Temperature Dependence on Tensile Mechanical Properties of Sintered Silver Film
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Determining p of the s-Ag
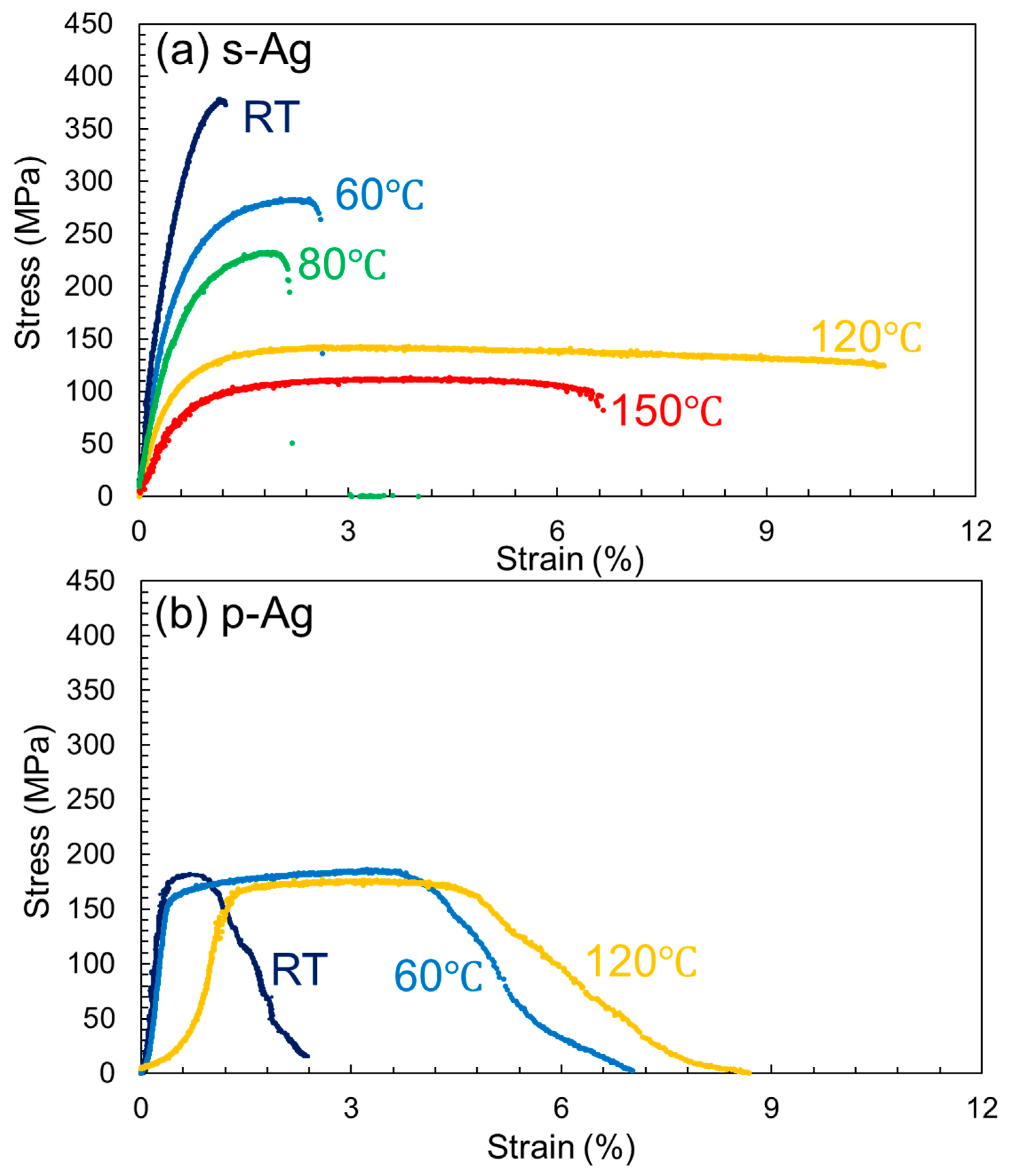
3.2. S-S Curves of the s-Ag and p-Ag
4. Fracture Mechanism
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siow, K.S. Are Sintered Silver Joints Ready for Use as Interconnect Material in Microelectronic Packaging? J. Electron. Mat. 2013, 43, 947–963. [Google Scholar] [CrossRef] [Green Version]
- Siow, K.S. Mechanical properties of nano-silver joints as die attach materials. J. Alloys Compd. 2012, 514, 6–19. [Google Scholar] [CrossRef]
- Peng, P.; Hu, A.; Gerlich, A.P.; Zou, G.; Liu, L.; Zhou, Y.N. Joining of Silver Nanomaterials at Low Tempertures: Processes, Properties, and Applications. Appl. Mater. Interfaces 2015, 7, 12597–12618. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.G.; Calata, J.N.; Lu, G.Q. Processing and Characterization of Nanosilver Pastes for Die-Attaching SiC Devices. IEEE Trans. Electron. Packag. Technol. 2007, 30, 241–245. [Google Scholar] [CrossRef]
- Zabihzadeh, S.; Petegem, S.V.; Duarte, L.I.; Mokso, R.; Cervellino, A.; Swygenhoven, H.V. Deformation behavior of sintered nanocrystalline silver layers. Acta Mater. 2015, 97, 116–123. [Google Scholar] [CrossRef]
- Zabihzadeh, S.; Petegem, S.V.; Holler, M.; Diaz, A.; Duarte, L.I.; Swygenhoven, H.V. Deformation behavior of nanoporous polycrystalline silver. Part I: Microstructure and mechanical properties. Acta Mater. 2017, 131, 467–474. [Google Scholar] [CrossRef]
- Gadaud, P.; Caccuri, V.; Bertheau, D.; Carr, J.; Milhet, X. Milhet. Aging sintered silver: Relationship between tensile behavior, mechanical properties and the nanoporous structure evolution. Mater. Sci. Eng. A 2016, 669, 379–386. [Google Scholar]
