± 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Galusková, D.; Kaňková, H.; Zheng, K.; Michálek, M.; Liverani, L.; Galusek, D.; Boccaccini, A.R. Electrospun PCL Fiber Mats Incorporating Multi-Targeted B and Co Co-Doped Bioactive Glass Nanoparticles for Angiogenesis. Materials 2020, 13, 4010. https://doi.org/10.3390/ma13184010
Chen S, Galusková D, Kaňková H, Zheng K, Michálek M, Liverani L, Galusek D, Boccaccini AR. Electrospun PCL Fiber Mats Incorporating Multi-Targeted B and Co Co-Doped Bioactive Glass Nanoparticles for Angiogenesis. Materials. 2020; 13(18):4010. https://doi.org/10.3390/ma13184010
Chicago/Turabian StyleChen, Si, Dagmar Galusková, Hana Kaňková, Kai Zheng, Martin Michálek, Liliana Liverani, Dušan Galusek, and Aldo R. Boccaccini. 2020. "Electrospun PCL Fiber Mats Incorporating Multi-Targeted B and Co Co-Doped Bioactive Glass Nanoparticles for Angiogenesis" Materials 13, no. 18: 4010. https://doi.org/10.3390/ma13184010
APA StyleChen, S., Galusková, D., Kaňková, H., Zheng, K., Michálek, M., Liverani, L., Galusek, D., & Boccaccini, A. R. (2020). Electrospun PCL Fiber Mats Incorporating Multi-Targeted B and Co Co-Doped Bioactive Glass Nanoparticles for Angiogenesis. Materials, 13(18), 4010. https://doi.org/10.3390/ma13184010











