Biologically Active Compounds of Plants: Structure-Related Antioxidant, Microbiological and Cytotoxic Activity of Selected Carboxylic Acids
Abstract
1. Introduction
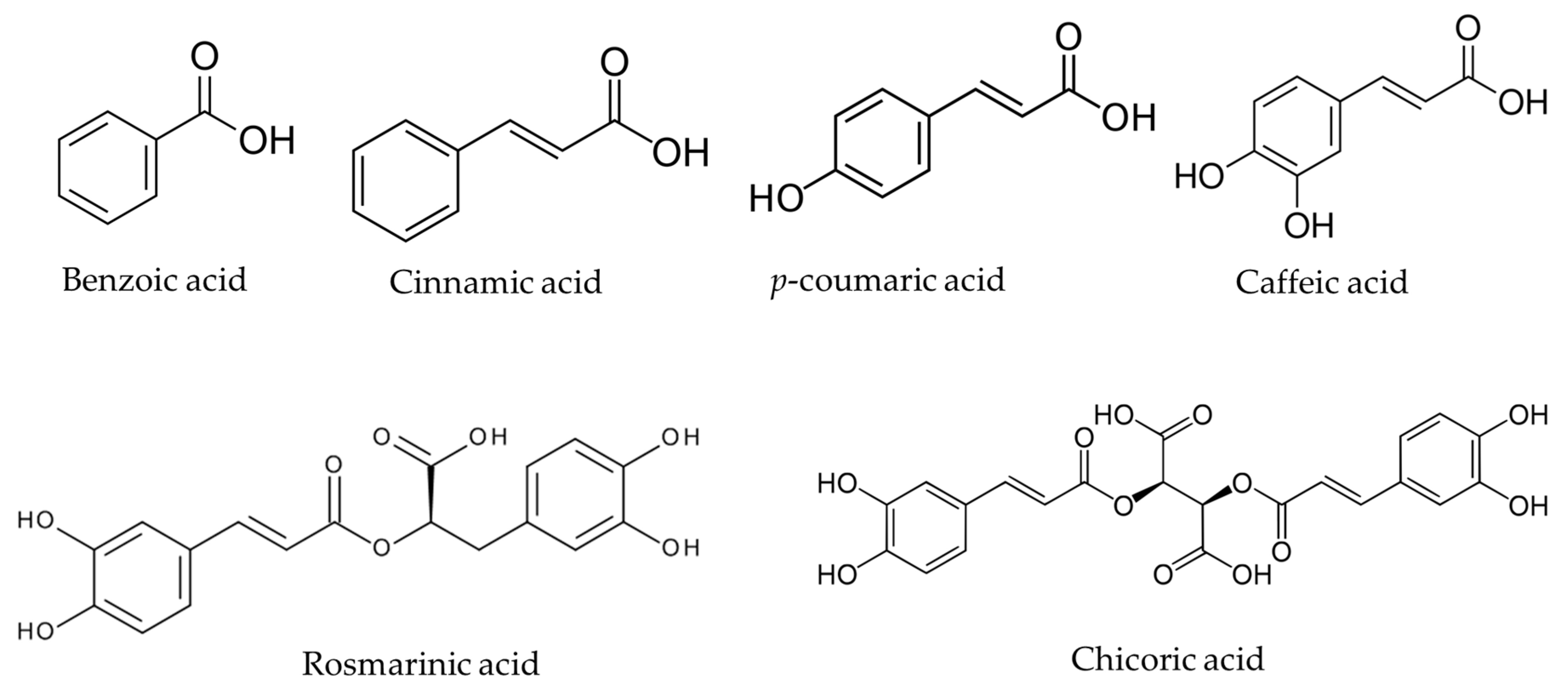
2. Chemical Structure, Occurrence, and Physicochemical Parameters of NCA
3. Antioxidant Activity of NCA Determined by Various Chemical Methods
3.1. Scavenging of the DPPH Radical
3.2. Scavenging of the ABTS Radical
3.3. Ferric Reducing Antioxidant Power (FRAP) Assay
3.4. The CUPRAC (Cupric Reducing Antioxidant Capacity) Method
3.5. The Oxygen Radical Antioxidant Capacity (ORAC)
3.6. Lipid Peroxidation Assay (LP)
3.7. Nitric Oxide Radical Scavenging Assay
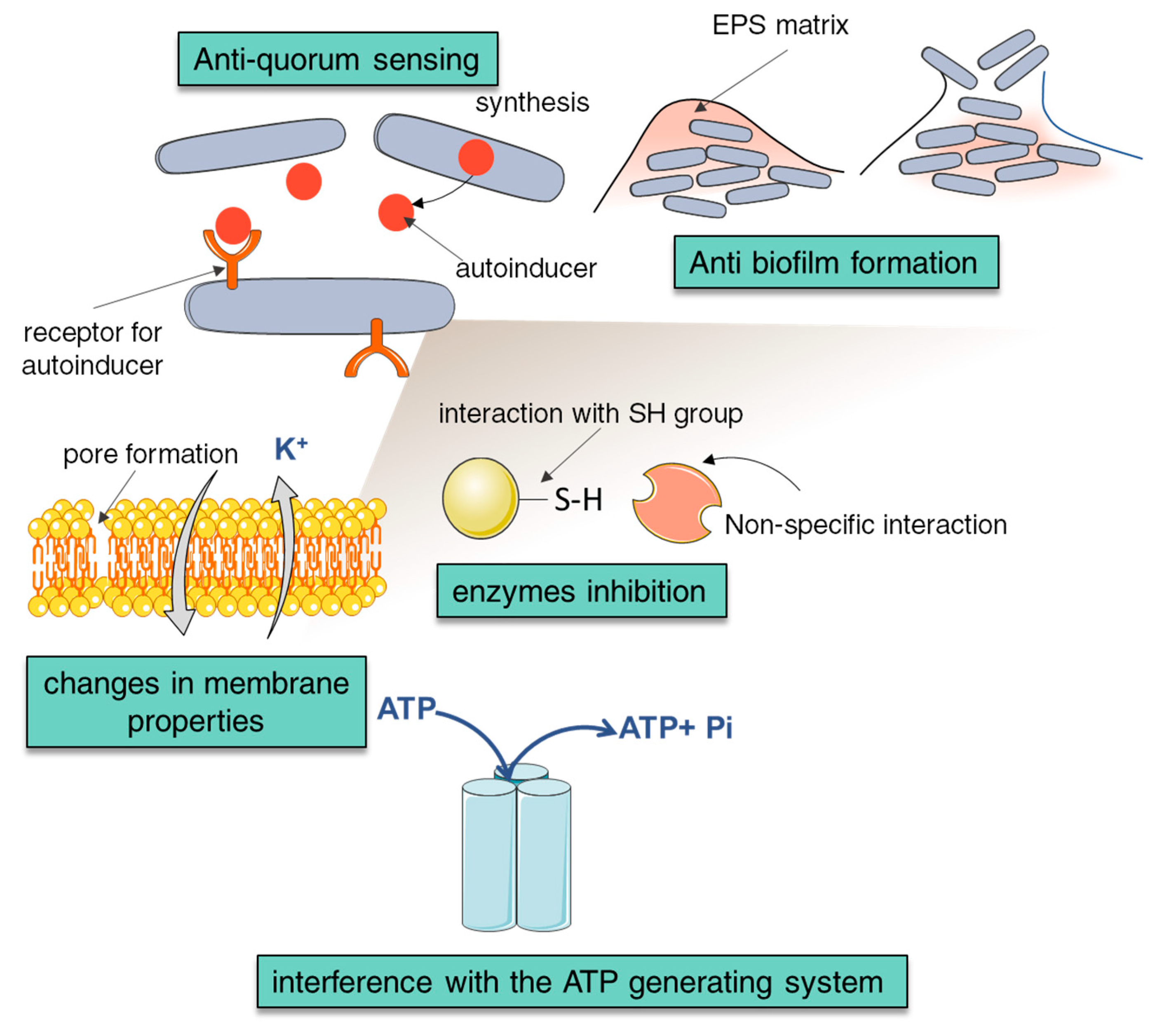
4. Antimicrobial Properties of NCA
5. Anticancer Activity of NCA
6. Structure Elements and Biological Activity
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial properties of selected essential oils in vapour phase against foodborne bacteria. Food Control 2009, 20, 157–160. [Google Scholar] [CrossRef]
- Mancini, E.; Camele, I.; Elshafie, H.S.; De Martino, L.; Pellegrino, C.; Grulova, D.; De Feo, V. Chemical composition and biological activity of the essential oil of Origanum vulgare ssp. hirtum from different areas in the Southern Apennines (Italy). Chem. Biodivers. 2014, 11, 639–651. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Matejczyk, M.; Świsłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Frolinger, T.; Sims, S.; Smith, C.; Wang, J.; Cheng, H.; Faith, J.; Ho, L.; Hao, K.; Pasinetti, G.M. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci. Rep. 2019, 9, 3546. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kwon, H.J.; Choi, J.; Seo, J.S.; Han, P.L. Aging increases vulnerability to stress-induced depression via upregulation of NADPH oxidase in mice. Commun. Biol. 2020, 3, 292. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ippoushi, K.; Nakayama, M.; Ito, H.; Higashio, H.; Terao, J. Absorption of chlorogenic acid and caffeic acid in rats after oral administration. J. Agric. Food Chem. 2000, 48, 5496–5500. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yu, F.; Li, Y.; Lou, Z.; Toure, S.L.; Wang, H. The inhibitory activity of p-coumaric acid on quorum sensing and its enhancement effect on meat preservation. CyTA J. Food 2020, 18, 61–67. [Google Scholar] [CrossRef]
- Wang, J.Z.; Yan, C.H.; Zhang, X.R.; Tu, Q.B.; Xu, Y.; Sheng, S.; Wu, F.A.; Wang, J. A novel nanoparticle loaded with methyl caffeate and caffeic acid phenethyl ester against: Ralstonia solanacearum—A plant pathogenic bacteria. RSC Adv. 2020, 10, 3978–3990. [Google Scholar] [CrossRef]
- Chung, C.H.; Jung, W.; Keum, H.; Kim, T.W.; Jon, S. Nanoparticles Derived from the Natural Antioxidant Rosmarinic Acid Ameliorate Acute Inflammatory Bowel Disease. ACS Nano 2020, 14, 6887–6896. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- He, Z.D.; Qiao, C.F.; Han, Q.B.; Cheng, C.L.; Xu, H.X.; Jiang, R.W.; But, P.P.H.; Shaw, P.C. Authentication and quantitative analysis on the chemical profile of Cassia Bark (Cortex cinnamomi) by high-pressure liquid chromatography. J. Agric. Food Chem. 2005, 53, 2424–2428. [Google Scholar] [CrossRef]
- del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2015, 57, 3084–3103. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid: Chemistry, distribution, and production. Front. Chem. 2013, 1, 40. [Google Scholar] [CrossRef]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid levels in commercial basil (Ocimum basilicum) and Echinacea purpurea products. J. Funct. Foods 2010, 2, 77–84. [Google Scholar] [CrossRef]
