Electrochemical Activation of Li2MnO3 Electrodes at 0 °C and Its Impact on the Subsequent Performance at Higher Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Li2MnO3
2.2. Preparation of Electrodes and Electrochemical Cells
2.3. Electrochemical Measurements
2.4. Structural and Surface Studies
2.5. Online Electrochemical Mass Spectrometry
3. Results and Discussion
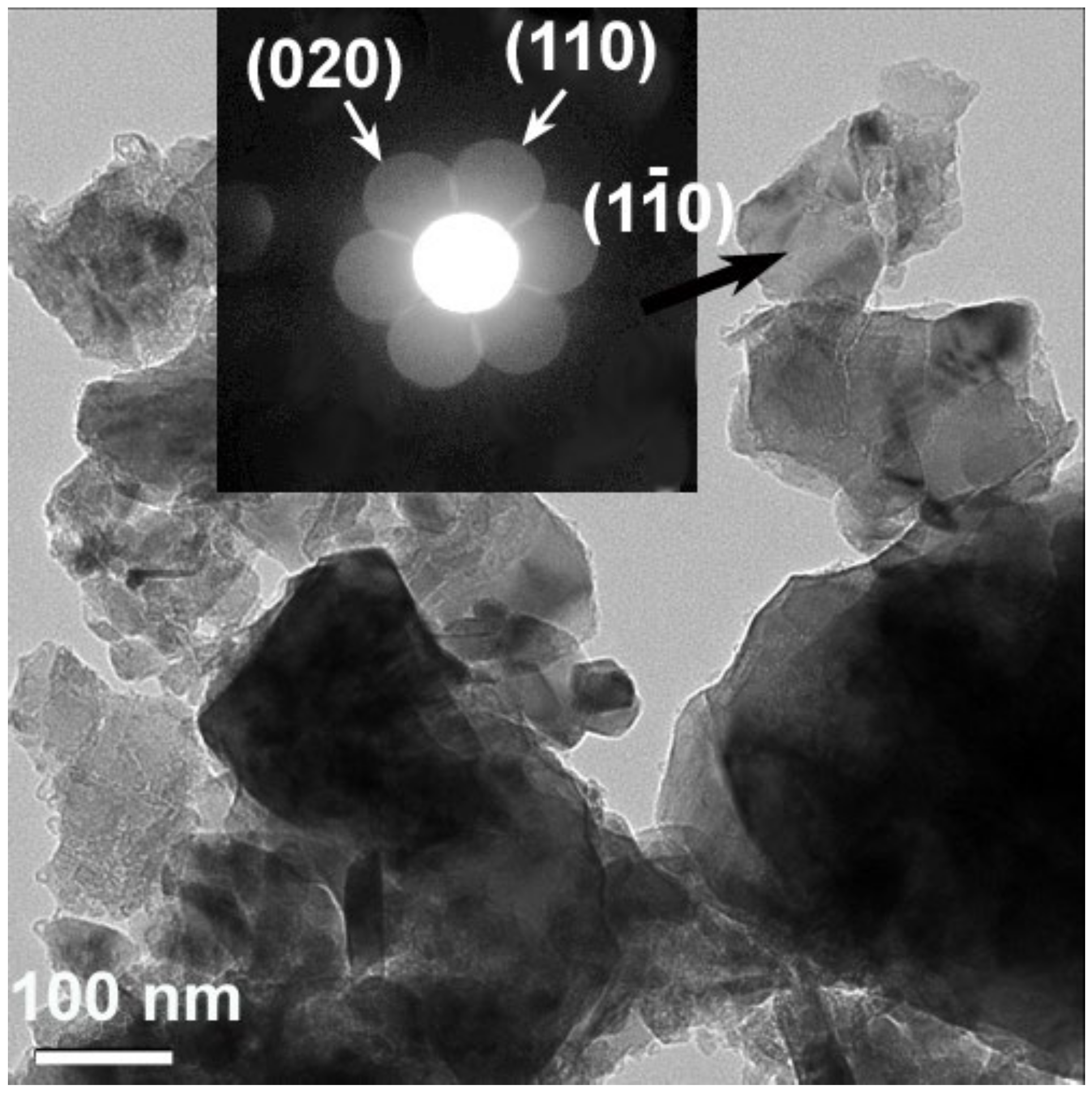
3.1. Structural and Morphological Characteristics of Li2MnO3
3.2. Electrochemical Behavior and Structural Evolution of Li2MnO3 Electrodes at 0 °C, 30 °C, and 45 °C. Impact of their Initial Activation Cycling at 0 °C
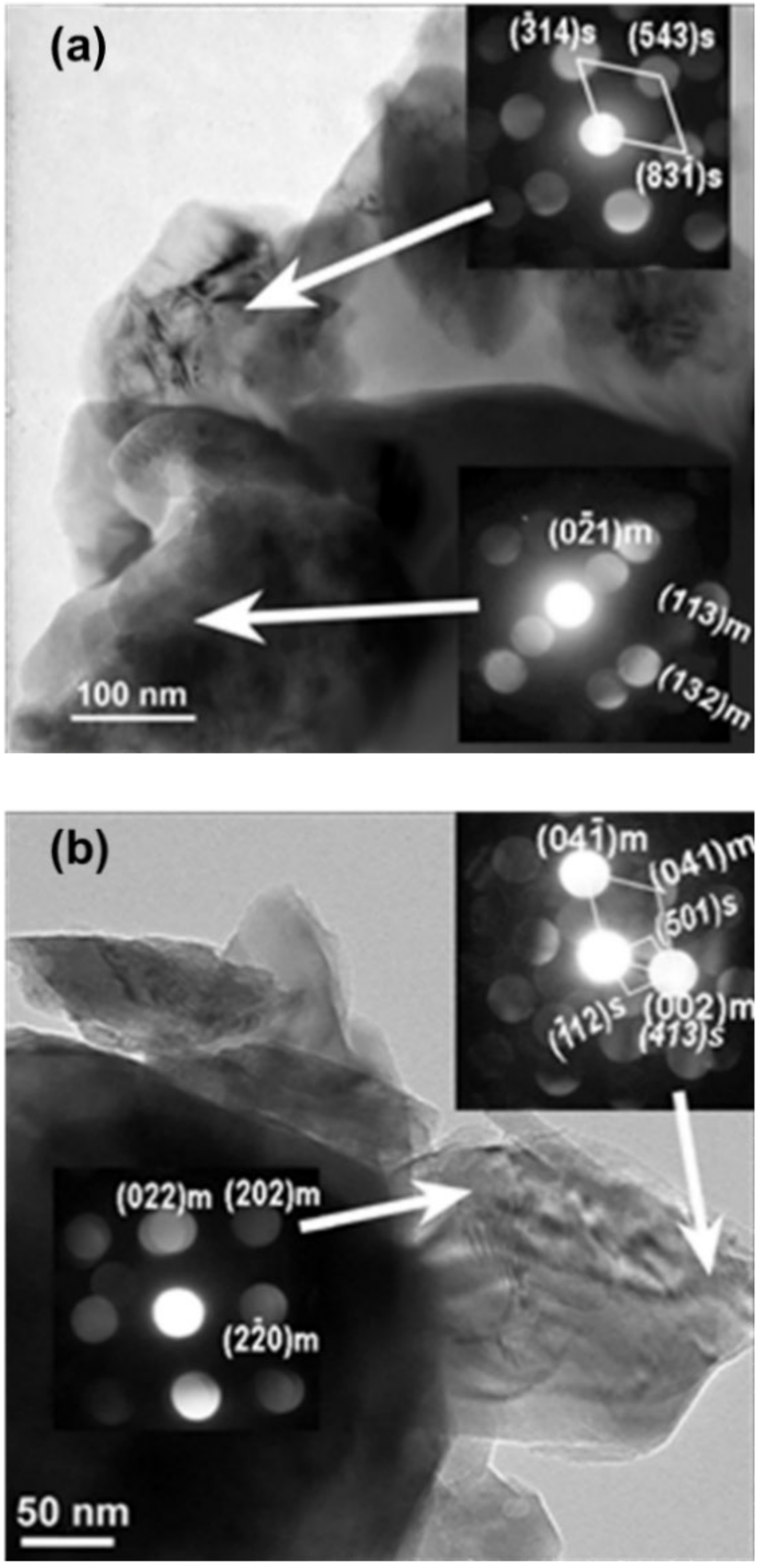
3.3. Structural Aspects of the Initial Cycling of Li2MnO3 Electrodes at 0 °C and 30 °C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thackeray, M.M.; Johnson, C.S.; Vaughey, J.T.; Li, N.; Hackney, S.A. Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J. Mater. Chem. 2005, 15, 2257–2267. [Google Scholar] [CrossRef]
- Nayak, P.K.; Erickson, E.M.; Schipper, F.; Penki, T.R.; Munichandraiah, N.; Adelhelm, P.; Sclar, H.; Amalraj, F.; Markovsky, B.; Aurbach, D. Review on challenges and recent advances in the electrochemical performance of high capacity Li and Mn-rich cathode materials for Li-Ion batteries. Adv. Energy Mater. 2018, 8, 1702397. [Google Scholar] [CrossRef]
- Amalraj, F.; Talianker, M.; Markovsky, B.; Sharon, D.; Burlaka, L.; Shafir, G.; Zinigrad, E.; Haik, O.; Aurbach, D.; Lampert, J.; et al. Study of the lithium-rich integrated compound xLi2MnO3·(1-x)LiMO2 (x around 0.5; M = Mn, Ni, Co; 2:2:1) and its electrochemical activity as positive electrode in lithium cells. J. Electrochem. Soc. 2013, 160, A324–A337. [Google Scholar] [CrossRef]
- Erickson, E.M.; Schipper, F.; Penki, T.R.; Shin, J.-Y.; Erk, C.; Chesneau, F.-F.; Markovsky, B.; Aurbach, D. Review—Recent advances and remaining challenges for lithium ion battery cathodes. J. Electrochem. Soc. 2017, 164, A6341–A6348. [Google Scholar] [CrossRef]
- Zheng, J.; Myeong, S.; Cho, W.; Yan, P.; Xiao, J.; Wang, C.; Cho, J.; Zhang, J. Li- and Mn-rich cathode materials: Challenges to commercialization. Adv. Energy Mater. 2017, 7, 1601284. [Google Scholar] [CrossRef]
- Kleiner, K.; Strehle, B.; Baker, A.R.; Day, S.J.; Tang, C.C.; Buchberger, I.; Chesneau, F.-F.; Gasteiger, H.A.; Piana, M. Origin of high capacity and poor cycling stability of Li-rich layered oxides: A long-duration in Situ synchrotron powder diffraction study. Chem. Mater. 2018, 30, 3656–3667. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2 (NMC) cathode materials for Li-Ion batteries. J. Electrochem. Soc. 2017, 164, A1361–A1377. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Z.; Dinh, K.N.; Chen, N.; Chen, G.; Du, F.; Yan, Q. Layered oxide cathode for potassium-Ion battery: Recent progress and prospective. Small 2020, 2002700. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, Y.; Chen, L.; Hu, Y. Flexible Na batteries. InfoMat 2020, 2, 126–138. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Recent progress in rechargeable potassium batteries. Adv. Funct. Mater. 2018, 28, 1802938. [Google Scholar] [CrossRef]
