Hydrogen Plasma Treatment of Aligned Multi-Walled Carbon Nanotube Arrays for Improvement of Field Emission Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
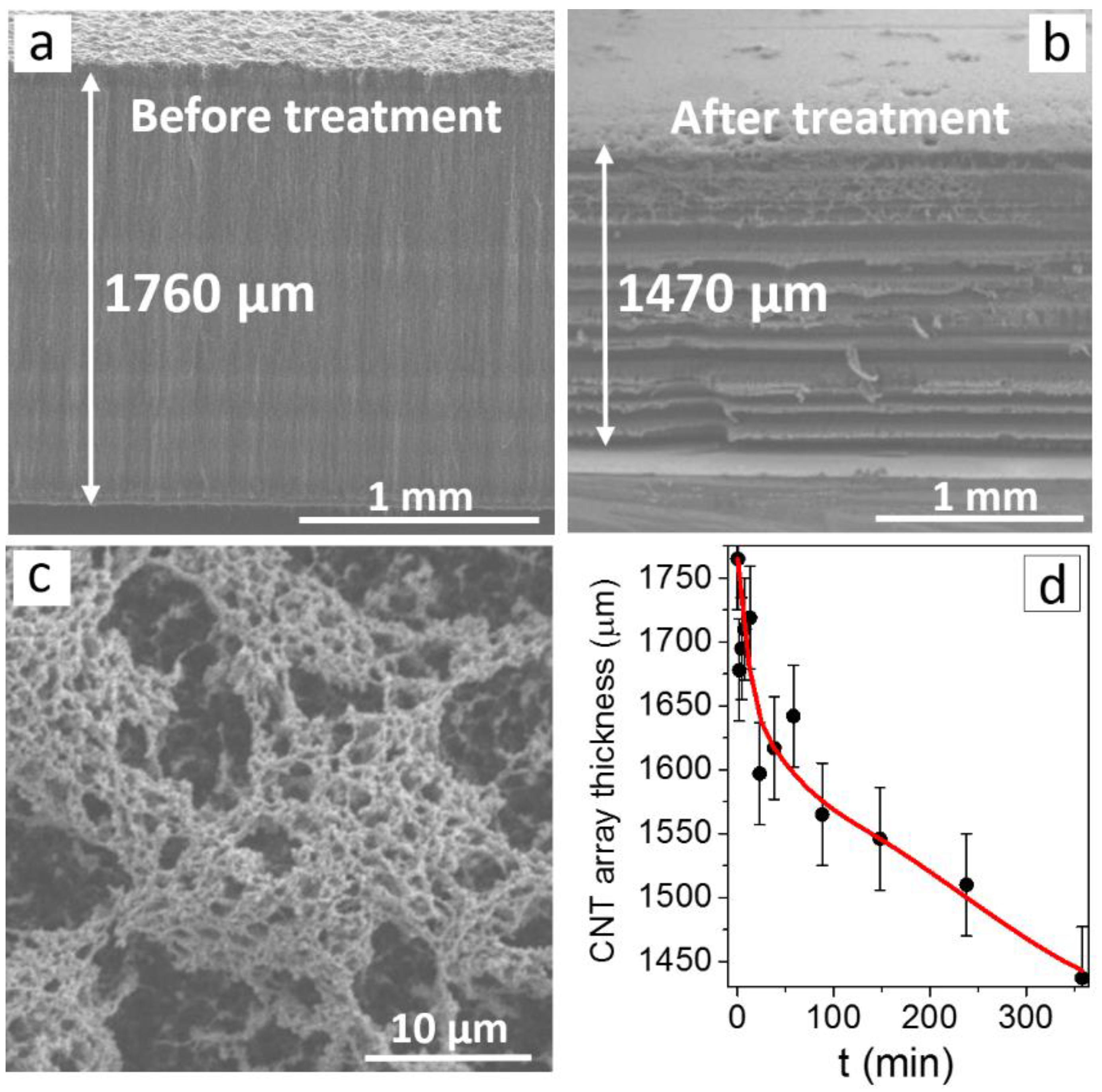
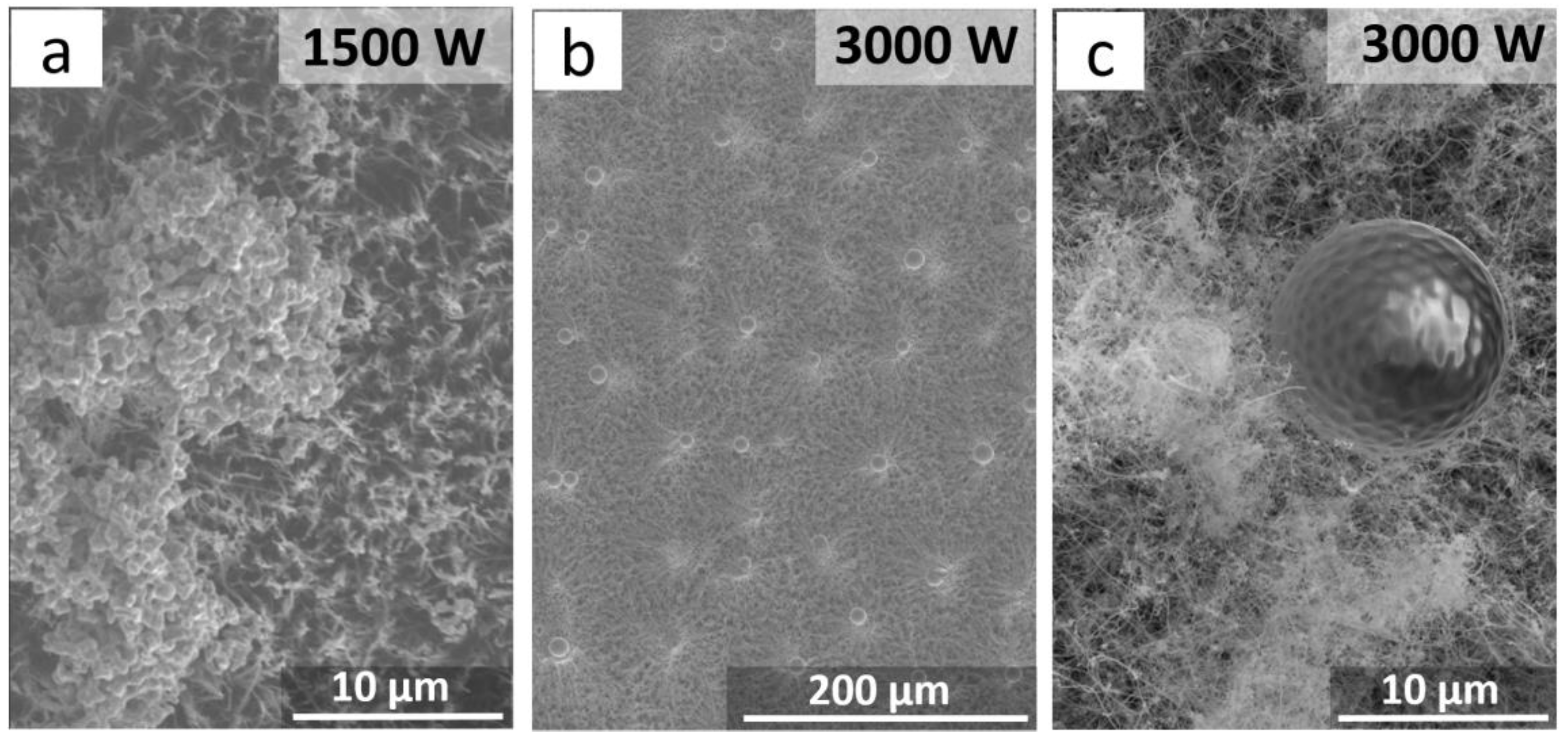
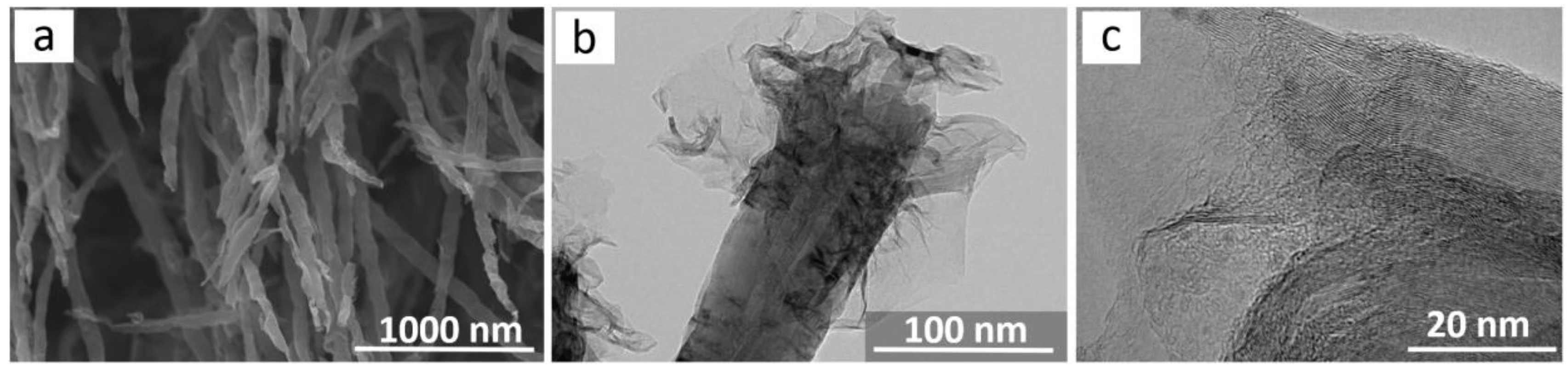
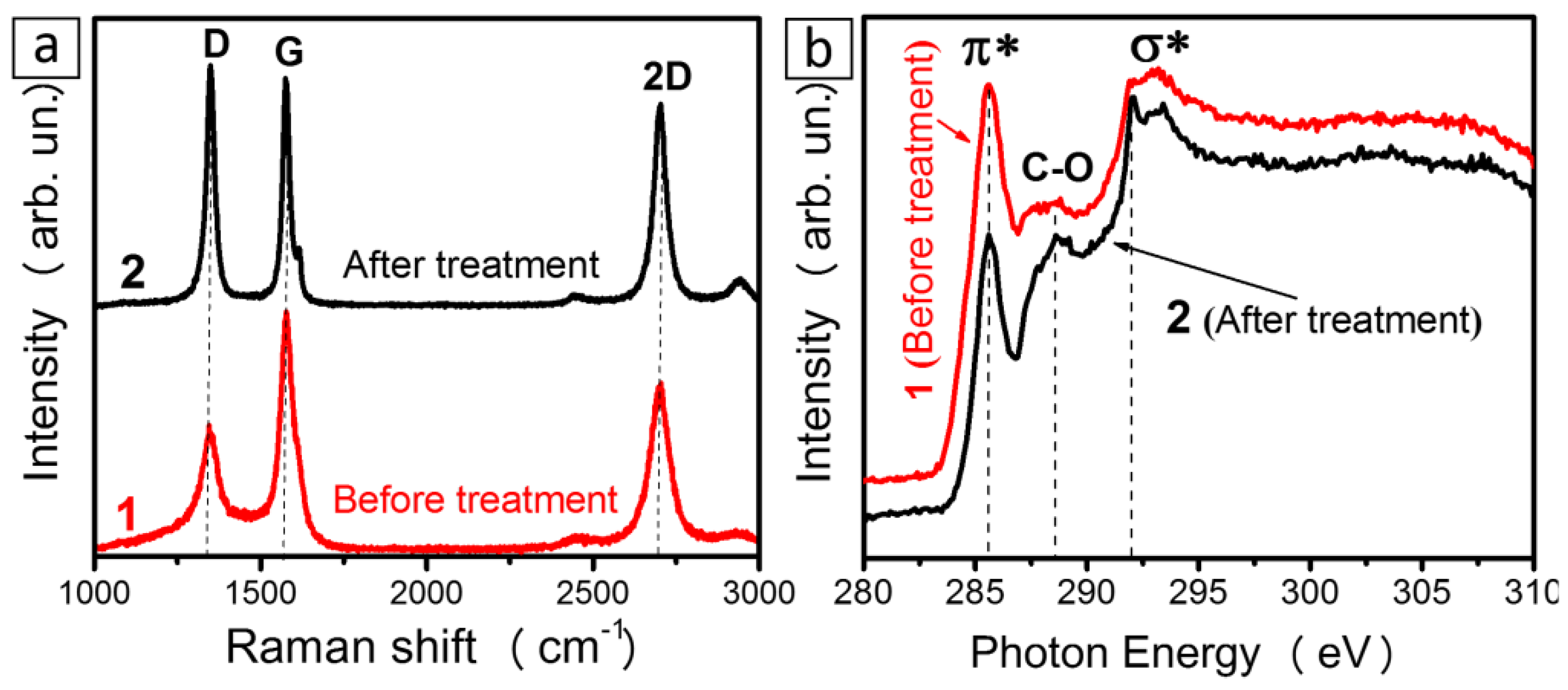
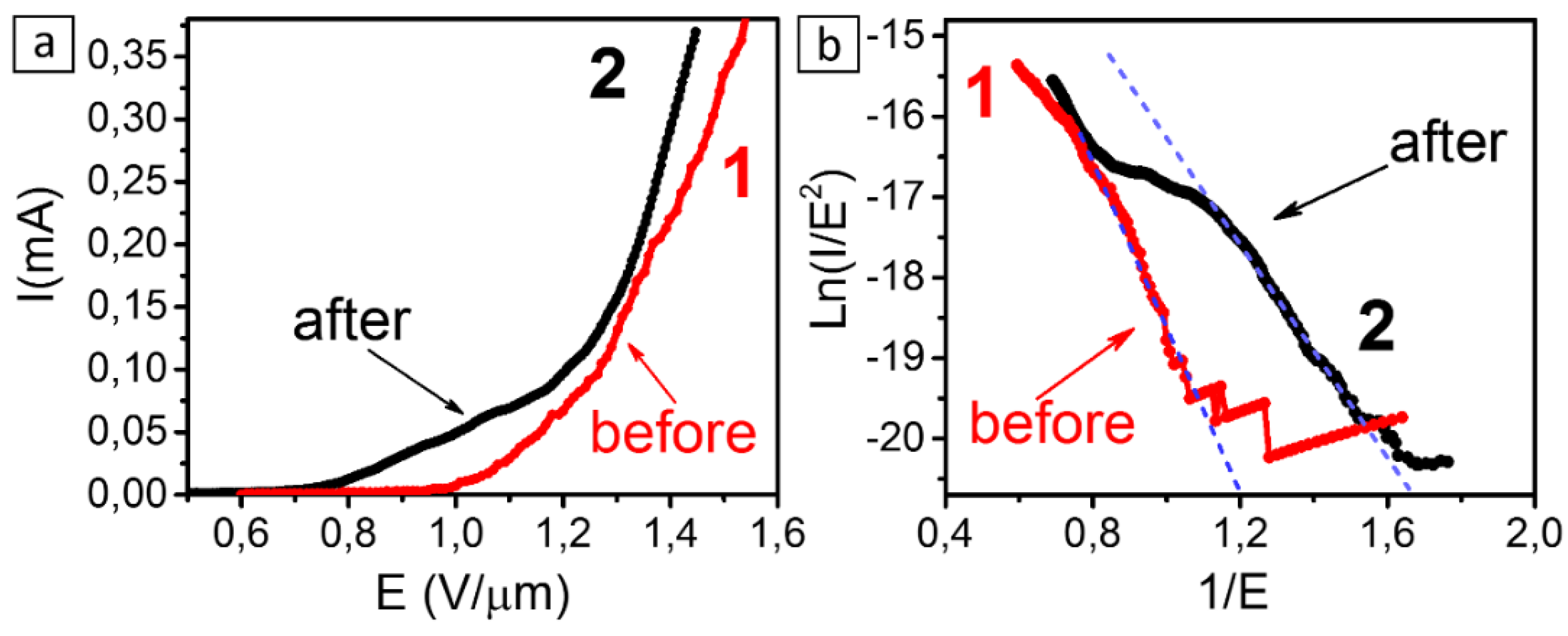
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Heer, W.A.; Chatelain, A.; Ugarte, D.A. Carbon nanotube field-emission electron source. Science 1995, 270, 1179–1180. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, O. Electron field emission from carbon nanotubes. Comptes Rendus Phys. 2003, 4, 1021–1033. [Google Scholar] [CrossRef]
- Milne, W.I.; Teo, K.B.K.; Amaratunga, G.A.J.; Legagneux, P.; Gangloff, L.; Schnell, J.-P.; Semet, V.; Binh, V.T.; Groeningd, O. Carbon nanotubes as field emission sources. J. Mater. Chem. 2004, 14, 933–943. [Google Scholar] [CrossRef]
- Li, X.; Zhou, J.; Wu, Q.; Liu, M.; Zhou, R.; Chen, Z. Fast microfocus X-ray tube based on carbon nanotube array. J. Vac. Sci. Technol. B 2019, 37, 051203. [Google Scholar] [CrossRef]
- Cheng, Z.; Sun, L.; Li, Z.Y.; Serbun, P.; Kargin, N.; Labunov, V.; Shulitski, B.; Kashko, I.; Grapov, D.; Gorokh, G. Field emission cathodes based on structured carbon nanotube arrays. World J. Hepatol. 2017, 4, 8–27. [Google Scholar]
- Lee, N.S.; Chung, D.S.; Han, I.T.; Kang, J.H.; Choi, Y.S.; Kim, H.Y.; Park, S.H.; Jin, Y.W.; Yi, W.K.; Yun, M.J.; et al. Application of carbon nanotubes to field emission displays. Diam. Relat. Mater. 2001, 10, 265–270. [Google Scholar] [CrossRef]
- Wang, Q.H.; Setlur, A.A.; Lauerhaas, J.M.; Dai, J.Y.; Seelig, E.W. A nanotube based field emission flat panel display. Appl. Phys. Lett. 1998, 72, 2912–2913. [Google Scholar] [CrossRef]
- Lee, S.; Oda, T.; Shin, P.-K.; Lee, B.-J. Chemical modification of carbon nanotube for improvement of field emission property. Microelectron. Eng. 2009, 86, 2110–2113. [Google Scholar] [CrossRef]
- Thapa, A.; Jungjohann, K.L.; Wang, X.; Li, W. Improving field emission properties of vertically aligned carbon nanotube arrays through a structure modification. J. Mater. Sci. 2020, 55, 2101–2117. [Google Scholar] [CrossRef]