- Yun, S.S.; Lee, S.J.; Lim, D.Y.; Lim, H.S.; Lee, G.; Kim, M. Monitoring of Benzoic Acid, Sorbic Acid, and Propionic Acid in Spices. J. Food Hyg. Saf. 2017, 32, 381–388. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.J.; Skowyra, M.; Almajano, M.P. Antioxidant properties of three aromatic herbs (rosemary, thyme and lavender) in oil-in-water emulsions. J. Am. Oil Chem. Soc. 2013, 90, 1559–1568. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Koldaş, S.; Demirtas, I.; Ozen, T.; Demirci, M.A.; Behçet, L. Phytochemical screening, anticancer and antioxidant activities of Origanum vulgare L. ssp. viride (Boiss.) Hayek, a plant of traditional usage. J. Sci. Food Agric. 2015, 95, 786–798. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Alvarenga, J.F.R.; Martinez-Huelamo, M.; Leal, L.N.; Lamuela-Raventos, R.M. Characterization of the phenolic and antioxidant profiles of selected culinary herbs and spices: Caraway, turmeric, dill, marjoram and nutmeg. Food Sci. Technol. 2015, 35, 189–195. [Google Scholar] [CrossRef]
- Sigma-Aldrich®. Available online: http://sigmaaldrich.com/catalog/search (accessed on 23 May 2020).
- Guillaume, M.; Botek, E.; Champagne, B.; Castet, F.; Ducasse, L. Substituent effects on the electronic structure and pKa benzoic acid. Int. J. Quantum Chem. 2002, 90, 1396–1403. [Google Scholar] [CrossRef]
- Barton, D.; Ollis, W.D. (Eds.) Comprehensive Organic Chemistry; Pergamon Press: Oxford, UK, 1979. [Google Scholar]
- Kealey, D. Experiments in Modern Analytical Chemistry; Springer: New York, NY, USA, 1986. [Google Scholar]
- Naseem, S.; Laurent, A.D.; Carroll, E.C.; Vengris, M.; Kumauchi, M.; Hoff, W.D.; Krylov, A.I.; Larsen, D.S. Photo-isomerization upshifts the pKa of the photoactive yellow protein chromophore to contribute to photocycle propagation. J. Photochem. Photobiol. A Chem. 2013, 270, 43–52. [Google Scholar] [CrossRef]
- Silva, F.A.; Borges, F.; Guimarães, C.; Lima, J.L.; Matos, C.; Reis, S. Phenolic Acids and Derivatives: Studies on the Relationship among Structure, Radical Scavenging Activity, and Physicochemical Parameters. J. Agric. Food Chem. 2000, 48, 2122–2126. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Kapcsándi, V.; Székelyhidi, R.; Hanczné, E.L.; Ajtony, Z. Recent advances in the analysis of rosmarinic acid from herbs in the Lamiaceae family. Nat. Prod. Commun. 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Dalton, B.R.; Blum, U.; Weed, S.B. Differential Sorption of Exogenously Applied Ferulic, p-Coumaric, p-Hydroxybenzoic, and Vanillic Acids in Soil. Soil Sci. Soc. Am. J. 1989, 53, 757–762. [Google Scholar] [CrossRef]
- Kiss, T.; Nagy, G.; Pécsi, M.; Kozlowski, H.; Micera, G.; Erre, L.S. Complexes of 3,4-dihydroxyphenyl derivatives-X. Copper(II) complexes of chlorogenic acid and related compounds. Polyhedron 1989, 8, 2345–2349. [Google Scholar] [CrossRef]
- Beiginejad, H.; Moradi, M.; Paziresh, S.; Farahani, H. Thermodynamic and Mechanistic Study of the Electrochemical Oxidation of Rosmarinic Acid. J. Electrochem. Soc. 2018, 165, H698–H704. [Google Scholar] [CrossRef]
- Muzafarov, E.N.; Zolotareva, E.K. Uncoupling Effect of Hydroxycinnamic Acid Derivatives on Pea Chloroplasts. Biochem. Physiol. Pflanz. 1989, 184, 363–369. [Google Scholar] [CrossRef]
- Linder, P.W.; Voyé, A. Potentiometric investigations of the equilibria between caffeic acid and copper(II), zinc(II), iron(II) and hydrogen ions in aqueous solution. Polyhedron 1987, 6, 53–60. [Google Scholar] [CrossRef]
- ACD/ChemSketch; Advanced Chemistry Development: Toronto, ON, Canada, 2020; Ver. 2019.2.2.
