Analysis of Selected Properties of Biocomposites Based on Polyethylene with a Natural Origin Filler
Abstract
:1. Introduction
2. Experimental Conditions
2.1. Test Stand
2.2. Materials
2.3. Research Programme and Methodology
- (1)
- Content by weight of bran: 0%, 5%, 10%, 15%;
- (2)
- The grain size of the bran fraction used <0.4mm, 0.4–0.6 mm and 0.6–0.8 mm.
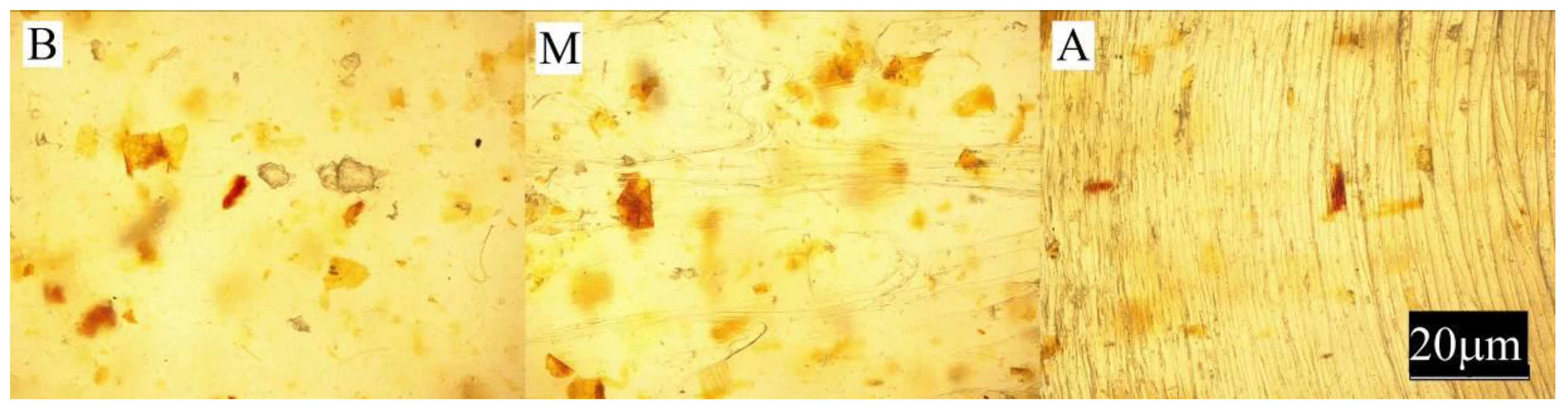
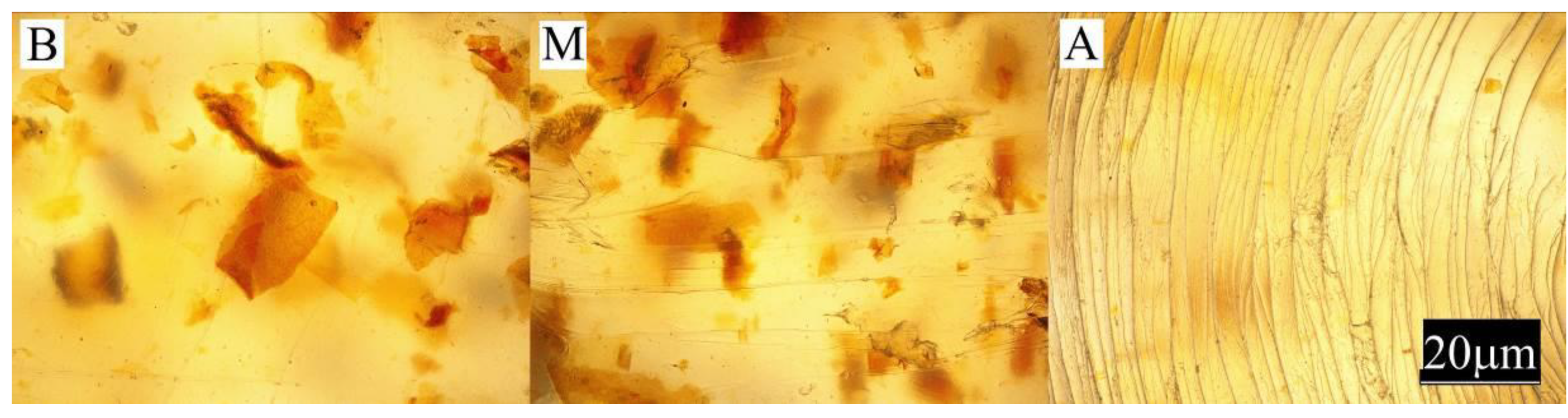
- Observation of the sample surface with the optical microscope (Nikon, Tokyo, Japan), model Eclipse LV100ND equipped with a DS-U3 camera using the NIS-Elements AR 4.20.00 software (Nikon, Tokyo, Japan). The transmitted light method was used for observation. Observations were made at three points of the sample, marked in Figure 1. All samples were observed in identical magnification and illumination parameters.
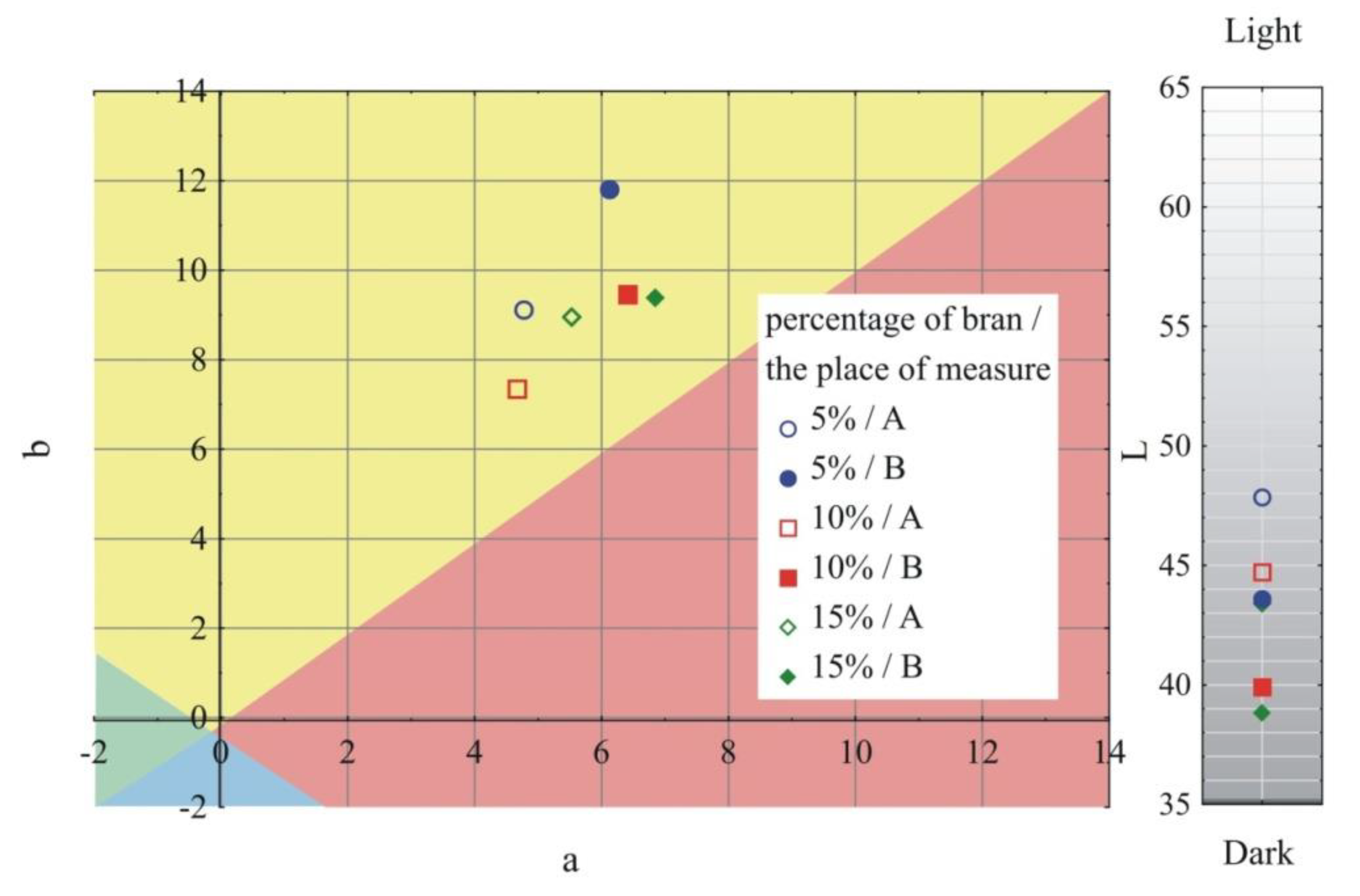
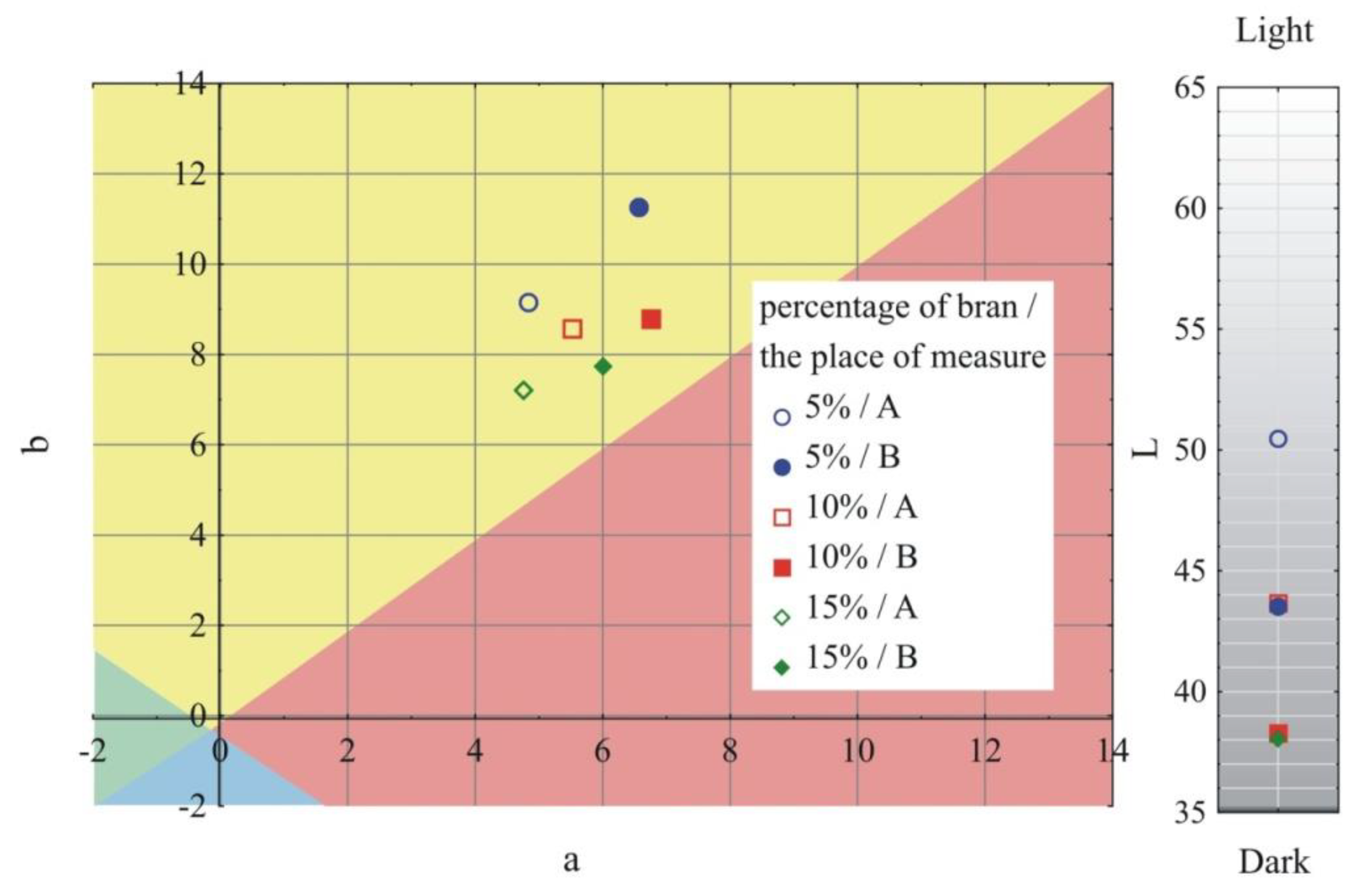
- Measurement of the color of samples in accordance with ASTM E308 [79], for which the Ci4200 spectrophotometer (X-Rite, Grand Rapids, MI, USA) was used. The color is described in the CIELab system, where it is defined in the L*, a*, b* area. Parameter a* describes the color from green (negative values) to red (positive values); parameter