- Sreekanth, M.; Ghosh, S.; Biswas, P.; Kumar, S.; Srivastava, P. Improved field emission from indium decorated multi-walled carbon nanotubes. Appl. Surf. Sci. 2016, 383, 84–89. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, Y.-H.; Shanov, V.; Tu, Y.; Subramaniam, S.; Schulz, M.J. Growth mechanism of long aligned multiwall carbon nanotube arrays by water-assisted chemical vapor deposition. J. Phys. Chem. B 2006, 110, 23920–23925. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.N. Properties, synthesis, and growth mechanisms of carbon nanotubes with special focus on thermal chemical vapor deposition. Nanoscale 2010, 2, 1306–1323. [Google Scholar]
- Castro, C.; Pinault, M.; Coste-Leconte, S.; Porterat, D.; Bendiab, N.; Reynaud, C.; Mayne-L’Hermite, M. Dynamics of catalyst particle formation and multi-walled carbon nanotube growth in aerosol-assisted catalytic chemical vapor deposition. Carbon 2010, 48, 3807–3816. [Google Scholar] [CrossRef]
- Seah, C.-M.; Chai, S.-P.; Mohamed, A.R. Synthesis of aligned carbon nanotubes. Carbon 2011, 49, 4613–4635. [Google Scholar] [CrossRef]
- Kurenya, A.G.; Gorodetskiy, D.V.; Arkhipov, V.E.; Okotrub, A.V. Evaluation of the optimal carrier gas flow rate for the carbon nanotubes growth. Tech. Phys. Lett. 2013, 39, 3. [Google Scholar] [CrossRef]
- Fedorovskaya, E.O.; Bulusheva, L.G.; Kurenya, A.G.; Asanov, I.P.; Rudina, N.A.; Funtov, K.O.; Lyubutin, I.S.; Okotrub, A.V. Supercapacitor performance of vertically aligned multiwall carbon nanotubes produced by aerosol-assisted CCVD method. Electrochim. Acta 2014, 139, 165–172. [Google Scholar] [CrossRef]
- Gorodetskiy, D.V.; Kurenya, A.G.; Gusel’nikov, A.V.; Kanygin, M.A.; Prokhorova, S.A.; Bulusheva, L.G.; Okotrub, A.V. Field emission characteristics of periodically structured carbon nanotube arrays. J. Nanoelectron. Optoelectron. 2013, 8, 52–57. [Google Scholar] [CrossRef]
- Zhi, C.Y.; Bai, X.D.; Wang, E.G. Enhanced field emission from carbon nanotubes by hydrogen plasma treatment. Appl. Phys. Lett. 2002, 81, 1690–1962. [Google Scholar] [CrossRef]