- Suzuki, T.; Terasaki, T.; Kawana, Y.; Ishikawa, D.; Nishimura, M.; Nakako, H.; Kurafuchi, K. Effect of Manufacturing Process on Micro-Deformation Behavior of Sintered-Silver Die-Attach Material. IEEE Trans. Device Mater. Reliab. 2016, 16, 588–593. [Google Scholar]
- Takesue, M.; Watanabe, T.; Tanaka, K.; Nakajima, N. Mechanical Properties and Reliability of Pressureless Sintered Silver Materials for Power Devices. In Proceedings of the PCIM Europe 2018 International Exhibition and Conference for Power Electronics, Intelligent Motion, Renewable Energy and Energy Management, Nuremberg, Germany, 5–7 June 2018. [Google Scholar]
- Bai, J.G.; Chang, Z.Z.; Calata, J.N.; Lu, G.Q. Low-Temperature Sintered Nanoscale Silver as a novel Semiconductor Device-Metallized Substrate Interconnect Material. IEEE Trans. Device Mater. Reliab. 2006, 29, 589–595. [Google Scholar]
- Wakamoto, K.; Mochizuki, Y.; Otsuka, T.; Nakahara, K.; Namazu, T. Tensile Mechanical properties of sintered porous silver films and their dependence on porosity. Jpn. J. Appl. Phys. 2019, 58, SDDL08-1–SDDL08-5. [Google Scholar] [CrossRef]
- Herboth, T.; Guenther, M.; Fix, A.; Wilde, J. Failure Mechanisms of Sintered Silver Interconnections for Power Electronic Applications. In Proceedings of the ECTC 2013 Electronic Components and Technology Conference, Las Vegas, NV, USA, 28–31 May 2013. [Google Scholar]
- Herboth, T.; Fruh, C.; Gunther, M.; Wilde, J. Assessment of Thermo-Mechanical Stresses in Low Temperature Joining Technology. In Proceedings of the EuroSimE 2012 International Thermal, Mechanical and Multi-Physics Simulation and Experiments in Microelectronics and Micro-systems, Lisbon, Portugal, 16–17 April 2012. [Google Scholar]
- Webber, C.; Dijk, M.V.; Walter, H.; Hutter, M.; Witter, O.; Lang, K.D. Combination of experimental and simulation methods for analysis of sintered Ag joints for high temperature applications. In Proceedings of the ECTC 2016 Electronic Components and Technology Conference, Las Vegas, NV, USA, 31 May–3 June 2016. [Google Scholar]