b*, the color from blue (negative values) to yellow (positive values); and parameter L* is luminance–brightness, representing the gray scale from black to white (0 is black and 100 is white). The difference between the two colors—two points in the three-dimensional space L*, a*, b* are described by the relationship:where: ΔL, Δa and Δb, respectively, represent the difference in color parameters between the compared samples. The measurements of the color of the injection mouldings were taken at points A and B and marked in Figure 1;
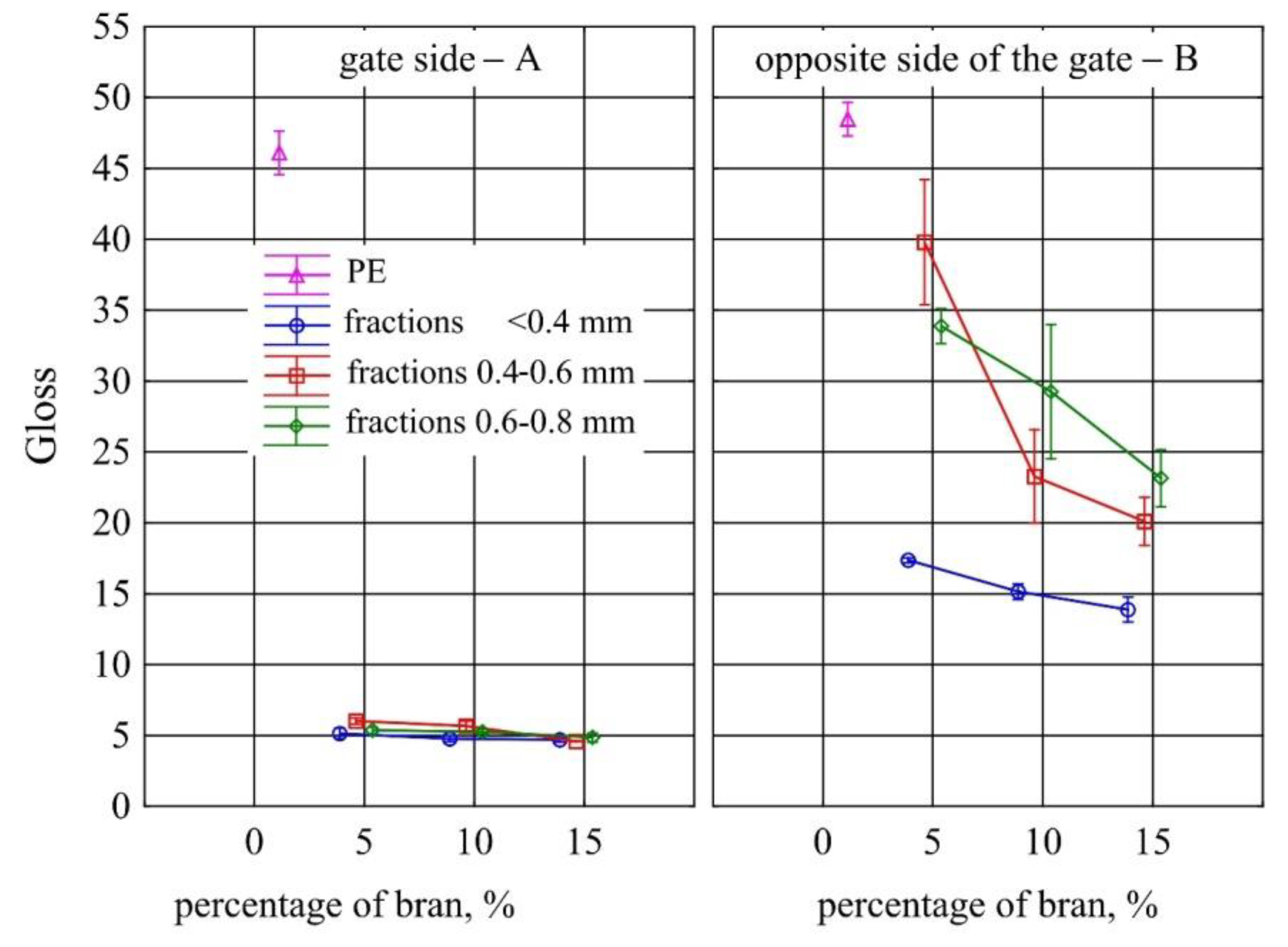
- Measurement of the gloss of the surface of samples using the X - Rite Ci4200 spectrophotometer, according to ISO 2813: 2001 [80] at an angle of 60° of the aperture of the image of the light source and the receiver. Gloss of injection moulded parts was measured at points A and B, as marked in Figure 1;
- Measurement of longitudinal processing shrinkage of samples according to ISO 294-4: 2005 [81];
- Determination of tensile strength σ (MPa), nominal elongation at maximum tensile stress ε (%) and Young’s modulus (MPa) using Z010 testing machine (Zwick/Roell, Ulm, Germany) according to ISO 527-2 [82] at a tensile speed of 50 mm/min;
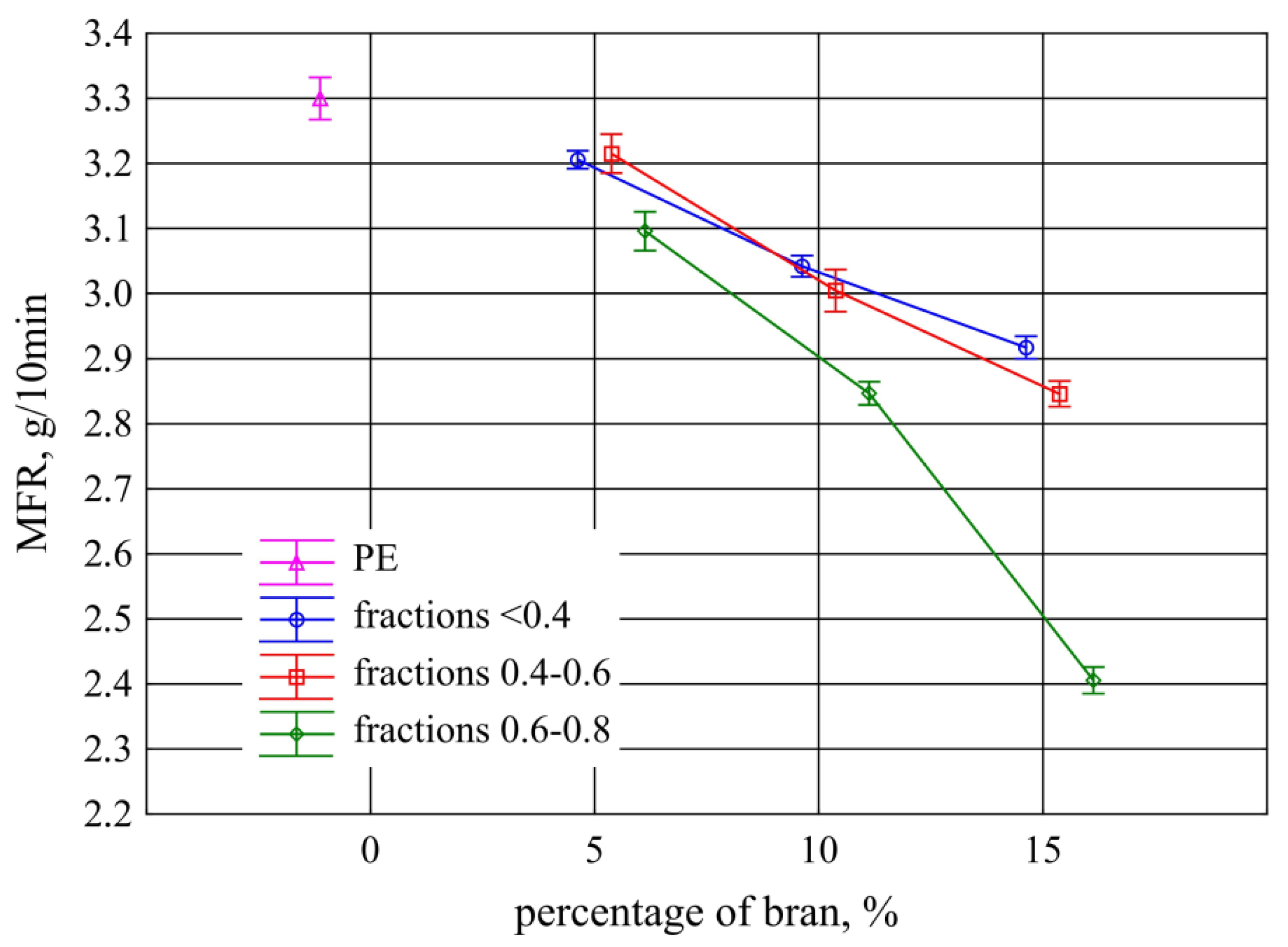