- Jones, J.G.; Waite, A.R.; Muratore, C.; Voevodin, A.A. Nitrogen and hydrogen plasma treatments of multiwalled carbon nanotubes. J. Vac. Sci. Technol. B 2008, 26, 995–1000. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Wang, W.; Liang, J.; Wang, Z.; Xia, Y.; Lei, D.; Ren, X.; Yao, N.; Zhang, B. The changes of morphology, structure and optical properties from carbon nanotubes treated by hydrogen plasma. Mater. Chem. Phys. 2008, 108, 82–87. [Google Scholar] [CrossRef]
- Yu, K.; Zhu, Z.; Xu, M.; Li, Q.; Lu, W. Electron field emission from soluble carbon nanotube films treated by hydrogen plasma. Chem. Phys. Lett. 2003, 373, 109–114. [Google Scholar] [CrossRef]
- Yu, K.; Zhu, Z.; Li, Q.; Lu, W. Electronic properties and field emission of carbon nanotube films treated by hydrogen plasma. Appl. Phys. A Mater. 2003, 77, 811–817. [Google Scholar] [CrossRef]
- Wang, S.; Sellin, P.J.; Lian, J.; Özsan, E.; Chang, S. Improvement of electron field emission in patterned carbon nanotubes by high temperature hydrogen plasma treatment. Curr. Nanosci. 2009, 5, 54–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.; Lei, D.; Wang, W.; Liang, J.; Wang, Z.; Yao, N.; Zhang, B. Preparation of carbon nanosheets deposited on carbon nanotubes by microwave plasma-enhanced chemical vapor deposition method. Appl. Surf. Sci. 2008, 254, 1700–1704. [Google Scholar] [CrossRef]
- Yua, K.; Zhua, Z.; Zhanga, Y.; Lia, Q.; Wanga, W.; Luoa, L.; Yub, X.; Mac, H.; Lid, Z.; Feng, T. Change of surface morphology and field emission property of carbon nanotube films treated using a hydrogen plasma. Appl. Surf. Sci. 2004, 225, 380–388. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, T.; Yu, W.; Liu, X.; Wang, X.; Li, Q. Enhancement of field emission from hydrogen plasma processed carbon nanotubes. Diam. Relat. Mater. 2004, 13, 54–59. [Google Scholar] [CrossRef]
- Abdia, Y.; Mohajerzadeha, S.; Koohshorkhia, J.; Robertsonb, M.D.; Andrei, C.M. A plasma enhanced chemical vapor deposition process to achieve branched carbon nanotubes. Carbon 2008, 46, 1611–1625. [Google Scholar] [CrossRef]
- Tung, F.-K.; Yoshimura, M.; Ueda, K.; Ohira, Y.; Tanji, T. Hydrogen plasma enhanced alignment on CNT-STM tips grown by liquid catalyst-assisted microwave plasma-enhanced chemical vapor deposition. Appl. Surf. Sci. 2008, 254, 7750–7754. [Google Scholar] [CrossRef]