- Yan, G.; Mariyappan, S.; Rousse, G.; Jacquet, Q.; Deschamps, M.; David, R.; Mirvaux, B.; Freeland, J.W.; Tarascon, J.-M. Higher energy and safer sodium ion batteries via an electrochemically made disordered Na3V2(PO4)2F3 material. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Tanibata, N.; Kondo, Y.; Yamada, S.; Maeda, M.; Takeda, H.; Nakayama, M.; Asaka, T.; Kitajou, A.; Okada, S. Nanotube-structured Na 2 V 3 O 7 as a cathode material for sodium-ion batteries with high-rate and stable cycle performances. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, D.; Lian, R.; Kan, D.; Mamoor, M.; Wang, C.; Chen, G.; Gao, C.; Wei, Y. Electronic properties, phase transformation and anionic redox of monoclinic Na2MnO3 cathode material for sodium-ion batteries: First-principle calculations. ChemElectroChem 2019, 6, 3987–3993. [Google Scholar] [CrossRef]
- Hosaka, T.; Kubota, K.; Hameed, A.S.; Komaba, S. Research development on K-Ion batteries. Chem. Rev. 2020, 120, 6358–6466. [Google Scholar] [CrossRef] [PubMed]
- Croy, J.R.; Gallagher, K.G.; Balasubramanian, M.; Long, B.R.; Thackeray, M.M. Quantifying hysteresis and voltage fade in xLi2MnO3●(1-x)LiMn0.5Ni0.5O2 electrodes as a function of Li2MnO3 content. J. Electrochem. Soc. 2014, 161, A318–A325. [Google Scholar] [CrossRef]
- Sathiya, M.; Abakumov, A.M.; Foix, D.; Rousse, G.; Ramesha, K.; Saubanère, M.; Doublet, M.L.; Vezin, H.; Laisa, C.P.; Prakash, A.S.; et al. Origin of voltage decay in high-capacity layered oxide electrodes. Nat. Mater. 2015, 14, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Lyu, Y.; Xin, H.L.; Liu, J.; Han, L.; Bak, S.-M.; Bai, J.; Yu, X.; Li, H.; Yang, X.-Q. Explore the effects of microstructural defects on voltage fade of Li- and Mn-rich cathodes. Nano Lett. 2016, 16, 5999–6007. [Google Scholar] [CrossRef] [PubMed]
- Croy, J.R.; Kim, D.; Balasubramanian, M.; Gallagher, K.; Kang, S.-H.; Thackeray, M.M. Countering the voltage decay in high capacity xLi2MnO3•(1–x)LiMO2 electrodes (M = Mn, Ni, Co) for Li+-Ion batteries. J. Electrochem. Soc. 2012, 159, A781–A790. [Google Scholar] [CrossRef]
- Yu, X.; Lyu, Y.; Gu, L.; Wu, H.; Bak, S.-M.; Zhou, Y.; Amine, K.; Ehrlich, S.N.; Li, H.; Nam, K.-W.; et al. Understanding the rate capability of high-energy-density Li-rich layered Li1.2Ni0.15Co0.1Mn0.55O2 cathode materials. Adv. Energy Mater. 2014, 4, 1300950. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.-C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef]