- Determination of the mass melt flow rate MFR (150 °C/2.16 kg) in g/10 min. The measurement was carried out using a load plastometer MeltFlow TQ6841 model (CEAST, Turin, Italy) based on the recommendations of ISO 1133-1: 2011 using method A [83];
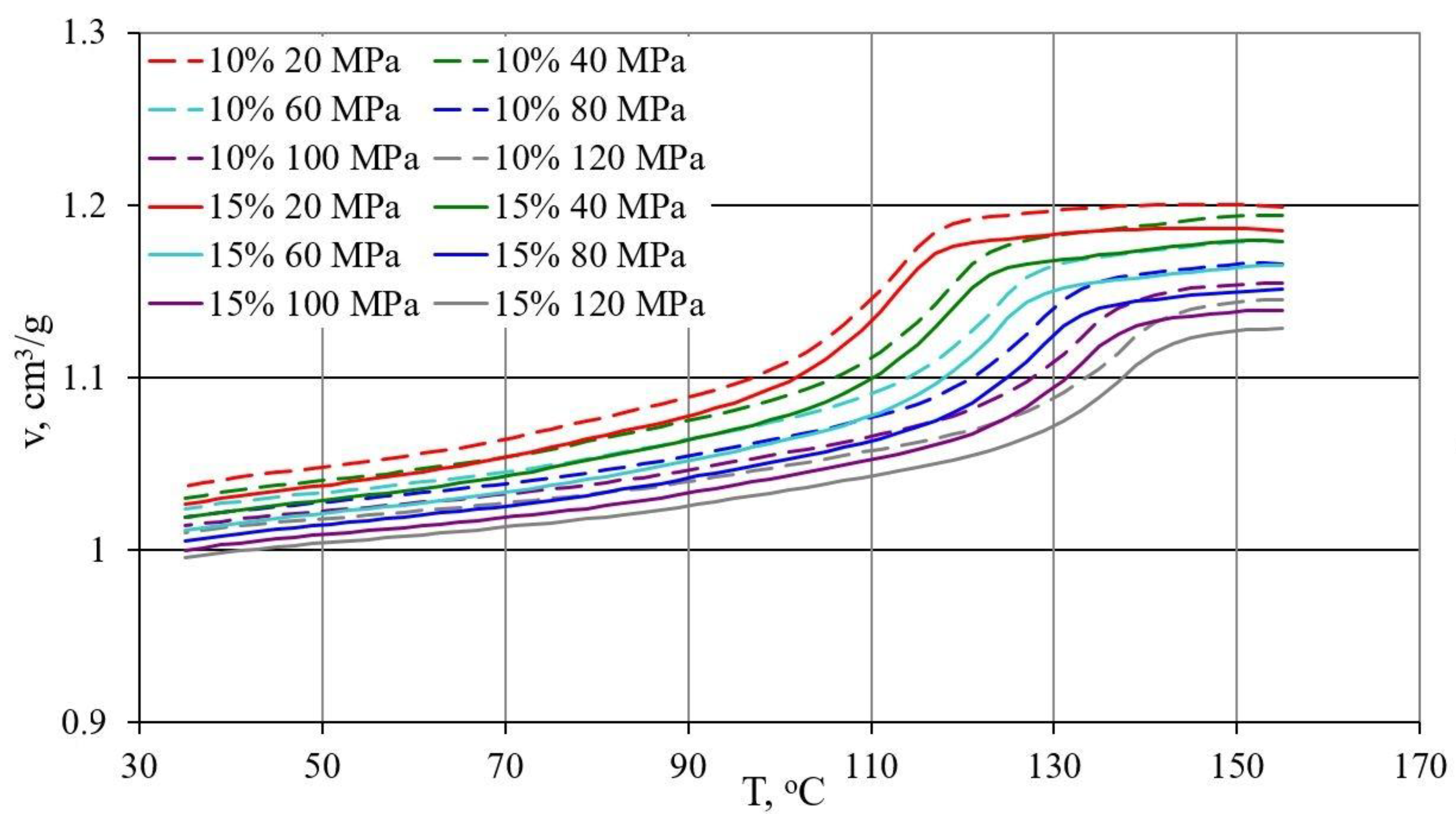
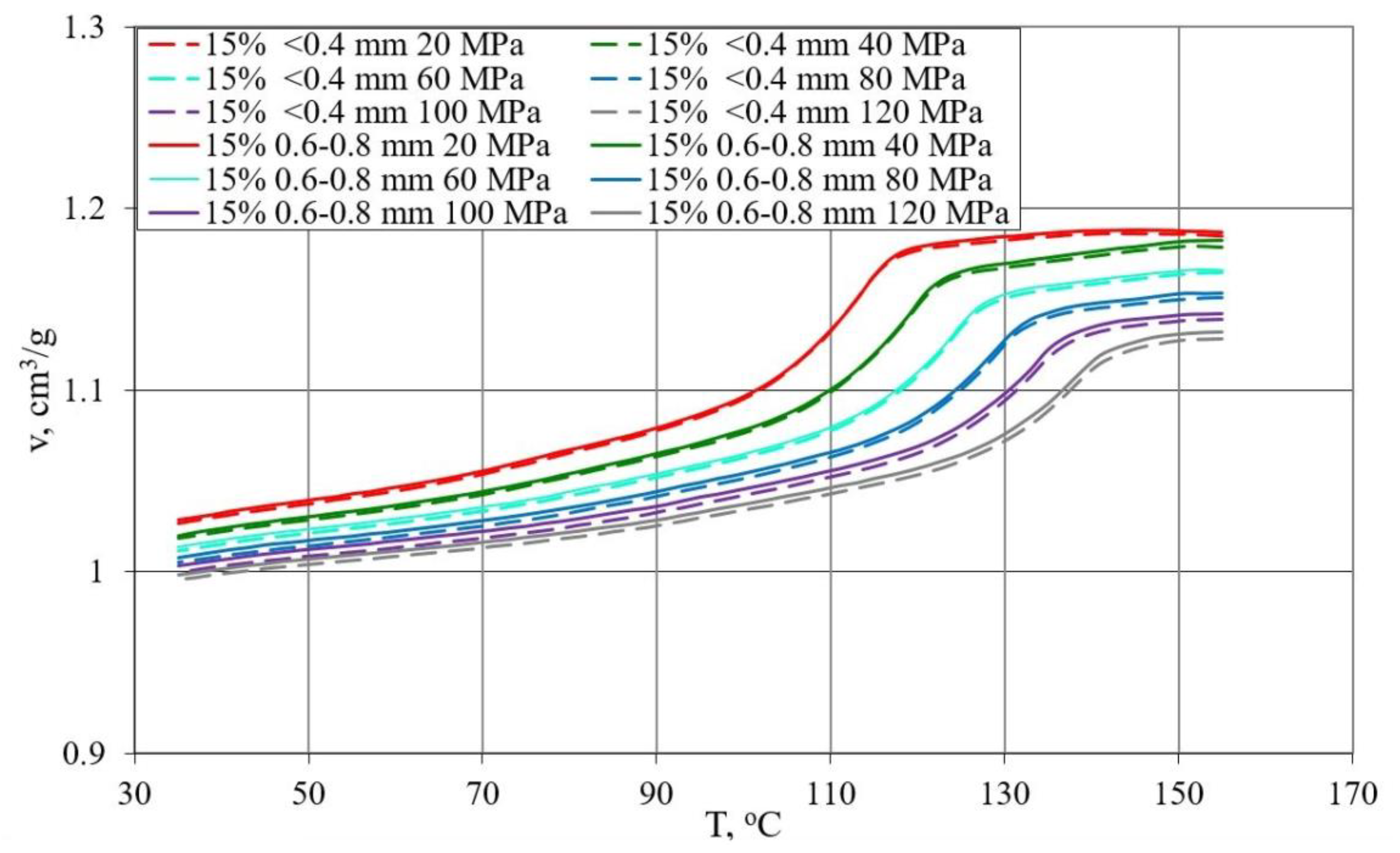
- Determination of the relationship between pressure p specific volume v and temperature T during isobaric cooling in accordance with ISO 17744: 2004 [84]. The measurements were carried out with the p-v-T 100 device from SWO Polymertechnik GmbH (Krefeld, Germany). The test samples were heated to the maximum measurement temperature of 155 °C and compressed to the preset pressure value (20, 40, 60, 80, 100 and 120 MPa). Then, the set pressure was kept constant and the samples were cooled at a rate of 5 °C/min to a temperature of 35 °C while the changes in specific volume were measured, and the process was repeated for the next higher compression pressure value.
- FTIR analysis of samples using a FTIR TENSOR 27 spectrophotometer (Bruker, Germany) equipped with an attenuated total reflection (ATR) attachment with diamond crystal. Spectra were collected in the range of 600–4000 cm−1 with 16 scans per sample with a resolution of 4 cm−1.