- Li, Y.; Ji, K.; Duan, Y.; Meng, G.; Dai, Z. Effect of hydrogen concentration on the growth of carbon nanotube arrays for gecko-inspired adhesive applications. Coatings 2017, 7, 221. [Google Scholar] [CrossRef] [Green Version]
- Brodoceanu, D.; Bauer, C.T.; Kroner, E.; Arzt, E.; Kraus, T. Hierarchical bioinspired adhesive surfaces—A review. Bioinspiration Biomim. 2016, 11, 051001. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Vankar, V.D.; Kumar, V. Effect of hydrogen plasma treatment on the growth and microstructures of multiwalled carbon nanotubes. Nano Micro Lett. 2010, 2, 42–48. [Google Scholar] [CrossRef]
- Choi, W.S.; Choi, S.-H.; Hong, B.; Lim, D.-G.; Yang, K.-J.; Lee, J.-H. Effect of hydrogen plasma pretreatment on growth of carbon nanotubes by MPECVD. Mater. Sci. Eng. C 2006, 26, 1211–1214. [Google Scholar] [CrossRef]
- Zhang, M.-C.; Guo, G.-C.; Wang, R.-Z.; Cui, Y.-L.; Feng, X.-Y.; Wang, B.-R. Coupling enhanced growth by nitrogen and hydrogen plasma of carbon nanotubes. CrystEngComm 2019, 21, 4653–4660. [Google Scholar] [CrossRef]
- Il’in, O.I.; Il’ina, M.V.; Rudyk, N.N.; Fedotov, A.A.; Ageev, O.A. Vertically Aligned Carbon Nanotubes Production by PECVD. In Perspective of Carbon Nanotubes; IntechOpen: London, UK, 2019; pp. 1–17. [Google Scholar]
- Choi, H.; Shin, Y.J.; Cha, S.I.; Kang, I.H.; Bahng, W. Enhanced field-emission capacity by density control of a CNT cathode using post-plasma treatment. Solid State Commun. 2013, 171, 50–54. [Google Scholar] [CrossRef]
- Gusel’nikov, A.V.; Safronov, A.V.; Kurenya, A.G.; Arkhipov, V.E.; Bulgarian, S.G.; Ivanov, A.E.; Kvashnin, A.G.; Okotrub, A.V. The automation of a CVD-reactor for the synthesis of vertically oriented carbon nanotube arrays. Instrum. Exp. Tech. 2018, 61, 482–485. [Google Scholar] [CrossRef]
- Kudashov, A.G.; Kurenya, A.G.; Okotrub, A.V.; Gusel’nikov, A.V.; Danilovich, V.S.; Bulusheva, L.G. Synthesis and structure of films consisting of carbon nanotubes oriented normally to the substrate. Tech. Phys. 2007, 52, 1627–1632. [Google Scholar] [CrossRef]