- Flores, T.P.; Shomer, B. The European Bioinformatics Institute. Available online: https://www.ebi.ac.uk/ (accessed on 28 May 2020).
- Benvidi, A.; Dadras, A.; Abbasi, S.; Tezerjani, M.D.; Rezaeinasab, M.; Tabaraki, R.; Namazian, M. Experimental and computational study of the pKa of coumaric acid derivatives. J. Chin. Chem. Soc. 2019, 66, 589–593. [Google Scholar] [CrossRef]
- Kloetzer, L.; Bompa, A.S.; Blaga, A.C.; Galaction, A.I.; Ca\cscaval, D.; Caşcaval, D. Study on rosmarinic acid separation by synergic extraction. Sep. Sci. Technol. 2017, 53, 645–654. [Google Scholar] [CrossRef]
- Sangster, J. Octanol Water Partition Coefficients of Simple Organic Compounds. J. Phys. Chem. Ref. Data 1989, 18, 1111–1229. [Google Scholar] [CrossRef]
- Barvinchenko, V.N.; Lipkovskaya, N.A.; Kulik, T.V.; Kartel, N.T. Adsorption of Natural 3-Phenylpropenic Acids on Cerium Dioxide Surface. Colloid J. 2019, 81, 1–7. [Google Scholar] [CrossRef]
- Hanai, T.; Hubert, J. Hydrophobicity and chromatographic behaviour of aromatic acids found in urine. J. Chromatogr. A 1982, 239, 527–536. [Google Scholar] [CrossRef]
- Alam, A.; Tamkeen, N.; Imam, N.; Farooqui, A.; Ahmed, M.M.; Tazyeen, S.; Ali, S.; Malik, M.Z.; Ishrat, R. Pharmacokinetic and molecular docking studies of plant-derived natural compounds to exploring potential anti-Alzheimer activity. In In Silico Approach for Sustainable Agriculture; Springer: Singapore, 2018; pp. 217–238. ISBN 9789811303470. [Google Scholar]
- Lu, P.-Y.Y.; Metcalf, R.L. Environmental fate and biodegradability of benzene derivatives as studied in a model aquatic ecosystem. Environ. Health Perspect. 1975, 10, 269–284. [Google Scholar] [CrossRef]
- Yaguzhinsky, L.S.; Smirnova, E.G.; Ratnikova, L.A.; Kolesova, G.M.; Krasinskaya, I.P. Hydrophobic sites of the mitochondrial electron transfer system. J. Bioenerg. 1973, 5, 163–174. [Google Scholar] [CrossRef]
- Cheah, H.L.; Lim, V.; Sandai, D. Inhibitors of the glyoxylate cycle enzyme ICL1 in Candida albicans for potential use as antifungal agents. PLoS ONE 2014, 9, e95951. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 28 May 2020).
- Jablonsky, M.; Haz, A.; Burcova, Z.; Kreps, F.; Jablonsky, J. Pharmacokinetic Properties of Biomass-extracted Substances Isolated by Green Solvents. BioResources 2019, 14, 6294–6303. [Google Scholar] [CrossRef]
- Silva, T.; Bravo, J.; Summavielle, T.; Remião, F.; Pérez, C.; Gil, C.; Martínez, A.; Borges, F. Biology-oriented development of novel lipophilic antioxidants with neuroprotective activity. RSC Adv. 2015, 5, 15800–15811. [Google Scholar] [CrossRef][Green Version]
- Ehigiator, B.E.; Adesida, A.S.; Omotuyi, I.O. Chicoric Acid, a Phytochemical Compound of Solenostemon monostachyus: Possible Drug Candidate for the Relief of Erectile Dysfunction. Int. J. Eng. Appl. Sci. Technol. 2020, 04, 509–518. [Google Scholar] [CrossRef]
- Cumming, H.; Rücker, C. Octanol-Water Partition Coefficient Measurement by a Simple 1H NMR Method. ACS Omega 2017, 2, 6244–6249. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, R.; Campos, D.; Costa, N.; Arbizu, C.; Pedreschi, R.; Larondelle, Y. Phenolic profiles of andean mashua (Tropaeolum tuberosum Ruíz & Pavón) tubers: Identification by HPLC-DAD and evaluation of their antioxidant activity. Food Chem. 2008, 106, 1285–1298. [Google Scholar] [CrossRef]
- Tsao, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. B 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Troncoso, A.M.; García-Parrilla, M.C. Comparison of antioxidant activity of wine phenolic compounds and metabolites in vitro. Anal. Chim. Acta 2005, 538, 391–398. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Wayner, D.D.M.; Burton, G.W.; Ingold, K.U.; Locke, S. Quantitative measurement of the total, peroxyl radical-trapping antioxidant capability of human blood plasma by controlled peroxidation. The important contribution made by plasma proteins. FEBS Lett. 1985, 187, 33–37. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Pielecki, J.; Targoński, Z. Antioxidant Activities of Cinnamic and Benzoic Acid Derivaties. Acta Sci. Pol. Technol. Aliment. 2005, 4, 129–142. [Google Scholar]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef]
- Sladkovský, R.; Solich, P.; Opletal, L. Simultaneous determination of quercetin, kaempferol and (E)-cinnamic acid in vegetative organs of Schisandra chinensis Baill. by HPLC. J. Pharm. Biomed. Anal. 2001, 24, 1049–1054. [Google Scholar] [CrossRef]
- Kiliç, I.; Yeşiloǧlu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hwang, I.Y.; Jeong, C.S. Protective effects of cinnamic acid derivatives on gastric lesion. Nat. Prod. Sci. 2017, 23, 299–305. [Google Scholar] [CrossRef]
- Xie, P.J.; Huang, L.X.; Zhang, C.H.; Zhang, Y.L. Phenolic compositions, and antioxidant performance of olive leaf and fruit (Olea europaea L.) extracts and their structure-activity relationships. J. Funct. Foods 2015, 16, 460–471. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Kyselka, J.; Rabiej, D.; Dragoun, M.; Kreps, F.; Burčová, Z.; Němečková, I.; Smolová, J.; Bjelková, M.; Szydłowska-Czerniak, A.; Schmidt, Š.; et al. Antioxidant and antimicrobial activity of linseed lignans and phenolic acids. Eur. Food Res. Technol. 2017, 243, 1633–1644. [Google Scholar] [CrossRef]
- Sova, M. Antioxidant and Antimicrobial Activities of Cinnamic Acid Derivatives. Mini-Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Ahmadvand, H.; Khosrobeigi, A.; Nemati, L.; Boshtam, M.; Jafari, N.; Hosseini, R.H.; Pournia, Y. Rosmarinic acid prevents the oxidation of low density lipoprotein (LDL) in vitro. J. Biol. Sci. 2012, 12, 301–307. [Google Scholar] [CrossRef][Green Version]
- Liu, Q.; Wang, Y.; Xiao, C.; Wu, W.; Liu, X. Metabolism of chicoric acid by rat liver microsomes and bioactivity comparisons of chicoric acid and its metabolites. Food Funct. 2015, 6, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Benvenuti, S.; Magro, L.; Melegari, M.; Soragni, F. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. J. Pharm. Biomed. Anal. 2004, 35, 289–301. [Google Scholar] [CrossRef]
- Takahashi, T.; Miyazawa, M. Tyrosinase inhibitory activities of cinnamic acid analogues. Pharmazie 2010, 65, 913–918. [Google Scholar] [CrossRef]