- Chen, C.; Nagao, S.; Zhang, H.; Suganuma, T.; Suganuma, K.; Iwashige, T.; Sugiura, K.; Tsuruta, K. Low-stress design for SiC power modules with sintered porous Ag. In Proceedings of the ECTC 2016 Electronic Components and Technology Conference, Las Vegas, NV, USA, 31 May–3 June 2016. [Google Scholar]
- Chen, C.; Nagao, S.; Zhang, H.; Jiu, J.; Sugahara, T.; Suganuma, K.; Iwashige, T.; Sugiura, K.; Tsuruta, K. Mechanical Deformation of Sintered Porous Ag Die Attach at High Temperature and Its Size Effect for Wide-Bandgap Power Device Design. J. Electron. Mater. 2017, 46, 1576–1586. [Google Scholar] [CrossRef]
- Letz, S.; Hutzler, A.; Waltrich, U.; Zischler, S.; Schletz, A. Mechanical properties of silver sintered bond lines: Aspects for a reliable material data base for numerical simulations. In Proceedings of the CIPS 2016 International Conference on Integrated Power Electronics Systems, Nuremberg, Germany, 8–10 March 2016. [Google Scholar]
- Chen, X.; Li, R.; Qi, K.; Lu, G.Q. Tensile Behaviors and Ratcheting Effects of Partially Sintered Chip-Attachment Films of a Nanoscale Silver Paste. J. Electron. Mater. 2008, 37, 1574–1579. [Google Scholar] [CrossRef]
- Kariya, Y.; Yamaguchi, H.; Itako, M.; Mizumura, N.; Sasaki, K. Mechanical Behaviors of Nano-sized Ag particles. J. Smart Process 2013, 2, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Kraft, S.; Zischler, S.; Tham, N.; Schletz, A. Properties of a novel silver sintering die attach material for high temperature-high lifetime applications. In Proceedings of the SENSOR 2013 International Conference on Sensors and Measurement Technology, Nurnberg, Germany, 14–16 May 2013. [Google Scholar]