- Gent, W.E.; Lim, K.; Liang, Y.; Li, Q.; Barnes, T.; Ahn, S.-J.; Stone, K.H.; McIntire, M.; Hong, J.; Song, J.H.; et al. Coupling between oxygen redox and cation migration explains unusual electrochemistry in lithium-rich layered oxides. Nat. Commun. 2017, 8, 2091. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Yu, X.; Lin, R.; Bi, X.; Lu, J.; Bak, S.; Nam, K.-W.; Xin, H.L.; Jaye, C.; Fischer, D.A.; et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 2018, 3, 690–698. [Google Scholar] [CrossRef]
- Hy, S.; Felix, F.; Rick, J.; Su, W.-N.; Hwang, B.J. Direct In situ observation of Li2O evolution on Li-rich high-capacity cathode material, Li[NixLi(1–2x )/3Mn(2– x)/3]O2 (0 ≤ x ≤0.5). J. Am. Chem. Soc. 2014, 136, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huo, H.; Jian, J.; Wang, L.; Zhu, H.; Xu, S.; He, X.; Yin, G.; Du, C.; Sun, X. Radially oriented single-crystal primary nanosheets enable ultrahigh rate and cycling properties of LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries. Adv. Energy Mater. 2019, 9, 1–9. [Google Scholar]
- Singer, A.; Zhang, M.; Hy, S.; Cela, D.; Fang, C.; Wynn, T.A.; Qiu, B.; Xia, Y.; Liu, Z.; Ulvestad, A.; et al. Nucleation of dislocations and their dynamics in layered oxide cathode materials during battery charging. Nat. Energy 2018, 3, 641–647. [Google Scholar] [CrossRef]
- Zhang, X.; Belharouak, I.; Li, L.; Lei, Y.; Elam, J.W.; Nie, A.; Chen, X.; Yassar, R.S.; Axelbaum, R.L. Structural and electrochemical study of Al2O3 and TiO2 coated Li1.2 Ni0.13Mn0.54Co0.13O2 cathode material using ALD. Adv. Energy Mater. 2013, 3, 1299–1307. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, Z.; Wang, P.; Tang, L.; An, C.; He, Z. Multiple linkage modification of lithium-rich layered oxide Li1.2Mn0.54Ni0.13Co0.13O2 for lithium ion battery. ACS Appl. Mater. Interfaces 2018, 10, 31324–31329. [Google Scholar] [CrossRef]
- Amalraj, F.; Talianker, M.; Markovsky, B.; Burlaka, L.; Leifer, N.; Goobes, G.; Erickson, E.M.; Haik, O.; Grinblat, J.; Zinigrad, E.; et al. Studies of Li and Mn-Rich Lix[MnNiCo]O2 electrodes: Electrochemical performance, structure and the effect of the aluminum fluoride coating. J. Electrochem. Soc. 2013, 160, A2220–A2233. [Google Scholar] [CrossRef]
- Nayak, P.K.; Grinblat, J.; Levi, M.; Levi, E.; Kim, S.; Choi, J.W.; Aurbach, D. Al Doping for mitigating the capacity fading and voltage decay of layered Li and Mn-rich cathodes for Li-Ion batteries. Adv. Energy Mater. 2016, 6, 1502398. [Google Scholar] [CrossRef]
- Chen, G.; An, J.; Meng, Y.; Yuan, C.; Matthews, B.; Dou, F.; Shi, L.; Zhou, Y.; Song, P.; Wu, G.; et al. Cation and anion Co-doping synergy to improve structural stability of Li- and Mn-rich layered cathode materials for lithium-ion batteries. Nano Energy 2019, 57, 157–165. [Google Scholar] [CrossRef]