- Sedelnikova, O.V.; Korovin, E.Y.; Dorozhkin, K.V.; Kanygin, M.A.; Arkhipov, V.E.; Shubin, Y.V.; Zhuravlev, V.A.; Suslyaev, V.I.; Bulusheva, L.G.; Okotrub, A.V. Iron-filled multi-walled carbon nanotubes for terahertz applications: Effects of interfacial polarization, screening and anisotropy. Nanotechnology 2018, 29, 174003. [Google Scholar] [CrossRef]
- Polyakov, O.V.; Gorodetskii, D.V.; Okotrub, A.V. The effect of number of carbon atoms in a molecular precursor on the crystallite size in diamond films prepared by plasma_enhanced chemical_vapor deposition. Tech. Phys. Lett. 2013, 39, 501–504. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef] [Green Version]
- Doniach, S.; Sunjic, M. Many-electron singularity in X-ray photoemission and X-ray line spectra from metals. J. Phys. C Solid State Phys. 1970, 3, 285–291. [Google Scholar] [CrossRef]
- Lyubutin, I.S.; Anosova, O.A.; Frolov, K.V.; Sulyanov, S.N.; Okotrub, A.V.; Kudashov, A.G.; Bulusheva, L.G. Iron nanoparticles in aligned arrays of pure and nitrogen-doped carbon nanotubes. Carbon 2012, 50, 2628–2634. [Google Scholar] [CrossRef]
- Behr, M.J.; Gaulding, E.A.; Mkhoyan, K.A.; Aydil, E.S. Hydrogen etching and cutting of multiwall carbon nanotubes. J. Vac. Sci. Technol. B 2010, 28, 1187–1194. [Google Scholar] [CrossRef]
- Aussems, D.U.B.; Bal, K.M.; Morgan, T.W.; van de Sanden, M.C.M.; Neyts, E.C. Mechanisms of elementary hydrogen ion-surface interactions during multilayer graphene etching at high surface temperature as a function of flux. Carbon 2018, 137, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Hanson, R.L. Plasma quenching reactions with laser pyrolysis of graphite and coal in helium or hydrogen. Carbon 1978, 16, 159–162. [Google Scholar] [CrossRef]
- Vietzke, E.; Philipps, V. Hydrocarbon formation on carbon surfaces facing a hydrogen plasma. Fusion Technol. 1989, 15, 108–117. [Google Scholar] [CrossRef]
- Blume, R.; Rosenthal, D.; Tessonnier, J.-P.; Li, H.; KnopGericke, A.; Schlçgl, R. Characterizing graphitic carbon with X-ray photoelectron spectroscopy: A step-by-step approach. ChemCatChem 2015, 7, 2871–2881. [Google Scholar] [CrossRef] [Green Version]