- Chang, M.Y.; Liu, C.M.; Shieh, D.E.; Chen, C.Y. Evaluation and analysis of phytochemical antioxidant capacity. Biomed. Res. 2017, 28, 6431–6434. [Google Scholar]
- Masek, A.; Chrzescijanska, E.; Latos, M. Determination of antioxidant activity of caffeic acid and p-coumaric acid by using electrochemical and spectrophotometric assays. Int. J. Electrochem. Sci. 2016, 11, 10644–10658. [Google Scholar] [CrossRef]
- Velkov, Z.A.; Kolev, M.K.; Tadjer, A.V. Modeling and statistical analysis of DPPH scavenging activity of phenolics. Collect. Czechoslov. Chem. Commun. 2007, 72, 1461–1471. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-M.; Yoon, B.-S.; Chung, J.-H.; Lee, S.-K.; Hwang, J.-K.; Ryang, R. Antioxidant Properties of Tannic Acid and its Inhibitory Effects on Paraquat-Induced Oxidative Stress in Mice. Korean Soc. Food Sci. Technol. 2006, 15, 728–734. [Google Scholar]
- Taner, G.; Özkan Vardar, D.; Aydin, S.; Aytaç, Z.; Başaran, A.; Başaran, N. Use of in vitro assays to assess the potential cytotoxic, genotoxic and antigenotoxic effects of vanillic and cinnamic acid. Drug Chem. Toxicol. 2017, 40, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Lee, C.Y. Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit. Rev. Food Sci. Nutr. 2004, 44, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, G.; Nishanthi, E.; Sharmila, R. Nephroprotective effect of vanillic acid against cisplatin induced nephrotoxicity in wistar rats: A biochemical and molecular study. Environ. Toxicol. Pharmacol. 2015, 39, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Verma, S.; Prabha, R. Investigations on Antioxidant Potential of Phenolic Acids and Flavonoids: The Common Phytochemical Ingredients in Plants. J. Plant Biochem. Physiol. 2018, 6. [Google Scholar] [CrossRef]
- Choi, K.H.; Nam, K.C.; Lee, S.Y.; Cho, G.; Jung, J.S.; Kim, H.J.; Park, B.J. Antioxidant potential and antibacterial efficiency of caffeic acid-functionalized ZnO nanoparticles. Nanomaterials 2017, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Chavarria, D.; Silva, T.; Martins, D.; Bravo, J.; Summavielle, T.; Garrido, J.; Borges, F. Exploring cinnamic acid scaffold: Development of promising neuroprotective lipophilic antioxidants. Medchemcomm 2015, 6, 1043–1053. [Google Scholar] [CrossRef]
- Bittner Fialová, S.; Kurin, E.; Trajčíková, E.; Jánošová, L.; Šušaníková, I.; Tekeľová, D.; Nagy, M.; Mučaji, P. Mentha Rhizomes as an Alternative Source of Natural Antioxidants. Molecules 2020, 25, 200. [Google Scholar] [CrossRef]
- Jitareanu, A.; Tataringa, G. Cinnamic acid Derivatives and 4-Aminoantipyrine Amides–Synthesis and Evaluation of Biological Properties. Res. J. Chem. Sci 2013, 3, 9–13. [Google Scholar]
- Kindl, M.; Blažeković, B.; Bucar, F.; Vladimir-Knežević, S. Antioxidant and anticholinesterase potential of six thymus species. Evid.-Based Complementary Altern. Med. 2015, 2015, 403950. [Google Scholar] [CrossRef]
- Thygesen, L.; Thulin, J.; Mortensen, A.; Skibsted, L.H.; Molgaard, P. Antioxidant activity of cichoric acid and alkamides from Echinacea purpurea, alone and in combination. Food Chem. 2007, 101, 74–81. [Google Scholar] [CrossRef]
- Blažeković, B.; Vladimir-Knežević, S.; Brantner, A.; Štefan, M.B. Evaluation of antioxidant potential of Lavandula x intermedia Emeric ex Loisel. “Budrovka”: A comparative study with L. angustifolia Mill. Molecules 2010, 15, 5971–5987. [Google Scholar] [CrossRef] [PubMed]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Benedec, D.; Hanganu, D.; Oniga, I.; Tiperciuc, B.; Olah, N.-K.; Raita, O.; Bischin, C.; Silaghi-Dumitrescu, R.; Vlase, L. Assessment of rosmarinic acid content in six Lamiaceae species extracts and their antioxidant and antimicrobial potential. Pak. J. Pharm. Sci. 2015, 28, 2297–2303. [Google Scholar] [PubMed]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Sidoryk, K.; Jaromin, A.; Filipczak, N.; Cmoch, P.; Cybulski, M. Synthesis and antioxidant activity of caffeic acid derivatives. Molecules 2018, 23, 2199. [Google Scholar] [CrossRef] [PubMed]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. Vitr. 2017, 40, 248–255. [Google Scholar] [CrossRef]
- Swisłocka, R.; Regulska, E.; Karpinska, J.; Swiderski, G.; Lewandowski, W. Molecular structure and antioxidant properties of alkali metal salts of rosmarinic acid. Experimental and DFT studies. Molecules 2019, 24, 2645. [Google Scholar] [CrossRef]
- Guleria, S.; Tiku, A.K.; Singh, G.; Koul, A.; Gupta, S.; Rana, S. In vitro antioxidant activity and phenolic contents in methanol extracts from medicinal plants. J. Plant Biochem. Biotechnol. 2013, 22, 9–15. [Google Scholar] [CrossRef]
- Ertas, A.; Boga, M.; Yilmaz, M.A.; Yesil, Y.; Tel, G.; Temel, H.; Hasimi, N.; Gazioglu, I.; Ozturk, M.; Ugurlu, P. A detailed study on the chemical and biological profiles of essential oil and methanol extract of Thymus nummularius (Anzer tea): Rosmarinic acid. Ind. Crops Prod. 2015, 67, 336–345. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, M.; Yang, B.; Shen, G.; Rao, G. Identification of bioactive compounds in Phyllenthus emblica L. fruit and their free radical scavenging activities. Food Chem. 2009, 114, 499–504. [Google Scholar] [CrossRef]
- Son, S.; Lewis, B.A. Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: Structure-activity relationship. J. Agric. Food Chem. 2002, 50, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Ho, C.T. Antioxidant Activities of Caffeic Acid and Its Related Hydroxycinnamic Acid Compounds. J. Agric. Food Chem. 1997, 45, 2374–2378. [Google Scholar] [CrossRef]
- Bacanll, M.; Aydln, S.; Taner, G.; Göktaş, H.G.; Şahin, T.; Başaran, A.A.; Başaran, N. Does rosmarinic acid treatment have protective role against sepsis-induced oxidative damage in Wistar Albino rats? Hum. Exp. Toxicol. 2016, 35, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.; Moon, S.; Chang, Y.; Ko, J.; Lee, Y.; Cho, G.; Kim, S.; Kim, J.; Kim, C. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: Complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004, 18, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S.; Lau, C.H.; Chew, C.Y.; Ismail, N.I.M.; Soontorngun, N. Phytochemical profile of Orthosiphon aristatus extracts after storage: Rosmarinic acid and other caffeic acid derivatives. Phytomedicine 2018, 39, 49–55. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Lin, J.; Li, Y.; Wang, T.; Jiang, Q.; Chen, D. Sarcandra glabra (Caoshanhu) protects mesenchymal stem cells from oxidative stress: A bioevaluation and mechanistic chemistry. BMC Complementary Altern. Med. 2016, 16. [Google Scholar] [CrossRef]