- Nayak, P.K.; Grinblat, J.; Levi, M.; Haik, O.; Levi, E.; Aurbach, D. Effect of Fe in suppressing the discharge voltage decay of high capacity Li-rich cathodes for Li-ion batteries. J. Solid State Electrochem. 2015, 19, 2781–2792. [Google Scholar] [CrossRef]
- Dong, X.; Xu, Y.; Xiong, L.; Sun, X.; Zhang, Z. Sodium substitution for partial lithium to significantly enhance the cycling stability of Li2MnO3 cathode material. J. Power Sources 2013, 243, 78–87. [Google Scholar] [CrossRef]
- Dong, X.; Xu, Y.; Yan, S.; Mao, S.; Xiong, L.; Sun, X. Towards low-cost, high energy density Li2MnO3 cathode materials. J. Mater. Chem. A 2015, 3, 670–679. [Google Scholar] [CrossRef]
- Breddemann, U.; Erickson, E.M.; Davis, V.; Schipper, F.; Ellwanger, M.; Daub, M.; Hoffmann, A.; Erk, C.; Markovsky, B.; Aurbach, D.; et al. Fluorination of Li-rich lithium ion battery cathode materials by fluorine gas: Chemistry, characterization and electrochemical performance in half cells. ChemElectroChem 2019, 3, 3337–3349. [Google Scholar] [CrossRef]
- Erickson, E.M.; Sclar, H.; Schipper, F.; Liu, J.; Tian, R.; Ghanty, C.; Burstein, L.; Leifer, N.; Grinblat, J.; Talianker, M.; et al. High-temperature treatment of Li-rich cathode materials with ammonia: Improved capacity and mean voltage stability during cycling. Adv. Energy Mater. 2017, 7, 1700708. [Google Scholar] [CrossRef]
- Shizuka, K.; Kiyohara, C.; Shima, K.; Takeda, Y. Effect of CO2 on layered Li1+zNi1−x−yCoxMyO2 (M=Al, Mn) cathode materials for lithium ion batteries. J. Power Sources 2007, 166, 233–238. [Google Scholar] [CrossRef]
- Sclar, H.; Sicklinger, J.; Erickson, E.M.; Maiti, S.; Grinblat, J.; Talianker, M.; Amalraj, S.F.; Burstein, L.; Beyer, H.; Hartmann, L.; et al. Enhancement of electrochemical performance of lithium and manganese-rich cathode materials via thermal treatment with SO2. J. Electrochem. Soc. 2020, 167, 110563. [Google Scholar] [CrossRef]
- Johnson, C.S.; Kim, J.-S.; Lefief, C.; Li, N.; Vaughey, J.T.; Thackeray, M.M. The significance of the Li2MnO3 component in ‘composite’ x Li2MnO3·(1−x)LiMn0.5Ni0.5O2 electrodes. Electrochem. Commun. 2004, 6, 1085–1091. [Google Scholar] [CrossRef]
- Rossouw, M.; Thackeray, M. Lithium manganese oxides from Li2MnO3 for rechargeable lithium battery applications. Mater. Res. Bull. 1991, 26, 463–473. [Google Scholar] [CrossRef]
- Maiti, S.; Sclar, H.; Rosy; Grinblat, J.; Talianker, M.; Burstein, L.; Noked, M.; Markovsky, B.; Aurbach, D. Modification of Li- and Mn-rich cathode materials via formation of the rock-salt and spinel surface layers for steady and high-rate electrochemical performances. ACS Appl. Mater. Interfaces 2020, 12, 32698–32711. [Google Scholar] [CrossRef]