- Fedoseeva, Y.V.; Bulusheva, L.G.; Okotrub, A.V.; Kanygin, M.A.; Gorodetskiy, D.V.; Asanov, I.P.; Vyalikh, D.V.; Puzyr, A.P.; Bondar, V.S. Field emission luminescence of nanodiamonds deposited on the aligned carbon nanotube array. Sci. Rep. 2015, 5, 9379. [Google Scholar] [CrossRef] [Green Version]
- Zhan, D.; Ni, Z.; Chen, W.; Sun, L.; Luo, Z.; Lai, L.; Yu, T.; Wee, A.T.S.; Shen, Z. Electronic structure of graphite oxide and thermally reduced graphite oxide. Carbon 2011, 49, 1362–1366. [Google Scholar] [CrossRef]
- Popov, K.M.; Fedoseeva, Y.V.; Kokhanovskaya, O.A.; Razd′yakonova, G.I.; Smirnov, D.A.; Bulusheva, L.G.; Okotrub, A.V. Functional composition and electrochemical characteristics of oxidized nanosized carbon. J. Struct. Chem. 2017, 58, 1187. [Google Scholar] [CrossRef]
- Fedorovskaya, E.O.; Bulusheva, L.G.; Kurenya, A.G.; Asanov, I.P.; Okotrub, A.V. Effect of oxidative treatment on the electrochemical properties of aligned multi-walled carbon nanotubes. Russ. J. Electrochem. 2016, 52, 441–448. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Duda, T.A.; Kurenya, A.G.; Gusel’nikov, A.V.; Zhuravlev, K.S.; Vilkov, O.Y.; Bulusheva, L.G.; Okotrub, A.V. An X-ray spectroscopy study of CdS nanoparticles formed by the Langmuir-Blodgett technique on the surface of carbon nanotube arrays. J. Struct. Chem. 2017, 58, 876–884. [Google Scholar] [CrossRef]
- Momose, Y.; Tsuruya, K.; Sakurai, T.; Nakayama, K. Photoelectron emission and XPS studies of real iron surfaces subjected to scratching in air, water, and organic liquids. Surf. Interface Anal. 2016, 48, 202–211. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Fedoseeva, Y.V.; Kurenya, A.G.; Vyalikh, D.V.; Okotrub, A.V. The role of defects in carbon nanotube walls in deposition of CdS nanoparticles from a chemical bath. J. Phys. Chem. C 2015, 119, 25898–25906. [Google Scholar] [CrossRef]
- Bruhwiler, P.; Maxwell, A.; Puglia, C.; Nilsson, A.; Andersson, S.; Martensson, N. π* and σ* Excitons in C1s-absorption of graphite. Phys. Rev. Lett. 1995, 74, 614–617. [Google Scholar] [CrossRef]
- Cancado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via Raman spectrodcopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, R.A.; Cai, M.; Outlaw, R.A.; Butler, S.M.; Miller, J.R.; Mansour, A.N. Investigation of defects generated in vertically oriented graphene. Carbon 2013, 64, 92–100. [Google Scholar] [CrossRef]