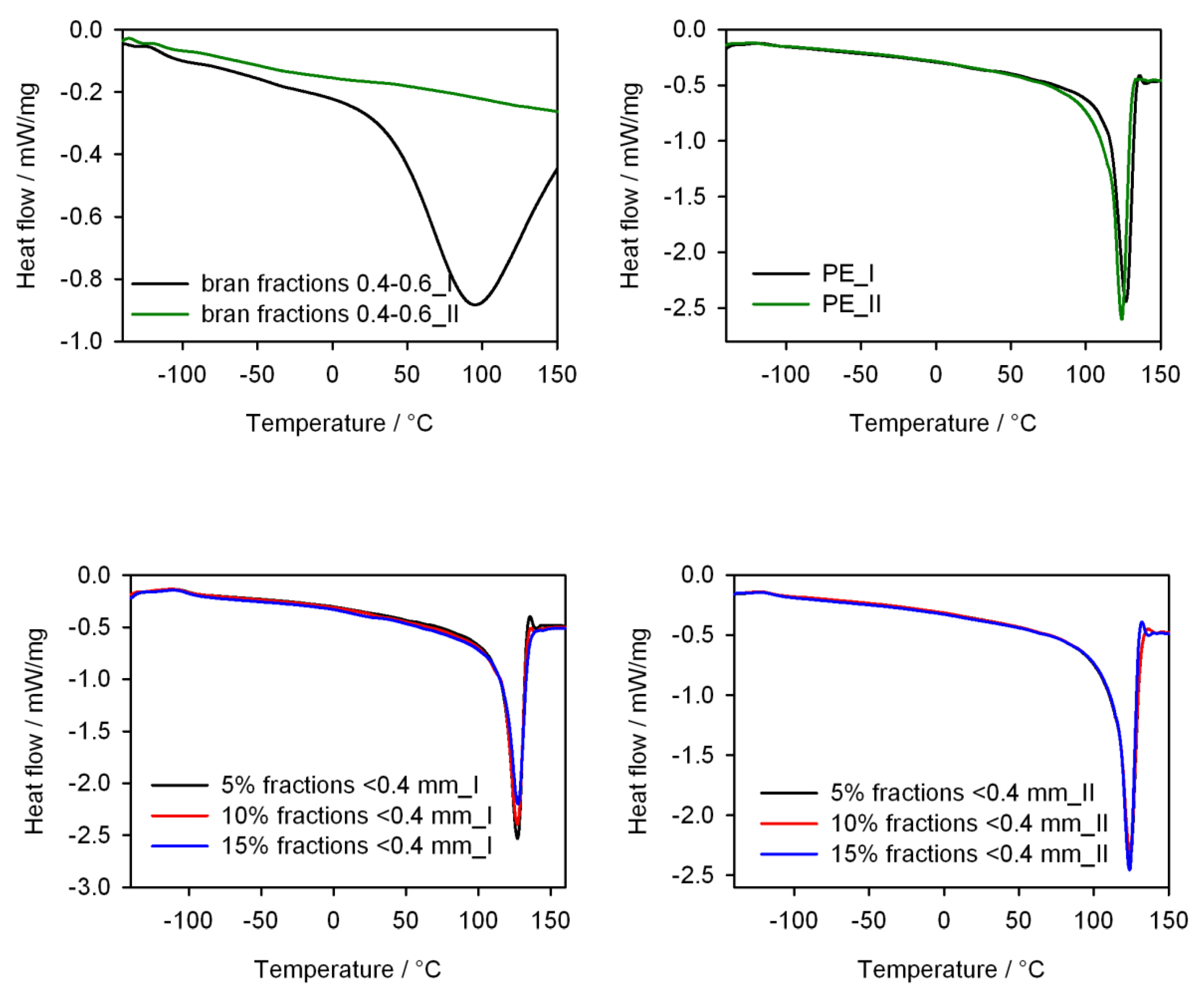
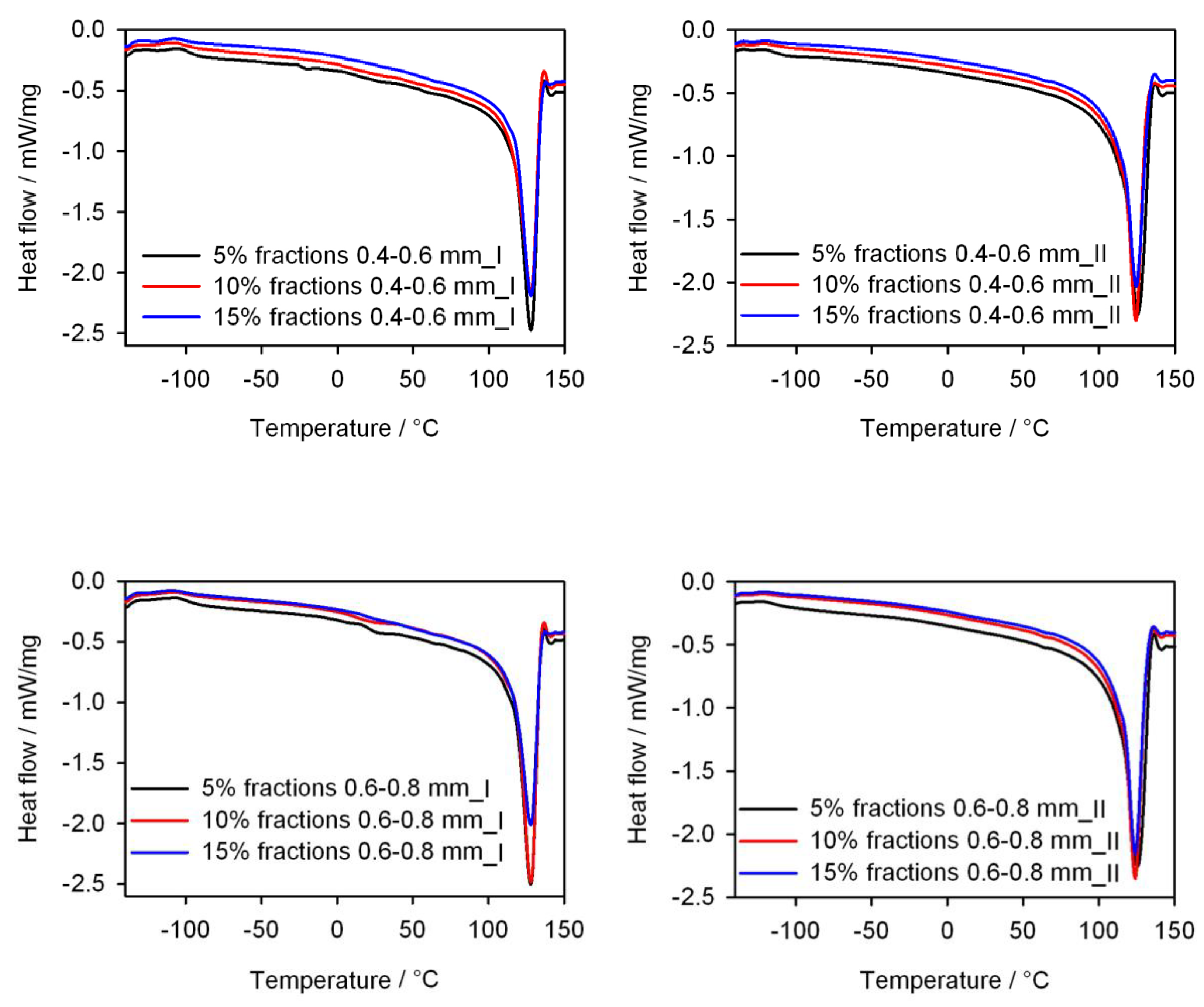
- Differential scanning calorimetry (DSC) tests of the obtained injection mouldings using the DSC 204 F1 Phoenix differential scanning calorimeter (NETZSCH, Günzbung, Germany) and the NETZSCH Proteus data processing software (NETZSCH, Günzbung, Germany), according to ISO 11357-1: 2016 [85]. The DSC curves were recorded in the heating (I) cycle from −150 to 150 °C (at a rate of 10 K/min), cooling from 150 to −150 °C (at a rate of 10 K/min) and heating (II) −150 to 150 °C (at a rate of 10 K/min). The samples were tested in aluminium crucibles with a pierced lid. On the basis of the DSC curves, the degree of crystallinity Xc, the enthalpy of melting ΔHm, the melting point Tm, the crystallization temperature and the glass transition temperature Tg of the injection mouldings were determined. The degree of crystallinity was calculated from the relationshipassuming that for PE ΔH100% = 293 J/g [86]. The inflection point of the DSC curve in the area of the glass transition was taken as the glass transition temperature.