- Suzuki, T.; Yasuda, Y.; Terasaki, T.; Morita, T.; Kawana, Y.; Ishikawa, D.; Nishimura, M.; Nakako, H.; Kurafuchi, K. Thermal cycling lifetime estimation of sintered metal die attachment. In Proceedings of the ICEP 2016 International Conference in Electronics Packaging, Hokkaido, Japan, 20–22 April 2016. [Google Scholar]
- Schmitt, W.; Chew, L.M. Silver sinter paste for SiC bonding with improved mechanical properties. In Proceedings of the ECTC 2017 Electronic Components and Technology Conference, Florida, America, 30 May–2 June 2017. [Google Scholar]
- Chen, C.; Nagao, S.; Suganuma, K.; Jiu, J.; Sugahara, T.; Zhang, H.; Iwashige, T.; Sugiura, K.; Tsuruta, K. Macroscale and microscale fracture toughness of microporous sintered Ag for applications in power devices. Acta Mater. 2017, 129, 41–51. [Google Scholar] [CrossRef]
- Kaibara, Y.; Fujii, H.; Namazu, T.; Tomizawa, Y.; Masunishi, K.; Inoue, S. Mechanical degradation mechanism of Aluminum-alloy structural films evaluated by environment-controlled tensile testing. In Proceedings of the TRANCEDUCERS, International Solid-State Sensors, Actuators and Microsystems Conference, Denver, CO, USA, 21–25 June 2009. [Google Scholar]
- Namazu, T.; Fujii, M.; Manusishi, K.; Tomizawa, Y.; Inoue, S. Thermal Anealing Effect on Elastic-Plastic Behavior of Al-Si-Cu Structual Films Under Uniaxial and Biaxial Tension. J. Microelectromech. Syst. 2013, 22, 1414–1427. [Google Scholar] [CrossRef]
- Goshima, Y.; Fujii, T.; Inoue, S.; Namazu, T. Influence of 700 °C vacuum annealing on fracture behavior of micro-nanoscale Si structures. Jpn. J. Appl. Phys. 2016, 55, 06GL03-1–06GL03-6. [Google Scholar] [CrossRef]
- Fujii, M.; Namazu, T.; Fujii, H.; Masunishi, K.; Tomizawa, Y.; Inoue, S. Quasistatic and dynamic mechanical properties of Al-Si-Cu structural films in uniaxial tension. J. Vac. Sci. Technol. B 2012, 30, 031804-1–031804-9. [Google Scholar] [CrossRef]