- Erickson, E.M.; Schipper, F.; Tian, R.; Shin, J.-Y.; Erk, C.; Chesneau, F.F.; Lampert, J.K.; Markovsky, B.; Aurbach, D. Enhanced capacity and lower mean charge voltage of Li-rich cathodes for lithium ion batteries resulting from low-temperature electrochemical activation. RSC Adv. 2017, 7, 7116–7121. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.-H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112–3125. [Google Scholar] [CrossRef]
- Shimoda, K.; Yazawa, K.; Matsunaga, T.; Murakami, M.; Yamanaka, K.; Ohta, T.; Matsubara, E.; Ogumi, Z.; Abe, T. Sequential delithiation behavior and structural rearrangement of a nanoscale composite-structured Li1.2Ni0.2Mn0.6O2 during charge–discharge cycles. Sci. Rep. 2020, 10, 10048. [Google Scholar] [CrossRef] [PubMed]
- Croy, J.R.; Gallagher, K.G.; Balasubramanian, M.; Chen, Z.; Ren, Y.; Kim, D.; Kang, S.-H.; Dees, D.W.; Thackeray, M.M. Examining hysteresis in composite xLi2MnO3·(1– x )LiMO2 cathode structures. J. Phys. Chem. C 2013, 117, 6525–6536. [Google Scholar] [CrossRef]
- Assat, G.; Iadecola, A.; Foix, D.; Dedryvère, R.; Tarascon, J.-M. Direct quantification of anionic redox over long cycling of Li-rich NMC via hard X-ray photoemission spectroscopy. ACS Energy Lett. 2018, 3, 2721–2728. [Google Scholar] [CrossRef]
- Lu, Z.; Dahn, J.R. Understanding the anomalous capacity of Li/Li[NixLi(1/3−2x/3)]Mn (2/3−x/3)]O2 cells using in situ X-Ray diffraction and electrochemical studies. J. Electrochem. Soc. 2002, 149, A815. [Google Scholar] [CrossRef]
- Yu, D.Y.W.; Yanagida, K.; Kato, Y.; Nakamura, H. Electrochemical Activities in Li2MnO3. J. Electrochem. Soc. 2009, 156, A417. [Google Scholar] [CrossRef]
- Phillips, P.J.; Bareño, J.; Li, Y.; Abraham, D.P.; Klie, R.F. On the localized nature of the structural transformations of Li2MnO3 following electrochemical cycling. Adv. Energy Mater. 2015, 5, 1501252. [Google Scholar] [CrossRef]
- Francis, A.S.; Markovsky, B.; Sharon, D.; Talianker, M.; Zinigrad, E.; Persky, R.; Haik, O.; Grinblat, J.; Lampert, J.; Schulz-Dobrick, M.; et al. Study of the electrochemical behavior of the “inactive” Li2MnO3. Electrochim. Acta 2012, 78, 32–39. [Google Scholar] [CrossRef]
- Yu, H.; Ishikawa, R.; So, Y.-G.; Shibata, N.; Kudo, T.; Zhou, H.; Ikuhara, Y. Direct atomic-resolution observation of two phases in the Li1.2Mn0.567Ni0.166Co0.067O2 cathode material for lithium-ion batteries. Angew. Chemie Int. Ed. 2013, 52, 5969–5973. [Google Scholar] [CrossRef]
- Xu, B.; Fell, C.R.; Chi, M.; Meng, Y.S. Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: A joint experimental and theoretical study. Energy Environ. Sci. 2011, 4, 2223–2233. [Google Scholar] [CrossRef]
- Rana, J.; Stan, M.; Kloepsch, R.; Li, J.; Schumacher, G.; Welter, E.; Zizak, I.; Banhart, J.; Winter, M. Structural changes in Li2MnO3 cathode material for Li-Ion batteries. Adv. Energy Mater. 2014, 4, 1300998. [Google Scholar] [CrossRef]