- Berker, K.I.; Demirata, B.; Apak, R. Determination of Total Antioxidant Capacity of Lipophilic and Hydrophilic Antioxidants in the Same Solution by Using Ferric-Ferricyanide Assay. Food Anal. Methods 2012, 5, 1150–1158. [Google Scholar] [CrossRef]
- Yildiz, L.; Başkan, K.S.; Tütem, E.; Apak, R. Combined HPLC-CUPRAC (cupric ion reducing antioxidant capacity) assay of parsley, celery leaves, and nettle. Talanta 2008, 77, 304–313. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Determination of antioxidants by a novel on-line HPLC-cupric reducing antioxidant capacity (CUPRAC) assay with post-column detection. Anal. Chim. Acta 2010, 674, 79–88. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Kadoma, Y.; Fujisawa, S. A comparative study of the radical-scavenging activity of the phenolcarboxylic acids caffeic acid, p-coumaric acid, chlorogenic acid and ferulic acid, with or without 2-mercaptoethanol, a thiol, using the induction period method. Molecules 2008, 13, 2488–2499. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Yadav, K.; Amist, N. Positive effects of nitric oxide on Solanum lycopersicum. J. Plant Interact. 2014, 9, 10–18. [Google Scholar] [CrossRef]
- Amist, N.; Singh, N.B. Comparative Effects of Benzoic Acid and Water Stress on Wheat Seedlings. Russ. J. Plant Physiol. 2018, 65, 709–716. [Google Scholar] [CrossRef]
- Ekinci Akdemir, F.N.E.; Albayrak, M.; Çalik, M.; Bayir, Y.; Gülçin, I. The protective effects of p-Coumaric acid on acute liver and kidney damages induced by cisplatin. Biomedicines 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lei, Z.; Zhong, Q.; Wu, W.; Zhang, H.; Min, T.; Wu, H.; Lai, F. Enrichment of caffeic acid in peanut sprouts and evaluation of its in vitro effectiveness against oxidative stress-induced erythrocyte hemolysis. Food Chem. 2017, 217, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, M.; Kalavagunta, P.K.; Li, J.; He, Q.; Zhang, Y.; Ahmad, O.; Yin, H.; Wang, T.; Shang, J. Protective effects of cichoric acid on H2O2-induced oxidative injury in hepatocytes and larval zebrafish models. Biomed. Pharmacother. 2018, 104, 679–685. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, F.; Zhang, L.; Niu, Y.; Liu, Z.; Liu, X. Comparison of chicoric acid, and its metabolites caffeic acid and caftaric acid: In vitro protection of biological macromolecules and inflammatory responses in BV2 microglial cells. Food Sci. Hum. Wellness 2017, 6, 155–166. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Hirshfield, I.N.; Terzulli, S.; O’Byrne, C. Weak organic acids: A panoply of effects on bacteria. Sci. Prog. 2003, 86, 245–269. [Google Scholar] [CrossRef]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wasik, T.J. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.; Benfeito, S.; Soares, P.; Magalhães e Silva, D.; Loureiro, J.; Borges, A.; Borges, F.; Simões, M. Fine-tuning of the hydrophobicity of caffeic acid: Studies on the antimicrobial activity against Staphylococcus aureus and Escherichia coli. RSC Adv. 2015, 5, 53915–53925. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N. The potential of systems biology to discover antibacterial mechanisms of plant phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Aldulaimi, O.A. General overview of phenolics from plant to laboratory, good antibacterials or not. Pharmacogn. Rev. 2017, 11, 123–127. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Muñoz-Cazares, N.; García-Contreras, R.; Pérez-López, M.; Castillo-Juárez, I. Phenolic Compounds with Anti-virulence Properties. In Phenolic Compounds—Biological Activity; InTech: London, UK, 2017; pp. 139–167. [Google Scholar]
- Rico-Munoz, E.; Bargiota, E.E.; Davidson, P.M. Effect of selected phenolic compounds on the membrane-bound adenosine triphosphatase of Staphylococcus aureus. Food Microbiol. 1987, 4, 239–249. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial synergy between rosmarinic acid and antibiotics against methicillin-resistant Staphylococcus aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Slobodníková, L.; Fialová, S.; Hupková, H.; Grančai, D. Rosmarinic acid interaction with planktonic and biofilm Staphylococcus aureus. Nat. Prod. Commun. 2013, 8, 1747–1750. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Doble, M. Synergistic interaction of phenylpropanoids with antibiotics against bacteria. J. Med. Microbiol. 2010, 59, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, F.; Hequet, A.; Voisin-Chiret, A.S.; Bouillon, A.; Lesnard, A.; Cresteil, T.; Jolivalt, C.; Rault, S. First identification of boronic species as novel potential inhibitors of the Staphylococcus aureus NorA efflux pump. J. Med. Chem. 2014, 57, 2536–2548. [Google Scholar] [CrossRef]
- Kubo, I.; Xiao, P.; Nihei, K.I.; Fujita, K.I.; Yamagiwa, Y.; Kamikawa, T. Molecular design of antifungal agents. J. Agric. Food Chem. 2002, 50, 3992–3998. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Andersen, P.C.; Marois, J.J.; Wright, D.L.; Srivastava, M.; Harmon, P.F. Effect of phenolic compounds on growth and ligninolytic enzyme production in Botryosphaeria isolates. Crop Prot. 2013, 43, 146–156. [Google Scholar] [CrossRef]
- Chen, F.C.; Peng, C.F.; Tsai, I.L.; Chen, I.S. Antitubercular constituents from the stem wood of Cinnamomum kotoense. J. Nat. Prod. 2005, 68, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Podobnik, B.; Stojan, J.; Lah, L.; Kraševec, N.; Seliškar, M.; Rižner, T.L.; Rozman, D.; Komel, R. CYP53A15 of Cochliobolus lunatus, a target for natural antifungal compounds. J. Med. Chem. 2008, 51, 3480–3486. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sood, S.; Sharma, N.; Rahi, P.; Kumar, R.; Sinha, A.K.; Gulati, A. Synthesis and SAR investigation of natural phenylpropene-derived methoxylated cinnamaldehydes and their novel Schiff bases as potent antimicrobial and antioxidant agents. Med. Chem. Res. 2013, 22, 5129–5140. [Google Scholar] [CrossRef]