- Matsuda, T.; Inami, K.; Motoyama, K.; Sano, T.; Hirose, A. Silver oxide decomposition mediated direct bonding of silicon-based materials. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Morita, T.; Yasuda, Y.; Ide, E.; Hirose, A. Direct Bonding to Aluminium with Silver-Oxide Microparticles. Mater. Trans. 2009, 50, 226–228. [Google Scholar] [CrossRef] [Green Version]
- Saada, G. Hall-petch revisited. Mater. Sci. Eng. A 2005, 400–401, 146–149. [Google Scholar] [CrossRef]
- Cordero, Z.C.; Knight, B.E.; Schuh, C.A. Six decades of the Hall-Petch effect—A survey of grain-size strengthening studies on pure metals. Int. Mater. Rev. 2016, 61, 495–512. [Google Scholar] [CrossRef]
- Zhou, M.; Wei, Z.; Qiao, H.; Zhu, L.; Yang, H.; Xia, T. Particle Size and Pore Structure Characterization of Silver Nanoparticles Prepared by Confined Arc Plasma. J. Nanomater. 2009, 2009, 968058. [Google Scholar] [CrossRef]
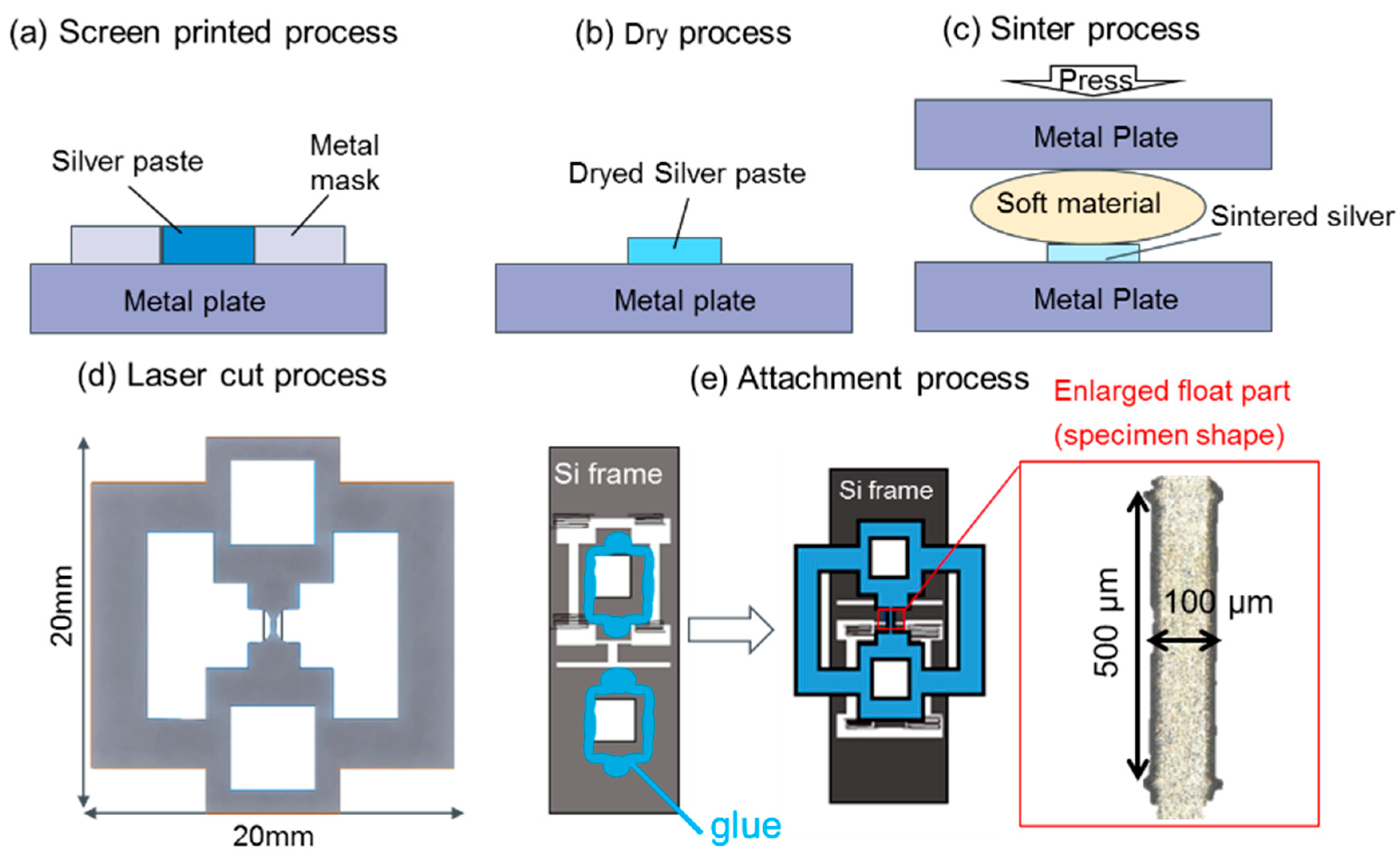





| Material | Test Temperature (°C) | Strain Speed (s−1) |
|---|---|---|
| s-Ag | RT, 60, 80, 120, 150 | 1 × 10−4 |
| p-Ag | RT, 60, 120 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakamoto, K.; Mochizuki, Y.; Otsuka, T.; Nakahara, K.; Namazu, T. Temperature Dependence on Tensile Mechanical Properties of Sintered Silver Film. Materials 2020, 13, 4061. https://doi.org/10.3390/ma13184061
Wakamoto K, Mochizuki Y, Otsuka T, Nakahara K, Namazu T. Temperature Dependence on Tensile Mechanical Properties of Sintered Silver Film. Materials. 2020; 13(18):4061. https://doi.org/10.3390/ma13184061
Chicago/Turabian StyleWakamoto, Keisuke, Yo Mochizuki, Takukazu Otsuka, Ken Nakahara, and Takahiro Namazu. 2020. "Temperature Dependence on Tensile Mechanical Properties of Sintered Silver Film" Materials 13, no. 18: 4061. https://doi.org/10.3390/ma13184061
APA StyleWakamoto, K., Mochizuki, Y., Otsuka, T., Nakahara, K., & Namazu, T. (2020). Temperature Dependence on Tensile Mechanical Properties of Sintered Silver Film. Materials, 13(18), 4061. https://doi.org/10.3390/ma13184061