- Chen, H.; Islam, M.S. Lithium extraction mechanism in Li-rich Li2MnO3 involving oxygen hole formation and dimerization. Chem. Mater. 2016, 28, 6656–6663. [Google Scholar] [CrossRef]
- Seo, D.H.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 2016, 8, 692–697. [Google Scholar] [CrossRef]
- Cao, T.; Shi, C.; Zhao, N.; He, C.; Li, J.; Liu, E. Understanding the electrochemical properties of Li-rich cathode materials from first-principles calculations. J. Phys. Chem. C 2015, 119, 28749–28756. [Google Scholar] [CrossRef]
- Lee, E.; Persson, K.A. Structural and chemical evolution of the layered Li-excess LixMnO3 as a function of Li content from first-principles calculations. Adv. Energy Mater. 2014, 4, 1400498. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, S.; Wang, B.; Yu, H. Local redox reaction of high valence manganese in Li2MnO3-based lithium battery cathodes. Cell Rep. Phys. Sci. 2020, 1, 100061. [Google Scholar] [CrossRef]
- Amalraj, S.F.; Sharon, D.; Talianker, M.; Julien, C.M.; Burlaka, L.; Lavi, R.; Zhecheva, E.; Markovsky, B.; Zinigrad, E.; Kovacheva, D.; et al. Study of the nanosized Li2MnO3: Electrochemical behavior, structure, magnetic properties, and vibrational modes. Electrochim. Acta 2013, 97, 259–270. [Google Scholar] [CrossRef]
- Sekar, M.M.A.; Patil, K.C. Combustion synthesis and properties of fine-particle dielectric oxide materials. J. Mater. Chem. 1992, 2, 739–743. [Google Scholar] [CrossRef]
- Hershkovitz, S.; Baltianski, S.; Tsur, Y. Electrochemical impedance analysis of SOFC cathode reaction using evolutionary programming. Fuel Cells 2012, 12, 77–85. [Google Scholar] [CrossRef]
- Oz, A.; Gelman, D.; Goren, E.; Shomrat, N.; Baltianski, S.; Tsur, Y. A novel approach for supercapacitors degradation characterization. J. Power Sources 2017, 355, 74–82. [Google Scholar] [CrossRef]
- Kalimuthu, V.S.; Attias, R.; Tsur, Y. Electrochemical impedance spectra of RuO2 during oxygen evolution reaction studied by the distribution function of relaxation times. Electrochem. Commun. 2020, 110, 106641. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Gabrisch, H.; Yi, T.; Yazami, R. Transmission electron microscope studies of LiNi1/3 Mn1/3Co1/3O2 before and after long-term aging at 70 °C. Electrochem. Solid-State Lett. 2008, 11, A119. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M. Lattice vibrations of materials for lithium rechargeable batteries III. Lithium manganese oxides. Mater. Sci. Eng. B 2003, 100, 69–78. [Google Scholar] [CrossRef]
- Strobel, P.; Lambert-Andron, B. Crystallographic and magnetic structure of Li2MnO3. J. Solid State Chem. 1988, 75, 90–98. [Google Scholar] [CrossRef]
- Ye, D.; Sun, C.; Chen, Y.; Ozawa, K.; Hulicova-Jurcakova, D.; Zou, J. Ni-induced stepwise capacity increase in Ni-poor Li-rich cathode materials for high performance lithium ion. Nano Res. 2015, 8, 808–820. [Google Scholar] [CrossRef]