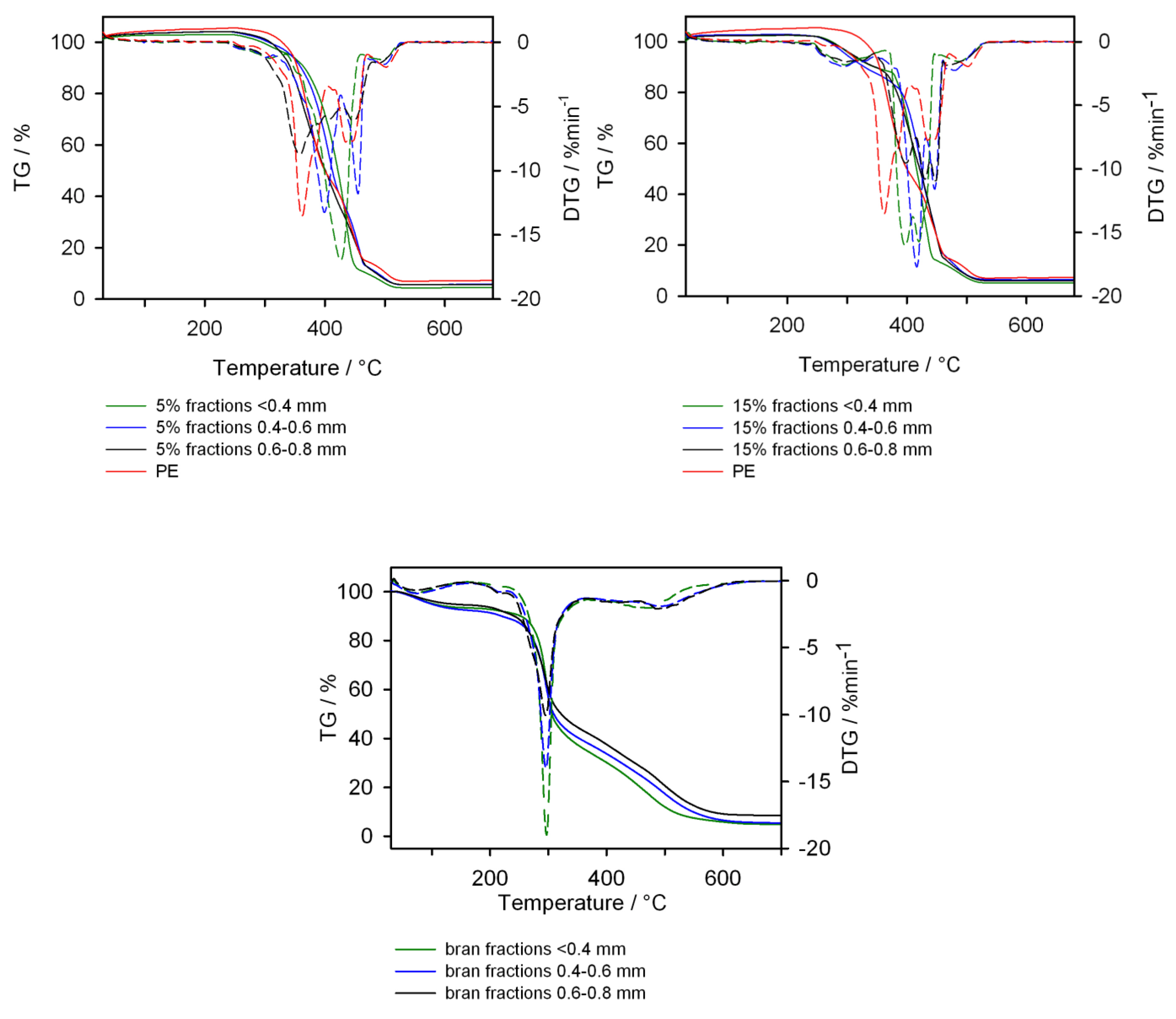
- Thermogravimetric analysis (TG), carried out using the STA 449 F1 Jupiter thermal analyzer (NETZSCH, Günzbung, Germany). The tests were carried out at temperatures of 30–700 °C in an atmosphere of synthetic air with a gas flow rate of 20 mL/min and the heating rate 10 °C/min. The samples weighing about 10 mg were tested in crucibles made of Al2O3.
3. Results
3.1. Visual Characteristics of Injection Mouldings
3.2. Mass Melt Flow Rate
3.3. p-v-T Diagrams
3.4. Chemical Structure
3.5. Thermal Properties
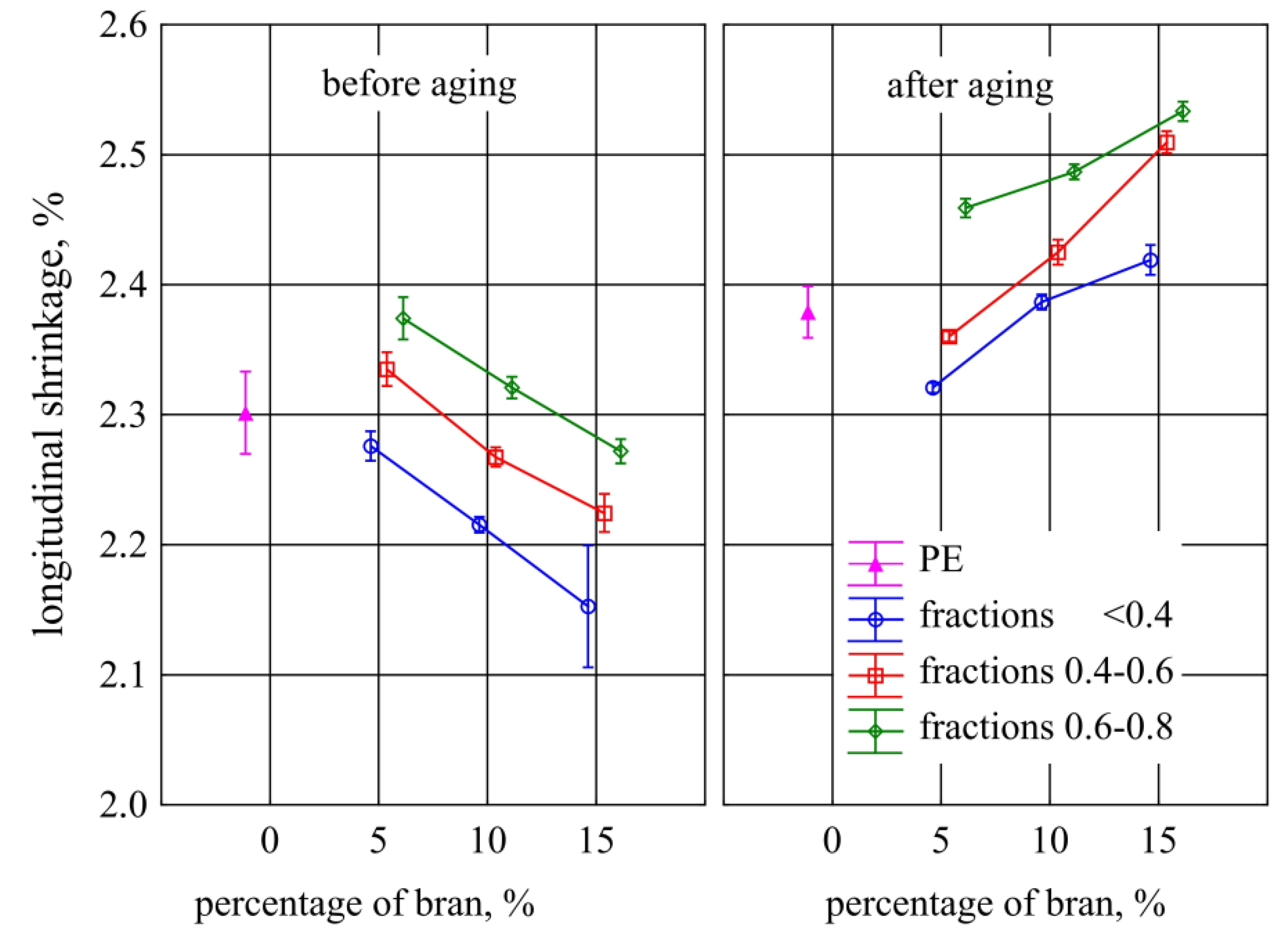
3.6. Longitudinal Contraction
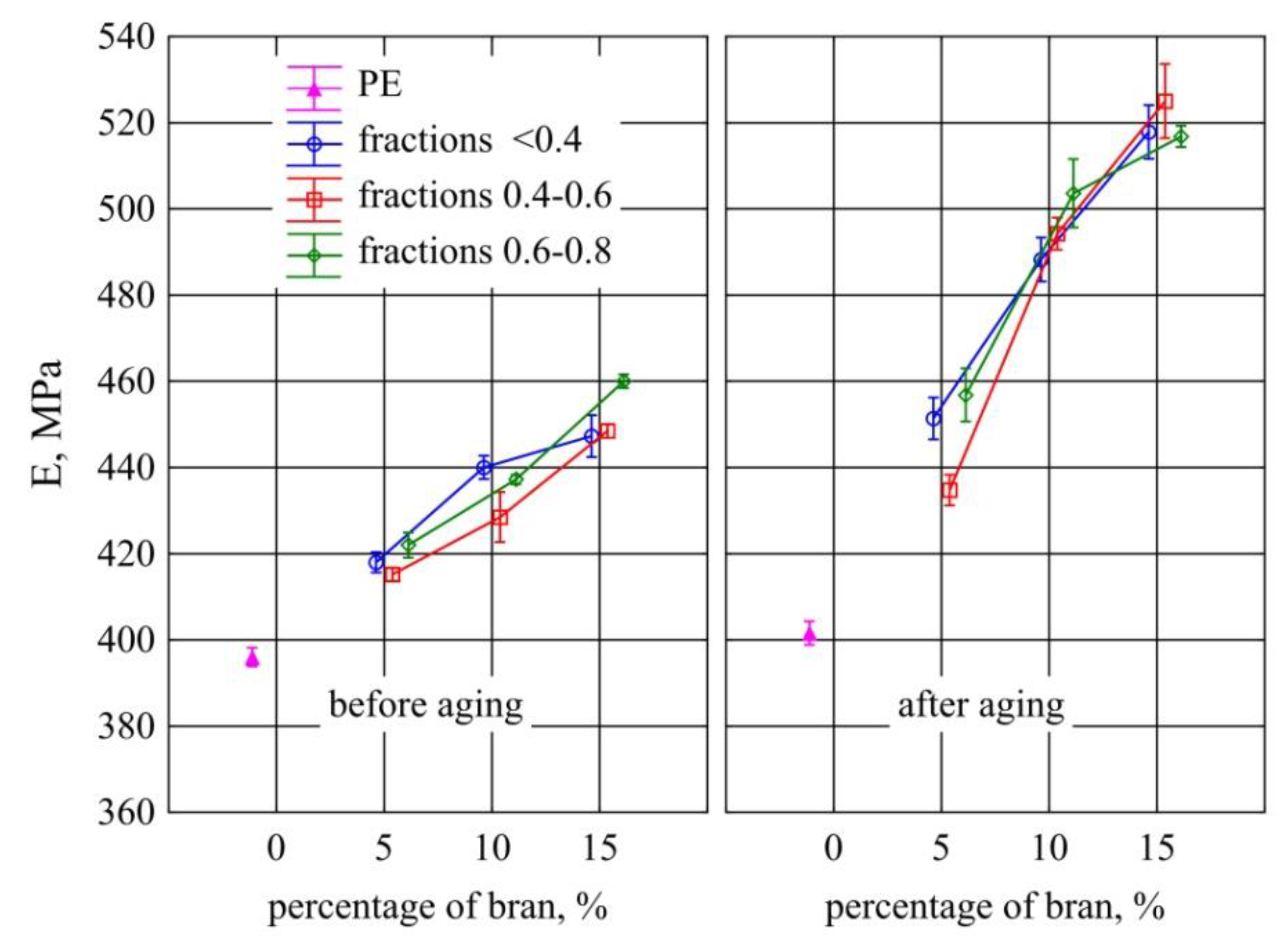
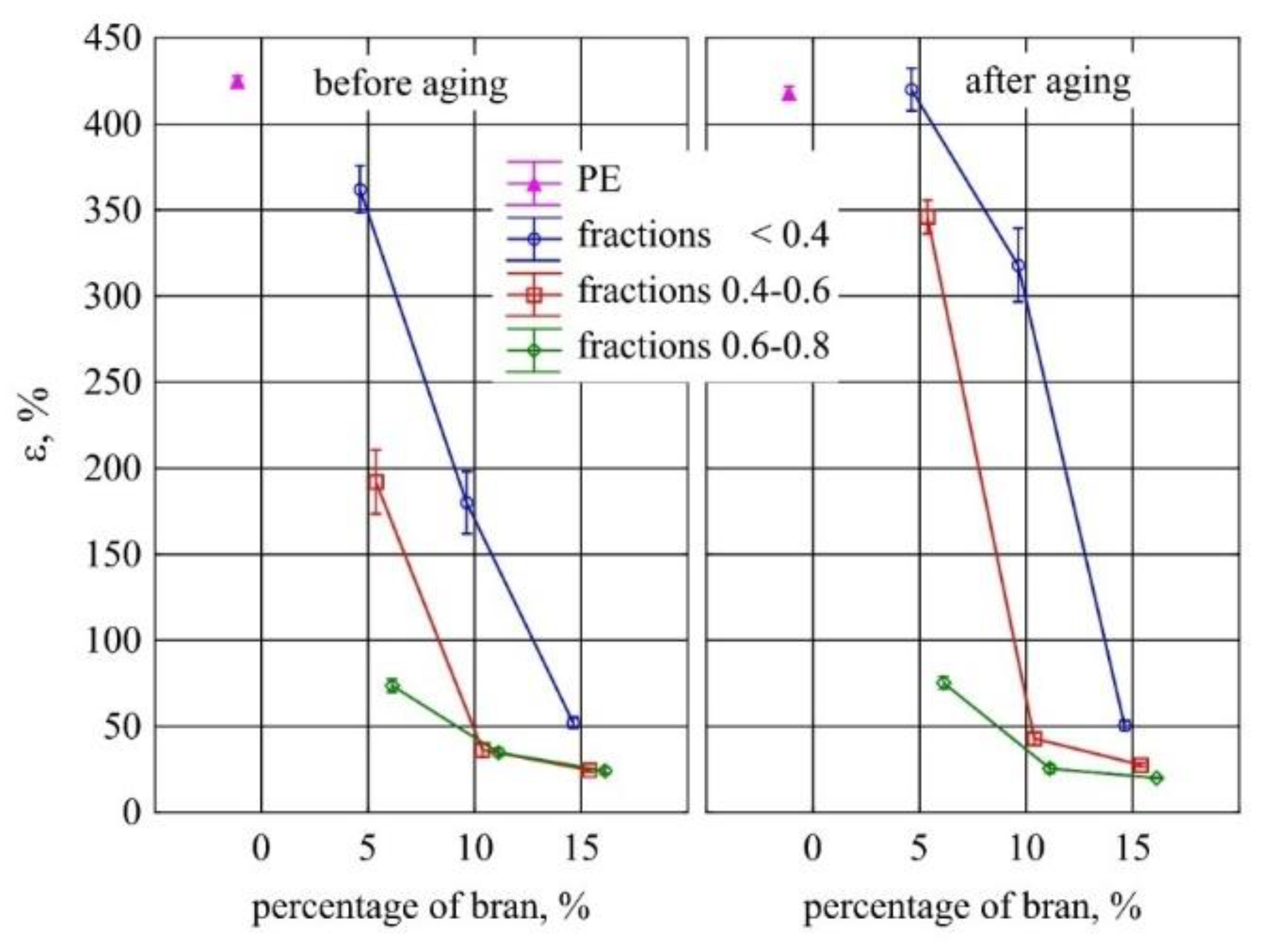
3.7. Static Tensile Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Melo, P.M.A.; Macêdo, O.B.; Barbosa, G.P.; Ueki, M.M.; Silva, L.B. High-Density Polyethylene/Mollusk Shell-Waste Composites: Effect of Particle Size and Coupling Agent on Morphology, Mechanical and Thermal Properties. J. Mater. Res. Technol. 2019, 8, 1915–1925. [Google Scholar] [CrossRef]
- Kumar, N.; Das, D. Fibrous Biocomposites from Nettle (Girardinia Diversifolia) and Poly(Lactic Acid) Fibres for Automotive Dashboard Panel Application. Compos. Part B Eng. 2017, 130, 54–63. [Google Scholar] [CrossRef]
- Xanthos, M. Functional Fillers for Plastics. Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010. [Google Scholar]
- Sasimowski, E.; Majewski, Ł. Effect of the Intensive Plasticizing Zone Design on the Effectiveness of Corotating Twin-Screw Extrusion. Adv. Polym. Technol. 2019, 8518341. [Google Scholar] [CrossRef]
- Liang, J.Z. Reinforcement and Quantitive Description of Inorganic Particulate-Filled Polymer Composites. Compos. Part B Eng. 2013, 51, 224–232. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Influence of the Conditions of Corotating Twin-Screw Extrusion for Talc-Filled Polypropylene on Selected Properties of the Extrudate. Polymers 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P. Polymer Modified Clay Minerals: A Review. Appl. Clay Sci. 2007, 38, 64–76. [Google Scholar] [CrossRef]
- Väisänen, T.; Das, O.; Tomppo, L. A Review on New Bio-Based Constituents for Natural Fiber-Polymer Composutes. J. Clean. Prod. 2017, 149, 582–592. [Google Scholar] [CrossRef]
- Bher, A.; Unalan, I.U.; Auras, R.; Rubino, M.; Schvezov, C.E. Roughening of Poly (Lactic Acid) and Thermoplastic Cassava Starch Reactive Blends Using Graphene Nanoplatelets. Polymers 2018, 10, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Acosta, D.; Rodriguez-Uribe, A.; Álvarez-Chávez, C.; Mohanty, A.K.; Misra, M.; López-Cervantes, J.; Madera-Santana, T.J. Physicochemical Characterization and Evaluation of Pecan Nutshell as Biofiller in A Matrix of Poly(Lactic Acid). J. Polym. Environ. 2019, 27, 521–532. [Google Scholar] [CrossRef]
- Obasi, H.C. Peanut Husk Filled Polyethylene Composites: Effect of Filler Content and Compatibilizer on Properties. J. Polym. 2015. [Google Scholar] [CrossRef] [Green Version]
- Sikora, J.; Majewski, Ł.; Puszka, A. Modern Biodegradable Plastics—Processing and Properties: Part I. Materials 2020, 13, 1986. [Google Scholar] [CrossRef] [PubMed]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites Reinforced with Natural Fibres: 2000-2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Tian, G.; Zhuang, J.; Fu, Y.; Wang, Z.; Li, Q. Enhanced Mechanical Strength of Polypropylene-Based Lignocellulosic-Plastic Composites by Cellulose Fibres. Bioresources 2019, 14, 1668–1678. [Google Scholar]
- Bekele, L.D.; Zhang, W.; Liu, Y.; Duns, G.J.; Yu, C.; Jin, X.; Li, X.; Jia, Q.; Chen, J. Preparation and Characterization of Lemongrass Fiber (Cymbopogon Species) for Reinforcing Application in Thermoplastic Composites. Bioresources 2017, 12, 5664–5681. [Google Scholar] [CrossRef]
- Baba, B.O.; Özmen, U. Preparation and Mechanical Characterization of Chicken Feather/PLA Composites. Polym. Compos. 2015, 36, 837–845. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of Biopolymeric Materials Based on Cellulose, Cellulose Nanofibres, and Cellulose Derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [Green Version]
- Merkel, K.; Rydarowski, H.; Kazimierczak, J.; Bloda, A. Processing and Characterization of Reinforced Polyethylene Composites Made with Lignocellulosic Fibres Isolated from Waste Plant Biomass Such As Hemp. Compos. Part B Eng. 2014, 67, 138–144. [Google Scholar] [CrossRef]
- Das, O.; Bhattacharyya, D.; Hui, D.; Lau, K.T. Mechanical and Flammability Characterisations of Biochar/Polypropylene Biocomposites. Compos. Part B Eng. 2016, 106, 120–128. [Google Scholar] [CrossRef]
- Visakh, P.M.; Thomas, S. Preparation of Bionanomaterials and Their Polymer Nanocomposites from Waste and Biomas. Waste Biomass Valorization 2010, 1, 121–134. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Codou, A.; Misra, M.; Mohanty, A.K. A Critical Review on the Fabrication Processes and Performance of Polyamide Biocomposites from a Biofiller Perspective. Mater. Today Sustain. 2019, 5, 100014. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolisis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Melo, R.P.; Marques, M.F.V.; Navard, P.; Duque, N. Degradation Studies and Mechanical Properties of Treated Curauá Fibres and Microcrystalline Cellulose in Composites with Polyamide 6. J. Compos. Mater. 2017, 51, 3481–3489. [Google Scholar] [CrossRef]
- Varshney, V.K.; Naithani, S. Chemical functionalization of cellulose derived from nonconventional sources. In Cellulose Fibres: Bio- and Nano-Polymer Composites; Kalia, S., Kaith, B., Kaur, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 43–60. [Google Scholar]
- Mondal, S. Preparation, Properties and Applications of Nanocellulosic Materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef]