- Kanygin, M.A.; Okotrub, A.V.; Bulusheva, L.G.; Vilkov, O.Y.; Hata, K. Revealing distortion of carbon nanotube walls via angle-resolved x-ray spectroscopy. Curr. Appl. Phys. 2015, 15, 1111–1116. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Belavin, V.V.; Kudashov, A.G.; Vyalikh, D.V.; Molodtsov, S.L. Determination of the texture of arrays of aligned carbon nanotubes from the angular dependence of the X-ray emission and X-ray absorption spectra. J. Exp. Theor. Phys. 2008, 107, 517–525. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Kanygin, M.A.; Kurenya, A.G.; Kudashov, A.G.; Bulusheva, L.G.; Molodtsov, S.L. NEXAFS detection of graphitic layers formed in the process of carbon nanotube arrays synthesis. Nucl. Instrum. Methods Phys. Res. B 2009, 603, 115–118. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Kanygin, M.A.; Sedelnikova, O.V.; Gusel’nikov, A.V.; Kotosonov, A.S.; Bulusheva, L.G. Interaction of ultrasoft X-rays with arrays of aligned carbon nanotubes. J. Nanophotonics 2010, 4, 041655. [Google Scholar] [CrossRef]
- Kanygin, M.A.; Okotrub, A.V.; Gusel’nikov, A.V.; Kurenya, A.G. Features of inelastic interaction of X-ray radiation with aligned carbon nanotube films. J. Nanoelectron. Optoelectron. 2012, 7, 60–64. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Belavin, V.V.; Bulusheva, L.G.; Kudashov, A.G.; Vyalikh, D.V.; Molodtsov, S.L. X-ray spectroscopy characterization of carbon nanotube film texture. In Electronic Properties of Novel Nanostructures; Kuzmany, H., Fink, J., Mehring, M., Roth, S., Eds.; AIP Conference Proceedings, Melville; AIP: New York, NY, USA, 2005; Volume 786, pp. 150–153. [Google Scholar]
- Belavin, V.V.; Okotrub, A.V.; Bulusheva, L.G.; Kotosonov, A.S.; Vyalykh, D.V.; Molodtsov, S.L. Determining misorientation of graphite grains from the angular dependence of x-ray absorption spectra. J. Exp. Theor. Phys. 2006, 103, 604–610. [Google Scholar] [CrossRef]