- Li, X.; Sheng, J.; Huang, G.; Ma, R.; Yin, F.; Song, D.; Zhao, C.; Ma, S. Design, synthesis and antibacterial activity of cinnamaldehyde derivatives as inhibitors of the bacterial cell division protein FtsZ. Eur. J. Med. Chem. 2015, 97, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.D.; Mortazavi, P.N.; Munshi, T.; Evangelopoulos, D.; McHugh, T.D.; Gibbons, S.; Malkinson, J.; Bhakta, S. 2-Hydroxy-substituted cinnamic acids and acetanilides are selective growth inhibitors of Mycobacterium tuberculosis. Medchemcomm 2014, 5, 47–50. [Google Scholar] [CrossRef]
- Fu, J.; Cheng, K.; Zhang, Z.M.; Fang, R.Q.; Zhu, H.L. Synthesis, structure and structure-activity relationship analysis of caffeic acid amides as potential antimicrobials. Eur. J. Med. Chem. 2010, 45, 2638–2643. [Google Scholar] [CrossRef] [PubMed]
- De Vita, D.; Friggeri, L.; D’Auria, F.D.; Pandolfi, F.; Piccoli, F.; Panella, S.; Palamara, A.T.; Simonetti, G.; Scipione, L.; Di Santo, R.; et al. Activity of caffeic acid derivatives against Candida albicans biofilm. Bioorganic Med. Chem. Lett. 2014, 24, 1502–1505. [Google Scholar] [CrossRef]
- Georgiev, L.; Chochkova, M.; Totseva, I.; Seizova, K.; Marinova, E.; Ivanova, G.; Ninova, M.; Najdenski, H.; Milkova, T. Anti-tyrosinase, antioxidant and antimicrobial activities of hydroxycinnamoylamides. Med. Chem. Res. 2013, 22, 4173–4182. [Google Scholar] [CrossRef]
- Takahashi, T.; Miyazawa, M. Synthesis and structure-activity relationships of serotonin derivatives effect on α-glucosidase inhibition. Med. Chem. Res. 2012, 21, 1762–1770. [Google Scholar] [CrossRef]
- Takahashi, T.; Miyazawa, M. Synthesis and structure-activity relationships of phenylpropanoid amides of serotonin on tyrosinase inhibition. Bioorganic Med. Chem. Lett. 2011, 21, 1983–1986. [Google Scholar] [CrossRef]
- Mencherini, T.; Picerno, P.; Scesa, C.; Aquino, R. Triterpene, antioxidant, and antimicrobial compounds from Melissa officinalis. J. Nat. Prod. 2007, 70, 1889–1894. [Google Scholar] [CrossRef]
- Mencherini, T.; Picerno, P.; Russo, P.; Meloni, M.; Aquino, R. Composition of the fresh leaves and stems of Melissa officinalis and evaluation of skin irritation in a reconstituted human epidermis model. J. Nat. Prod. 2009, 72, 1512–1515. [Google Scholar] [CrossRef]
- Šilhár, P.; Lardy, M.A.; Hixon, M.S.; Shoemaker, C.B.; Barbieri, J.T.; Struss, A.K.; Lively, J.M.; Javor, S.; Janda, K.D. C-Terminus of botulinum a protease has profound and unanticipated kinetic consequences upon the catalytic cleft. ACS Med. Chem. Lett. 2013, 4, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Document Report Card. Available online: https://www.ebi.ac.uk/chembl/document_report_card/CHEMBL1201862/ (accessed on 5 April 2020).
- Sammar, M.; Abu-Farich, B.; Rayan, I.; Falah, M.; Rayan, A. Correlation between cytotoxicity in cancer cells and free radical-scavenging activity: In vitro evaluation of 57 medicinal and edible plant extracts. Oncol. Lett. 2019, 18, 6563–6571. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.X.; Li, W.X.; Han, F.F.; Guo, Y.C.; Zheng, J.J.; Liu, J.Q.; Wang, Q.; Gao, Y.D.; Li, G.H.; Huang, J.F. In silico identification of anti-cancer compounds and plants from traditional Chinese medicine database. Sci. Rep. 2016, 6, 25462. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Kim, C.S.; Subedi, L.; Kwon, O.W.; Park, H.B.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Wasabisides A-E, lignan glycosides from the roots of Wasabia japonica. J. Nat. Prod. 2016, 79, 2652–2657. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Mallareddy, A.; Suresh, P.; Lakshma Nayak, V.; Shetti, R.V.C.R.N.C.; Sankara Rao, N.; Tamboli, J.R.; Shaik, T.B.; Vishnuvardhan, M.V.P.S.; Ramakrishna, S. Synthesis and anticancer activity of 4β-alkylamidochalcone and 4β-cinnamido linked podophyllotoxins as apoptotic inducing agents. Eur. J. Med. Chem. 2012, 47, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Bruyre, C.; Genovese, S.; Lallemand, B.; Ionescu-Motatu, A.; Curini, M.; Kiss, R.; Epifano, F. Growth inhibitory activities of oxyprenylated and non-prenylated naturally occurring phenylpropanoids in cancer cell lines. Bioorganic Med. Chem. Lett. 2011, 21, 4174–4179. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, R.; Riveiro, M.E.; Vermeulen, M.; Alonso, E.; Mondillo, C.; Facorro, G.; Piehl, L.; Gómez, N.; Moglioni, A.; Fernández, N.; et al. Structure-anti-leukemic activity relationship study of ortho-dihydroxycoumarins in U-937 cells: Key role of the δ-lactone ring in determining differentiation-inducing potency and selective pro-apoptotic action. Bioorganic Med. Chem. 2012, 20, 5537–5549. [Google Scholar] [CrossRef]
- Wang, S.Y.; Mao, W.W.; She, Z.G.; Li, C.R.; Yang, D.Q.; Lin, Y.C.; Fu, L.W. Synthesis and biological evaluation of 12 allenic aromatic ethers. Bioorganic Med. Chem. Lett. 2007, 17, 2785–2788. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Z.; Li, X.; Wang, A.; Wu, H.; Liu, J.; Cao, S.; Liu, Q. Salviachinensines A-F, Antiproliferative Phenolic Derivatives from the Chinese Medicinal Plant Salvia chinensis. J. Nat. Prod. 2018, 81, 2531–2538. [Google Scholar] [CrossRef]
- Ben Salem, S.; Jabrane, A.; Harzallah-Skhiri, F.; Ben Jannet, H. New bioactive dihydrofuranocoumarins from the roots of the Tunisian Ferula lutea (Poir.) Maire. Bioorganic Med. Chem. Lett. 2013, 23, 4248–4252. [Google Scholar] [CrossRef] [PubMed]
- Iraji, A.; Firuzi, O.; Khoshneviszadeh, M.; Tavakkoli, M.; Mahdavi, M.; Nadri, H.; Edraki, N.; Miri, R. Multifunctional iminochromene-2H-carboxamide derivatives containing different aminomethylene triazole with BACE1 inhibitory, neuroprotective and metal chelating properties targeting Alzheimer’s disease. Eur. J. Med. Chem. 2017, 141, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Koufaki, M.; Theodorou, E.; Galaris, D.; Nousis, L.; Katsanou, E.S.; Alexis, M.N. Chroman/catechol hybrids: Synthesis and evaluation of their activity against oxidative stress induced cellular damage. J. Med. Chem. 2006, 49, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.J.; Le Lamer, A.C.; Castillo, D.; Arevalo, J.; Rojas, R.; Odonne, G.; Bourdy, G.; Moukarzel, B.; Sauvain, M.; Fabre, N. Caffeic acid esters and lignans from Piper sanguineispicum. J. Nat. Prod. 2010, 73, 1884–1890. [Google Scholar] [CrossRef]