- Wu, C.S. Renewable Resource-Based Green Composites of Surface-Treated Spent Coffee Grounds and Polylactide: Characterization and Biodegradability. Polym. Degrad. Stab. 2015, 121, 51–59. [Google Scholar] [CrossRef]
- Habibi, Y.; El-Zawawy, W.K.; Ibrahim, M.M.; Dufresne, A. Processing and Characterization of Reinforced Polyethylene Composites Made with Lignocellulosic Fibres from Egyptian Agro-Industrial Residues. Compos. Sci. Technol. 2008, 68, 1877–1885. [Google Scholar] [CrossRef] [Green Version]
- Tisserat, B.; Reifschneider, L.; Núñez, J.C.L.; Hughes, S.R.; Selling, G.; Finkenstadt, V.L. Evaluation of the Mechanical and Thermal Properties of Coffee Tree Wood Flour—Polypropylene Composites. Bioresources 2014, 9, 4449–4467. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Szostak, M.; Barczewski, M.; Łuczak, P. Cork-Wood Hybryd Filler System for Polypropylene and Poly (Lactic Acid) Based Injection Molded Composites. Structure Evaluation and Mechanical Performance. Compos. Part B Eng. 2019, 163, 655–668. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Piszczyk, Ł.; Saeb, M.R.; Colom, X. Processing and Structure-Property Relationship of Natural Rubber/Wheat Bran Biocomposite. Cellulose 2016, 23, 3157–3175. [Google Scholar] [CrossRef]
- Avérous, L.; Le Digabel, F. Properties of Biocomposites Based on Lignocellulosic Fillers. Carbohydr. Polym. 2006, 66, 480–493. [Google Scholar] [CrossRef]
- Montanes, N.; Garcia-Sanoguera, D.; Segui, V.J.; Fenollar, O.; Boronat, T. Processing and Characterization of Environmentally Friendly Composites from Biobased Polyethylene and Natural Fillers from Thyme Herbs. J. Polym. Environ. 2018, 26, 1218–1230. [Google Scholar] [CrossRef]
- Chafidz, A.; Rizal, M.; RM, F.; Kaavessina, M.; Hartanto, D.; Al Zahrani, S.M. Processing and Properties of High Density Polyethylene/Date Palm Fiber Composites Prepared by A Laboratory Mixing Extruder. J. Mech. Eng. Sci. 2018, 12, 3771–3785. [Google Scholar] [CrossRef]
- Jawaid, M.; Khalil, H.P.S.A. Cellulosic/Synthetic Fiber Reinforced Polymer Hybrid Composites: A Review. Carbohydr. Polym. 2011, 86, 1–18. [Google Scholar] [CrossRef]
- Sarsari, N.A.; Pourmousa, S.; Tajdini, A. Physical and Mechanical Properties of Walnut Shell Flour-Filled Thermoplastic Starch Composites. Bioresources 2016, 11, 6968–6983. [Google Scholar]
- Zhang, Q.; Li, Y.; Cai, H.; Lin, X.; Yi, W.; Zhang, J. Properties Comparison of High Density Polyethylene Composites Filled with Three Kinds of Shell Fibres. Results Phys. 2019, 12, 1542–1546. [Google Scholar] [CrossRef]
- Tufan, M.; Ayrilmis, N. Potential Use of Halzenut Husk in Recycled High-Density Polyethylene Composites. Bioresources 2016, 11, 7476–7489. [Google Scholar] [CrossRef] [Green Version]
- Rahmat, W.; Sin, T.L.; Rahmat, A.R.; Isa, N.M.; Salleh, M.S.N.; Mokhtar, M. Comparison of Rice Husk-Filled Polyethylene Composite and Natural Wood Under Weathering Effects. J. Compos. Mater. 2010, 45, 1403–1410. [Google Scholar] [CrossRef]
- Majewski, Ł.; Gaspar Cunha, A. Evaluation of Suitability of Wheat Bran as A Natural Filler in Polymer Processing. Bioresources 2018, 13, 7037–7052. [Google Scholar]
- Šimkovic, I.; Kelnar, I.; Mendichi, R.; Bertok, T.; Filip, J. Composite Films Prepared from Agricultural By-Products. Carbohydr. Polym. 2017, 156, 77–85. [Google Scholar] [CrossRef]
- Matkó, S.; Toldy, A.; Keszei, S.; Anna, P.; Bertalan, G.; Marosi, G. Flame Retardancy of Biodegradable Polymers and Biocomposites. Polym. Degrad. Stab. 2005, 88, 138–145. [Google Scholar] [CrossRef]
- Gowman, A.C.; Picard, M.C.; Lim, L.T.; Misra, M.; Mohanty, A.K. Fruit Waste Valorization for Biodegradable Biocomposites Applications: A Review. Bioresources 2019, 14, 10047–10092. [Google Scholar]
- Yan, L.; Chouw, N.; Jayaraman, K. Flax Fibre and Its Composites—A Review. Compos. Part B Eng. 2014, 56, 296–317. [Google Scholar] [CrossRef]
- Lau, K.; Hung, P.; Zhu, M.H.; Hui, D. Properties of Natural Fibre Composites for Structural Engineering Applications. Compos. Part B Eng. 2018, 136, 222–233. [Google Scholar] [CrossRef]
- Saba, J.; Tahir, P.M.; Jawaid, M. A Review on Potentiality of Nano Filler/Natural Fiber Filled Polymer Hybrid Composites. Polymers 2014, 6, 2247–2273. [Google Scholar] [CrossRef]
- Mirmehdi, S.M.; Zeinaly, F.; Dabbagh, F. Date Palm Wood Flour As A Filler of Linear Low-Density Polyethylene. Compos. Part B Eng. 2014, 56, 137–141. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Monteiro, S.N.; Lopes, F.P.D.; Margem, F.M.; Santafe, H.P.G.; da Costa, L.L. Dimensional analysis and surface morphology as selective criteria of lignocellulosic fibres as reinforcement in polymeric matrices. In Cellulosic Fibres: Bio- and Nano-Polymer Composites; Kalia, S., Kaith, B., Kaur, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 215–240. [Google Scholar]
- Murali Mohan Rao, K.; Mohana Rao, K.; Ratna Prasad, A.V. Fabrication and Testing of Natural Fibre Composites: Vakka, Sisal, Bamboo and Banana. Mater. Des. 2010, 31, 508–513. [Google Scholar] [CrossRef]
- Jagadish, R.S.; Raj, B.; Asha, M.R. Blending of Low-Density Polyethylene with Vanillin for Improved Barrier and Aroma-Releasing Properties in Food Packaging. J. Appl. Polym. Sci. 2009, 113, 3732–3741. [Google Scholar] [CrossRef]
- Santi, C.R.; Hage, E.; Vlachopoulos, J.; Correa, C.A. Rheology and Processing of HDPE/Wood Flour Composites. Int. Polym. Process. 2009, 24, 346–353. [Google Scholar] [CrossRef]
- Yang, H.S. Thermal and Dynamic Mechanical Thermal Analysis of Lignocellulosic Material-Filled Polyethylene Biocomposites. J. Therm. Anal. Calorim. 2017, 130, 1345–1355. [Google Scholar] [CrossRef]
- Yang, H.S.; Kim, H.J.; Park, H.J.; Lee, B.J.; Hwang, T.S. Water Absorption Behaviour and Mechanical Properties of Lignocellulosic Filler-Polyolefin Biocomposites. Compos. Struct. 2006, 72, 429–437. [Google Scholar] [CrossRef]