- Teufl, T.; Strehle, B.; Müller, P.; Gasteiger, H.A.; Mendez, M.A. Oxygen release and surface degradation of Li- and Mn-rich layered oxides in variation of the Li2MnO3 content. J. Electrochem. Soc. 2018, 165, A2718–A2731. [Google Scholar] [CrossRef]
- Strehle, B.; Kleiner, K.; Jung, R.; Chesneau, F.; Mendez, M.; Gasteiger, H.A.; Piana, M. The role of oxygen release from Li- and Mn-rich layered oxides during the first cycles investigated by on-line electrochemical mass spectrometry. J. Electrochem. Soc. 2017, 164, A400–A406. [Google Scholar] [CrossRef]
- Muhammad, S.; Kim, H.; Kim, Y.; Kim, D.; Song, J.H.; Yoon, J.; Park, J.-H.; Ahn, S.-J.; Kang, S.-H.; Thackeray, M.M.; et al. Evidence of reversible oxygen participation in anomalously high capacity Li- and Mn-rich cathodes for Li-ion batteries. Nano Energy 2016, 21, 172–184. [Google Scholar] [CrossRef]
- Saubanère, M.; McCalla, E.; Tarascon, J.-M.; Doublet, M.-L. The intriguing question of anionic redox in high-energy density cathodes for Li-ion batteries. Energy Environ. Sci. 2016, 9, 984–991. [Google Scholar] [CrossRef]
- Rozier, P.; Tarascon, J.M. Review—Li-rich layered oxide cathodes for next-generation Li-Ion batteries: Chances and challenges. J. Electrochem. Soc. 2015, 162, A2490–A2499. [Google Scholar] [CrossRef]
- Chen, Q.; Pei, Y.; Chen, H.; Song, Y.; Zhen, L.; Xu, C.-Y.; Xiao, P.; Henkelman, G. Highly reversible oxygen redox in layered compounds enabled by surface polyanions. Nat. Commun. 2020, 11, 3411. [Google Scholar] [CrossRef] [PubMed]
- Ohzuku, T.; Nagayama, M.; Tsuji, K.; Ariyoshi, K. High-capacity lithium insertion materials of lithium nickel manganese oxides for advanced lithium-ion batteries: Toward rechargeable capacity more than 300 mAhg−1. J. Mater. Chem. 2011, 21, 10179–10188. [Google Scholar] [CrossRef]
- Watanabe, A.; Matsumoto, F.; Fukunishi, M.; Kobayashi, G.; Ito, A.; Hatano, M.; Ohsawa, Y.; Sato, Y. Relationship between electrochemical pre-treatment and cycle performance of a Li-rich solid-solution layered Li1−α[Ni0.18Li0.20+αCo0.03Mn0.58]O2 cathode for Li-Ion secondary batteries. Electrochemistry 2012, 80, 561–565. [Google Scholar] [CrossRef]
- Ito, A.; Li, D.; Ohsawa, Y.; Sato, Y. A new approach to improve the high-voltage cyclic performance of Li-rich layered cathode material by electrochemical pre-treatment. J. Power Sources 2008, 183, 344–346. [Google Scholar] [CrossRef]
- Ito, A.; Li, D.; Sato, Y.; Arao, M.; Watanabe, M.; Hatano, M.; Horie, H.; Ohsawa, Y. Cyclic deterioration and its improvement for Li-rich layered cathode material Li[Ni0.17Li0.2Co0.07Mn0.56]O2. J. Power Sources 2010, 195, 567–573. [Google Scholar] [CrossRef]
- Van Bommel, A.; Krause, L.J.; Dahn, J.R. Investigation of the irreversible capacity loss in the lithium-rich oxide Li[Li1/5Ni1/5Mn3/5]O2. J. Electrochem. Soc. 2011, 158, A731. [Google Scholar] [CrossRef]