- Arkhipov, V.E.; Smirnov, A.L.; Grachev, G.N.; Bagayev, S.N.; Gusel’nikov, A.V.; Bulusheva, L.G.; Okotrub, A.V. Continuous synthesis of aligned carbon nanotube arrays on copper substrates using laser-activated gas jet. Appl. Phys. Lett. 2018, 113, 223102. [Google Scholar] [CrossRef]
- Gorodetskiy, D.V.; Shevchenko, S.N.; Gusel’nikov, A.V.; Okotrub, A.V. A memristive model for graphene emitters: Hysteresis and self-crossing. Phys. Status Solidi B 2020, 257, 2000020. [Google Scholar] [CrossRef]
- Shiraishi, M.; Ata, M. Work function of carbon nanotubes. Carbon 2001, 39, 1913–1917. [Google Scholar] [CrossRef]
- Lim, S.C.; Jeong, H.J.; Kim, K.S.; Lee, I.B.; Bae, D.J.; Lee, Y.H. Extracting independently the work function and field enhancement factor from thermal-field emission of multi-walled carbon nanotube tips. Carbon 2005, 43, 2801–2807. [Google Scholar] [CrossRef]
- Yu, Y.-J.; Zhao, Y.; Ryu, S.; Brus, L.E.; Kim, K.S.; Kim, P. Tuning the graphene work function by electric field effect. Nano Lett. 2009, 9, 3430–3434. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorodetskiy, D.V.; Gusel’nikov, A.V.; Kurenya, A.G.; Smirnov, D.A.; Bulusheva, L.G.; Okotrub, A.V. Hydrogen Plasma Treatment of Aligned Multi-Walled Carbon Nanotube Arrays for Improvement of Field Emission Properties. Materials 2020, 13, 4420. https://doi.org/10.3390/ma13194420
Gorodetskiy DV, Gusel’nikov AV, Kurenya AG, Smirnov DA, Bulusheva LG, Okotrub AV. Hydrogen Plasma Treatment of Aligned Multi-Walled Carbon Nanotube Arrays for Improvement of Field Emission Properties. Materials. 2020; 13(19):4420. https://doi.org/10.3390/ma13194420
Chicago/Turabian StyleGorodetskiy, Dmitriy V., Artem V. Gusel’nikov, Alexander G. Kurenya, Dmitry A. Smirnov, Lyubov G. Bulusheva, and Alexander V. Okotrub. 2020. "Hydrogen Plasma Treatment of Aligned Multi-Walled Carbon Nanotube Arrays for Improvement of Field Emission Properties" Materials 13, no. 19: 4420. https://doi.org/10.3390/ma13194420
APA StyleGorodetskiy, D. V., Gusel’nikov, A. V., Kurenya, A. G., Smirnov, D. A., Bulusheva, L. G., & Okotrub, A. V. (2020). Hydrogen Plasma Treatment of Aligned Multi-Walled Carbon Nanotube Arrays for Improvement of Field Emission Properties. Materials, 13(19), 4420. https://doi.org/10.3390/ma13194420