- Balint, T.; Chang, B.P.; Mohanty, A.K.; Misra, M. Underutilized Agricultural Co-Product As A Sustainable Biofiller for Polyamide6, 6: Effect of Carbonization Temperature. Molecules 2020, 25, 1455. [Google Scholar] [CrossRef] [Green Version]
- Codou, A.; Guigo, N.; van Berkel, J.G.; de Jong, E.; Sbirrazzuoli, N. Preparation and Crystallization Behavior of Poly(Ethylene 2,5-Furandicarboxylate)/Cellulose Composites by Twin Screw Extrusion. Carbohydr. Polym. 2017, 174, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Kaseem, M.; Hamad, K.; Deri, F.; Ko, Y.G. Material Properties of Polyethylene/Wood Composites: A Review of Recent Works. Polym. Sci. Ser. A 2015, 57, 689–703. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Barczewski, M.; Szostak, M. Injection Moulding of Highly Filled Polypropylene-Based Biocomposites. Buckwheat Husk and Wood Flour Filler: A Comparison of Agricultural and Wood Industry Waste Utilization. Polymers 2019, 11, 1881. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.S.; Wolcott, M.P.; Kim, H.S.; Kim, H.J. Thermal Properties of Lignocellulosic Filler-Thermoplastic Polymer Biocomposites. J. Therm. Anal. Calorim. 2005, 82, 157–160. [Google Scholar] [CrossRef]
- Väisänen, T.; Haapala, A.; Lappalainen, R.; Tomppo, L. Utilization of Agricultural and Forest Industry Waste and Residues in Natural Fiber-Polymer Composites: A Review. Waste Manag. 2016, 54, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, Properties and Applications of Polysaccharide Nanocrystals in Advanced Functional Nanomaterials: A Review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef]
- Maneesh, T.; Singh, V.K.; Gope, P.C.; Chaudhary, A.K. Evaluation of Mechanical Properties of Bagasse-Glass Fibre Reinforced Composites. J. Mater. Environ. Sci. 2012, 3, 171–184. [Google Scholar]
- Abba, H.A.; Zahari, I.N.; Sapuan, S.M.; Leman, Z. Characterization of Millet (Pennisetum Glaucum) Husk Fiber (MHF) and Its Use As Filler for High Density Polyethylene (HDPE) Composites. Bioresources 2017, 12, 9287–9301. [Google Scholar]
- Arifuzzaman Khan, G.M.; Alam Shams, M.S.; Kabir, M.R.; Gafur, M.A.; Terano, M.; Alam, M.S. Influence of Chemical Treatment on the Properties of Banana Stem Fiber and Banana Stem Fiber/Coir Hybrid Fiber Reinforced Maleic Anhydride Grafted Polypropylene/Low-Density Polyethylene Composites. J.Appl. Polym. Sci. 2013, 128, 1020–1029. [Google Scholar] [CrossRef]
- Essabir, H.; Raji, M.; Laaziz, S.A.; Rodrique, D.; Bouhfid, R.; Qaiss, A. Thermo-Mechanical Performances of Polypropylene Biocomposites Based on Untreated, Treated and Compatybilized Spent Coffee Grounds. Compos. Part B Eng. 2018, 149, 1–11. [Google Scholar] [CrossRef]
- Arrakhiz, F.Z.; El Achaby, M.; Bouhfid, R.; Vaudreuil, S.; Essassi, M.; Qaiss, A. Mechanical and Thermal Properties of Polypropylene Reinforced with Alfa Fiber under Different Chemical Treatment. Mater. Des. 2012, 35, 318–322. [Google Scholar] [CrossRef]
- Kaewkuk, S.; Sutapun, W.; Jarukumjorn, K. Effects of Interfacial Modification and Fiber Content on Physical Properties of Sisal Fiber/Polypropylene Composites. Compos. Part B Eng. 2013, 45, 544–549. [Google Scholar] [CrossRef]
- Menezes, A.J.; Siqueira, G.; Curvelo, A.A.S.; Dufresne, A. Extrusion and Characterization of Functionalized Cellulose Whiskers Reinforced Polyethylene Nanocomposites. Polymer 2009, 50, 4552–4563. [Google Scholar] [CrossRef]
- Kuciel, S.; Jakubowska, P.; Kuźniar, P. A Study on the Mechanical Properties and the Influence of Water Uptake and Temperature on Biocomposites Based on Polyethylene from Renewable Sources. Compos. Part B Eng. 2014, 64, 72–77. [Google Scholar] [CrossRef]
- Sobczak, L.; Brüggermann, O.; Putz, R.F. Polyolefin Composites with Natural Fibres and Wood-Modification of the Fiber/Filler-Matrix Interactions. J. Appl. Polym. Sci. 2013, 127, 1–17. [Google Scholar] [CrossRef]
- Yu, T.; Sun, F.; Lu, M.; Li, Y. Water Absorption and Hygrothermal Aging Behavior of Short Ramie Fiber-Reinforced Poly (Lactic Acid) Composites. Polym. Compos. 2018, 39, 1098–1104. [Google Scholar] [CrossRef]
- Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Influence of the Design Solutions of Extruder Screw Mixing Tip on Selected Properties of Wheat Bran-Polyethylene Biocomposite. Polymers 2019, 11, 2120. [Google Scholar] [CrossRef] [Green Version]
- Yeh, S.K.; Gupta, R.K. Improved Wood-Plastic Composites Through Better Processing. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1694–1699. [Google Scholar] [CrossRef]
- Fernandes, E.M.; Correlo, V.M.; Mano, J.F.; Reis, R.L. Polypropylene-Based Cork-Polymer Composites: Processing Parameters and Properties. Compos. Part B Eng. 2014, 66, 210–223. [Google Scholar] [CrossRef]
- Tazi, M.; Erchiqui, F.; Godard, F.; Kaddami, H.; Ajji, A. Characterization of Rheological and Thermophysical Properties of HDPE-Wood Composite. J. Appl. Polym. Sci. 2014. [Google Scholar] [CrossRef]
- Li, H.; Law, S.; Sain, M. Process Rheology and Mechanical Property Correlationship of Wood Flour-Polypropylene Composites. J. Reinf. Plast. Compos. 2004, 23, 1153–1158. [Google Scholar] [CrossRef]
- Dittenber, D.B.; GangaRao, H.V.S. Critical Review of Recent Publications on Use of Natural Composites in Infrastructure. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1419–1429. [Google Scholar] [CrossRef]
- ISO 294-1:2017. Plastics—Injection Moulding of Test Specimens of Thermoplastic Materials—Part 1: General Principles, and Moulding of Multipurpose and Bar Test Specimens; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Dowlex 2631.10EU Polyethylene Resin—Technical Data Sheet. Available online: https://www.dow.com/en-us/document-viewer.html?ramdomVar=9174234036439704424&docPath=/content/dam/dcc/ocuments/en-us/productdatasheet/400/400-00089061en-dowlex-2631ue-tds.pdf (accessed on 14 July 2020).