- Zheng, H.C.; Lu, Y.; Chen, D.F. Anticomplement compounds from Polygonum chinense. Bioorganic Med. Chem. Lett. 2018, 28, 1495–1500. [Google Scholar] [CrossRef]
- Tani, H.; Hasumi, K.; Tatefuji, T.; Hashimoto, K.; Koshino, H.; Takahashi, S. Inhibitory activity of Brazilian green propolis components and their derivatives on the release of cys-leukotrienes. Bioorganic Med. Chem. 2010, 18, 151–157. [Google Scholar] [CrossRef]
- Bailly, F.; Toillon, R.A.; Tomavo, O.; Jouy, N.; Hondermarck, H.; Cotelle, P. Antiproliferative and apoptotic effects of the oxidative dimerization product of methyl caffeate on human breast cancer cells. Bioorganic Med. Chem. Lett. 2013, 23, 574–578. [Google Scholar] [CrossRef]
- Sanderson, J.T.; Clabault, H.; Patton, C.; Lassalle-Claux, G.; Jean-François, J.; Paré, A.F.; Hébert, M.J.G.; Surette, M.E.; Touaibia, M. Antiproliferative, antiandrogenic and cytotoxic effects of novel caffeic acid derivatives in LNCaP human androgen-dependent prostate cancer cells. Bioorganic Med. Chem. 2013, 21, 7182–7193. [Google Scholar] [CrossRef]
- Cai, H.; Huang, X.; Xu, S.; Shen, H.; Zhang, P.; Huang, Y.; Jiang, J.; Sun, Y.; Jiang, B.; Wu, X.; et al. Discovery of novel hybrids of diaryl-1,2,4-triazoles and caffeic acid as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase for cancer therapy. Eur. J. Med. Chem. 2016, 108, 89–103. [Google Scholar] [CrossRef]
- Park, S.H.; Oh, H.S.; Kang, M.A.; Cho, H.; Prasad, J.B.; Won, J.; Lee, K.H. The structure-activity relationship of the series of non-peptide small antagonists for p56lck SH2 domain. Bioorganic Med. Chem. 2007, 15, 3938–3950. [Google Scholar] [CrossRef]
- Kim, J.; Song, S.; Lee, I.; Kim, Y.; Yoo, I.; Ryoo, I.; Bae, K. Anti-inflammatory activity of constituents from Glechoma hederacea var. longituba. Bioorganic Med. Chem. Lett. 2011, 21, 3483–3487. [Google Scholar] [CrossRef]
- Ye, X.; Yu, S.; Liang, Y.; Huang, H.; Lian, X.Y.; Zhang, Z. Bioactive triterpenoid saponins and phenolic compounds against glioma cells. Bioorganic Med. Chem. Lett. 2014, 24, 5157–5163. [Google Scholar] [CrossRef]
- Lee, M.A.; Kim, W.K.; Park, H.J.; Kang, S.S.; Lee, S.K. Anti-proliferative activity of hydnocarpin, a natural lignan, is associated with the suppression of Wnt/β-catenin signaling pathway in colon cancer cells. Bioorganic Med. Chem. Lett. 2013, 23, 5511–5514. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Spíchal, L.; Grúz, J.; Kadlecová, A.; Voller, J.; Svobodová, A.R.; Vostálová, J.; Ulrichová, J.; Doležal, K.; et al. New cytokinin derivatives possess UVA and UVB photoprotective effect on human skin cells and prevent oxidative stress. Eur. J. Med. Chem. 2018, 150, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Bailly, F.; Mbemba, G.; Mouscadet, J.F.; Debyser, Z.; Witvrouw, M.; Cotelle, P. Reaction of rosmarinic acid with nitrite ions in acidic conditions: Discovery of nitro- and dinitrorosmarinic acids as new anti-HIV-1 agents. J. Med. Chem. 2008, 51, 2575–2579. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Neamati, N.; Zhao, H.; Kiryu, Y.; Turpin, J.A.; Aberham, C.; Strebel, K.; Kohn, K.; Witvrouw, M.; Pannecouque, C.; et al. Chicoric acid analogues as HIV-1 integrase inhibitors. J. Med. Chem. 1999, 42, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Queffélec, C.; Bailly, F.; Mbemba, G.; Mouscadet, J.F.; Debyser, Z.; Witvrouw, M.; Cotelle, P. The total synthesis of fukiic acid, an HIV-1 integrase inhibitor. Eur. J. Med. Chem. 2008, 43, 2268–2271. [Google Scholar] [CrossRef]
- Russo, G.I.; Campisi, D.; Mauro, M.D.; Regis, F.; Reale, G.; Marranzano, M.; Ragusa, R.; Solinas, T.; Madonia, M.; Cimino, S.; et al. Dietary consumption of phenolic acids and prostate cancer: A case-control study in sicily, Southern Italy. Molecules 2017, 22, 2159. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, A.H.; Perks, C.M.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J.M.P.; Jernström, H. Caffeine and caffeic acid inhibit growth and modify estrogen receptor and insulin-like growth factor i receptor levels in human breast cancer. Clin. Cancer Res. 2015, 21, 1877–1887. [Google Scholar] [CrossRef]
- Ganmaa, D.; Willett, W.C.; Li, T.Y.; Feskanich, D.; Van Dam, R.M.; Lopez-Garcia, E.; Hunter, D.J.; Holmes, M.D. Coffee, tea, caffeine and risk of breast cancer: A 22-year follow-up. Int. J. Cancer 2008, 122, 2071–2076. [Google Scholar] [CrossRef]
- Alicandro, G.; Tavani, A.; La Vecchia, C. Coffee and cancer risk: A summary overview. Eur. J. Cancer Prev. 2017, 26, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Bøhn, S.K.; Blomhoff, R.; Paur, I. Coffee and cancer risk, epidemiological evidence, and molecular mechanisms. Mol. Nutr. Food Res. 2014, 58, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Birosová, L.; Mikulásová, M.; Vaverková, S. Antimutagenic effect of phenolic acids. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 2005, 149, 489–491. [Google Scholar] [CrossRef]
- Fadillioglu, E.; Oztas, E.; Erdogan, H.; Yagmurca, M.; Sogut, S.; Ucar, M.; Irmak, M.K. Protective effects of caffeic acid phenethyl ester on doxorubicin-induced cardiotoxicity in rats. J. Appl. Toxicol. 2004, 24, 47–52. [Google Scholar] [CrossRef]
- Tavares, D.C.; Lira, W.M.; Santini, C.B.; Takahashi, C.S.; Bastos, J.K. Effects of propolis crude hydroalcoholic extract on chromosomal aberrations induced by doxorubicin in rats. Planta Med. 2007, 73, 1531–1536. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Liu, L.; Cheng, X.L.; Cai, J.; Zhou, J.; Wang, T. Anticancer effects of Rosmarinic acid in OVCAR-3 ovarian cancer cells are mediated via induction of apoptosis, suppression of cell migration and modulation of lncRNA MALAT-1 expression. J. BUON 2018, 23, 763–768. [Google Scholar]
- Han, Y.; Ma, L.; Zhao, L.; Feng, W.; Zheng, X. Rosmarinic inhibits cell proliferation, invasion and migration via up-regulating miR-506 and suppressing MMP2/16 expression in pancreatic cancer. Biomed. Pharmacother. 2019, 115, 108878. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Reddy, D.B.; Padukudru, M.A.; Chitturi, C.M.K.; Vimalambike, M.G.; Madhunapantula, S.R.V. Induction of colon and cervical cancer cell death by cinnamic acid derivatives is mediated through the inhibition of Histone Deacetylases (HDAC). PLoS ONE 2017, 12, e0186208. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhang, J.; Jiang, Q.; Zhang, L.L.; Song, W. Zinc binding groups for histone deacetylase inhibitors. J. Enzyme Inhib. Med. Chem. 2018, 33, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [PubMed]