- Wills, A.S.; Raju, N.P.; Greedan, J.E. Low-temperature structure and magnetic properties of the spinel LiMn2O4: A frustrated antiferromagnet and cathode material. Chem. Mater. 1999, 11, 1510–1518. [Google Scholar] [CrossRef]
- Nakashima, S.; Nakatake, Y.; Harima, H.; Katsuno, M.; Ohtani, N. Detection of stacking faults in 6H-SiC by Raman scattering. Appl. Phys. Lett. 2000, 77, 3612–3614. [Google Scholar] [CrossRef]
- Leifer, N.; Schipper, F.; Erickson, E.M.; Ghanty, C.; Talianker, M.; Grinblat, J.; Julien, C.M.; Markovsky, B.; Aurbach, D. Studies of spinel-to-layered structural transformations in LiMn2O4 electrodes charged to high voltages. J. Phys. Chem. C 2017, 121, 9120–9130. [Google Scholar] [CrossRef]
- Amalraj, S.F.; Burlaka, L.; Julien, C.M.; Mauger, A.; Kovacheva, D.; Talianker, M.; Markovsky, B.; Aurbach, D. Phase Transitions in Li2MnO3 electrodes at various states-of-charge. Electrochim. Acta 2014, 123, 395–404. [Google Scholar] [CrossRef]
- Marusczyk, A.; Albina, J.-M.; Hammerschmidt, T.; Drautz, R.; Eckl, T.; Henkelman, G. Oxygen activity and peroxide formation as charge compensation mechanisms in Li2MnO3. J. Mater. Chem. A 2017, 5, 15183–15190. [Google Scholar] [CrossRef]
- Kantcheva, M.; Kucukkal, M.; Suzer, S. XPS and IR characterization of manganese ions deposited on alumina. J. Mol. Struct. 1999, 482–483, 19–22. [Google Scholar] [CrossRef][Green Version]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susai, F.A.; Talianker, M.; Liu, J.; Rosy; Paul, T.; Grinblat, Y.; Erickson, E.; Noked, M.; Burstein, L.; Frenkel, A.I.; et al. Electrochemical Activation of Li2MnO3 Electrodes at 0 °C and Its Impact on the Subsequent Performance at Higher Temperatures. Materials 2020, 13, 4388. https://doi.org/10.3390/ma13194388
Susai FA, Talianker M, Liu J, Rosy, Paul T, Grinblat Y, Erickson E, Noked M, Burstein L, Frenkel AI, et al. Electrochemical Activation of Li2MnO3 Electrodes at 0 °C and Its Impact on the Subsequent Performance at Higher Temperatures. Materials. 2020; 13(19):4388. https://doi.org/10.3390/ma13194388
Chicago/Turabian StyleSusai, Francis Amalraj, Michael Talianker, Jing Liu, Rosy, Tanmoy Paul, Yehudit Grinblat, Evan Erickson, Malachi Noked, Larisa Burstein, Anatoly I. Frenkel, and et al. 2020. "Electrochemical Activation of Li2MnO3 Electrodes at 0 °C and Its Impact on the Subsequent Performance at Higher Temperatures" Materials 13, no. 19: 4388. https://doi.org/10.3390/ma13194388
APA StyleSusai, F. A., Talianker, M., Liu, J., Rosy, Paul, T., Grinblat, Y., Erickson, E., Noked, M., Burstein, L., Frenkel, A. I., Tsur, Y., Markovsky, B., & Aurbach, D. (2020). Electrochemical Activation of Li2MnO3 Electrodes at 0 °C and Its Impact on the Subsequent Performance at Higher Temperatures. Materials, 13(19), 4388. https://doi.org/10.3390/ma13194388