- Stevenson, L.; Philips, F.; O’Sullivan, K.; Walton, J. Wheat Bran: Its Composition and Benefits to Health, A European Perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASTM E308. Standard Practice for Computing the Colour of Objects by Using the CIE System; ASTM: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ISO 2813:2001. Paints and Varnishes—Determination of Gloss Value at 20 Degrees, 60 Degrees and 85 Degrees; ISO: Geneva, Switzerland, 2001. [Google Scholar]
- ISO 294-4:2001. Plastics—Injection Moulding of Test Specimens of Thermoplastic Materials—Part 4: Determination of Moulding Shrinkage; ISO: Geneva, Switzerland, 2001. [Google Scholar]
- ISO 527-2:2012. Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- ISO 1133-1:2011. Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics—Part 1: Standard Method; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- ISO 17744:2004. Plastics—Determination of Specific Volume as A Function of Temperature and Pressure (pvT Diagram)—Piston Apparatus Method; ISO: Geneva, Switzerland, 2004. [Google Scholar]
- ISO 11357-1:2016. Plastics—Differential Scanning Calorimetry (DSC)—Part 1: General Principles; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Mathot, V.B.F. Calorimetry and Thermal Analysis of Polymers; Carl Hanser Verlag: Munich, Germany, 1994. [Google Scholar]
- Montanes, N.; Quiles-Carrillo, L.; Ferrandiz, S.; Fenollar, O.; Boronat, T. Effects of Lignocellulosic Fillers from Waste Thyme on Melt Flow Behavior and Processability of Wood Plastic Composites (WPC) with Biobased Poly (Ethylene) by Injection Moulding. J. Polym. Environ. 2019, 27, 747–756. [Google Scholar] [CrossRef]
- Orji, B.O.; McDonald, A.G. Evaluation of the Mechanical, Thermal and Rheological Properties of Recycled Polyolefins Rice-Hull Composites. Materials 2020, 13, 667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teuber, L.; Militz, H.; Krause, A. Processing of Wood Plastic Composites: The Influence of Feeding Method and Polymer Melt Flow Rate on Particle Degradation. J. Appl. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Pozo, C.; Rodríguez-Llamazares, S.; Bouza, R.; Barral, L.; Castaño, J.; Müller, N. Study of the Structural Order of Native Starch Granules Using Combined FTIR and XRD Analysis. J. Polym. Res. 2018, 25, 266. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables along the Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Piotrowicz, I.B.B.; Salas-Mellado, M.M. Protein Concentrates from Defatted Rice Bran: Preparation and Characterization. Food Sci. Technol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Poletto, M.; Zattera, A.J.; Santana, R.M.C. Structural Differences between Wood Species: Evidence from Chemical Composition, FTIR Spectroscopy, and Thermogravimetric Analysis. J. Appl. Polym. Sci. 2012, 126, E337–E344. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.J.; Sun, Z. Effects of Polyethylene-Grafted Maleic Anhydride (PE-g-MA) on Thermal Properties, Morphology, and Tensile Properties of Low Density Polyethylene (LDPE) and Corn Starch Blends. J. Appl. Polym. Sci. 2003, 88, 2904–2911. [Google Scholar] [CrossRef]
- Pabiot, J.; Verdu, J. The Change in Mechanical Behavior of Linear Polymers during Photochemical Aging. Polym. Eng. Sci. 1981, 21, 32–38. [Google Scholar] [CrossRef]
- Sikora, J.W.; Gajdoš, I.; Puszka, A. Polyethylene-Matrix Composites with Halloysite Nanotubes with Enhanced Physical/Thermal Properties. Polymers 2019, 11, 787. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, A.; Isaac, D.H. Weathering of lignocellulosic polymer composites. In Lignocellulosic Polymer Composites. Processing, Characterisation and Properties; Thakur, V.K., Ed.; John Willey & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Ashori, A. Wood-Plastic Composites as Promising Green-Composites for Automotive Industries. Bioresour. Technol. 2008, 99, 4661–4667. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Kloziński, A. Complex Modification Effect of linseed cake as an Agricultural Waste Filler Used in High Density Polyethylene Composites. Iran. Polym. J. 2018, 27, 677–688. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, S.Y.; Choi, P. Effects of Wood Fiber Content and Coupling Agent Content on Tensile Properties of Wood Fiber Polyethylene Composites. Holz Roh Werkst 2008, 66, 267–274. [Google Scholar] [CrossRef]










| Parameter | Unit | Value |
|---|---|---|
| Injection pressure | MPa | 100 |
| Temperature of heating zones in plasticising system | °C | I—105 II—130 III—140 |
| Injection nozzle temperature | °C | 150 |
| Mould temperature | °C | 40 |
| Injection cycle time | s | 30 |
| Cooling time in mould | s | 20 |
| Sample | Heating I | Cooling | Heating II | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tg (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) | Tg (°C) | Tc (°C) | Tm (°C) | ΔHm (J/g) | Xc (%) | |
| PE | −109.1 | 126.5 | 161.6 | 55.2 | −123.3 | 109.0 | 124.7 | 174.0 | 59.4 |
| PE <04 5% | −101.2 | 127.0 | 164.7 | 59.2 | −123.9 | 108.0 | 124.8 | 168.7 | 60.6 |
| PE <04 10% | −102.1 | 128.2 | 157.4 | 59.7 | −123.2 | 108.2 | 123.7 | 162.5 | 61.6 |
| PE <04 15% | −101.8 | 126.7 | 150.4 | 60.4 | −123.3 | 108.3 | 123.8 | 153.0 | 61.4 |
| PE 04–06 5% | −97.8 | 128.3 | 161.1 | 57.9 | −113.8 | 106.3 | 125.3 | 168.4 | 60.5 |
| PE 04–06 10% | −100.5 | 127.8 | 150.8 | 57.2 | −112.5 | 106.3 | 124.7 | 155.8 | 59.1 |
| PE 04–06 15% | −100.9 | 128.6 | 146.8 | 59.0 | −111.1 | 106.9 | 124.1 | 150.9 | 60.6 |
| PE 06–08 5% | −99.0 | 128.3 | 161.6 | 58.1 | −115.0 | 106.7 | 126.4 | 172.6 | 62.0 |
| PE 06–08 10% | −97.8 | 126.9 | 164.6 | 62.4 | −111.2 | 107.2 | 124.9 | 167.1 | 63.4 |
| PE 06–08 15% | −101.0 | 128.3 | 144.7 | 58.1 | −112.0 | 107.9 | 124.7 | 153.6 | 61.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasimowski, E.; Majewski, Ł.; Grochowicz, M. Analysis of Selected Properties of Biocomposites Based on Polyethylene with a Natural Origin Filler. Materials 2020, 13, 4182. https://doi.org/10.3390/ma13184182
Sasimowski E, Majewski Ł, Grochowicz M. Analysis of Selected Properties of Biocomposites Based on Polyethylene with a Natural Origin Filler. Materials. 2020; 13(18):4182. https://doi.org/10.3390/ma13184182
Chicago/Turabian StyleSasimowski, Emil, Łukasz Majewski, and Marta Grochowicz. 2020. "Analysis of Selected Properties of Biocomposites Based on Polyethylene with a Natural Origin Filler" Materials 13, no. 18: 4182. https://doi.org/10.3390/ma13184182