- Bora-Tatar, G.; Dayangaç-Erden, D.; Demir, A.S.; Dalkara, S.; Yelekçi, K.; Erdem-Yurter, H. Molecular modifications on carboxylic acid derivatives as potent histone deacetylase inhibitors: Activity and docking studies. Bioorganic Med. Chem. 2009, 17, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Waldecker, M.; Kautenburger, T.; Daumann, H.; Busch, C.; Schrenk, D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 2008, 19, 587–593. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Singh, R.; Westfall, S.; Herman, F.; Faith, J.; Ho, L. The Role of the Gut Microbiota in the Metabolism of Polyphenols as Characterized by Gnotobiotic Mice. J. Alzheimer’s Dis. 2018, 63, 409–421. [Google Scholar] [CrossRef]
- Haldar, S.; Lee, S.H.; Tan, J.J.; Chia, S.C.; Henry, C.J.; Chan, E.C.Y. Dose-dependent increase in unconjugated cinnamic acid concentration in plasma following acute consumption of polyphenol rich curry in the polyspice study. Nutrients 2018, 10, 934. [Google Scholar] [CrossRef]
- Zhu, B.; Shang, B.; Li, Y.; Zhen, Y. Inhibition of histone deacetylases by trans-cinnamic acid and its antitumor effect against colon cancer xenografts in athymic mice. Mol. Med. Rep. 2016, 13, 4159–4166. [Google Scholar] [CrossRef]
- Zambonin, L.; Caliceti, C.; Vieceli Dalla Sega, F.; Fiorentini, D.; Hrelia, S.; Landi, L.; Prata, C. Dietary phenolic acids act as effective antioxidants in membrane models and in cultured cells, exhibiting proapoptotic effects in leukaemia cells. Oxid. Med. Cell. Longev. 2012, 2012, 839298. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is a question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Raza, M.H.; Siraj, S.; Arshad, A.; Waheed, U.; Aldakheel, F.; Alduraywish, S.; Arshad, M. ROS-modulated therapeutic approaches in cancer treatment. J. Cancer Res. Clin. Oncol. 2017, 143, 1789–1809. [Google Scholar] [CrossRef]
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant activity of phenolic acids and their metabolites: Synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef] [PubMed]
- Valadão Vicente, S.J.; Ishimoto, E.Y.; Cruz, R.J.; Seabra Pereira, C.D.; Torres, E.A.F.D.S. Increase of the activity of phase II antioxidant enzymes in rats after a single dose of coffee. J. Agric. Food Chem. 2011, 59, 10887–10892. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-T.; Yen, G.-C. Induction of Hepatic Antioxidant Enzymes by Phenolic Acids in Rats Is Accompanied by Increased Levels of Multidrug Resistance–Associated Protein 3 mRNA Expression. J. Nutr. 2006, 136, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Uesawa, Y.; Sakagami, H.; Okudaira, N.; Toda, K.; Takao, K.; Kagaya, H.; Sugita, Y. Quantitative structure-cytotoxicity relationship of cinnamic acid phenetyl esters. Anticancer Res. 2018, 38, 817–823. [Google Scholar] [CrossRef]
- Fiuza, S.M.; Gomes, C.; Teixeira, L.J.; Girão Da Cruz, M.T.; Cordeiro, M.N.D.S.; Milhazes, N.; Borges, F.; Marques, M.P.M. Phenolic acid derivatives with potential anticancer properties—A structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorganic Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef]
- Li, F.; Awale, S.; Tezuka, Y.; Kadota, S. Cytotoxicity of constituents from Mexican propolis against a panel of six different cancer cell lines. Nat. Prod. Commun. 2010, 5, 1601–1606. [Google Scholar] [CrossRef]
- Phan, H.T.T.; Yoda, T.; Chahal, B.; Morita, M.; Takagi, M.; Vestergaard, M.C. Structure-dependent interactions of polyphenols with a biomimetic membrane system. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2670–2677. [Google Scholar] [CrossRef]
- Sirk, T.W.; Brown, E.F.; Sum, A.K.; Friedman, M. Molecular dynamics study on the biophysical interactions of seven green tea catechins with lipid bilayers of cell membranes. J. Agric. Food Chem. 2008, 56, 7750–7758. [Google Scholar] [CrossRef]
- Kruszewski, M.A.; Kotyńska, J.; Kusaczuk, M.; Gál, M.; Naumowicz, M. The modulating effect of p-coumaric acid on the surface charge density of human glioblastoma cell membranes. Int. J. Mol. Sci. 2019, 20, 5286. [Google Scholar] [CrossRef]
- Jin, X.L.; Wei, X.; Qi, F.M.; Yu, S.S.; Zhou, B.; Bai, S. Characterization of hydroxycinnamic acid derivatives binding to bovine serum albumin. Org. Biomol. Chem. 2012, 10, 3424–3431. [Google Scholar] [CrossRef]
- Kanagaratnam, R.; Sheikh, R.; Alharbi, F.; Kwon, D.H. An efflux pump (MexAB-OprM) of Pseudomonas aeruginosa is associated with antibacterial activity of Epigallocatechin-3-gallate (EGCG). Phytomedicine 2017, 36, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Tóth, I.V.; Rangel, A.O.S.S.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Shamsi, A.; Shahbaaz, M.; Queen, A.; Khan, P.; Hasan, G.M.; Islam, A.; Alajmi, M.F.; Hussain, A.; Ahmad, F.; et al. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci. Rep. 2020, 10, 10300. [Google Scholar] [CrossRef] [PubMed]
- Kuban-Jankowska, A.; Sahu, K.K.; Gorska, M.; Tuszynski, J.A.; Wozniak, M. Chicoric acid binds to two sites and decreases the activity of the YopH bacterial virulence factor. Oncotarget 2016, 7, 2229–2238. [Google Scholar] [CrossRef]
- Deshmukh, P.; Unni, S.; Krishnappa, G.; Padmanabhan, B. The Keap1–Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017, 9, 41–56. [Google Scholar] [CrossRef]

| Plant | Total Phenols | BA | CinA | p-CA | CFA | RA | ChA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [mg GAE/g DW] | [mg/g DW] | |||||||||||||
| Cinnamon —Cinnamomum verum J.Presl—Lauraceae | 5.82 ± 0.44 | [21] | 0.461 | [15] | _ | 0.0022 | [21] | 0.153 e | [19] | 0.00073 | [21] | _ | ||
| 54.40–391.99 * | [25] | 0.00045 | [21] | |||||||||||
| —Cinnamomum cassia (L.) J.Presl—Lauraceae | _ | _ | 0.01–1.91 | [14] | _ | _ | _ | _ | ||||||
| Rosemary —Rosmarinus officinalis L.—Lamiaceae | 5.02 ± 0.43 | [21] | n.s. | [25] | _ | 0.0056 | [21] | L: 41.42 ± 51 *,a R: 112.40 ± 51 * | [26] | L: 15.14 ± 19 *,a R: 156.61 ± 65 *,a | [26] | _ | ||
| 0.401 e | [19] | 12.86 e | [19] | |||||||||||
| 0.0126 | [21] | 0.157 | [21] | |||||||||||
| 4.06 | [18] | 328 ± 16.9 ** | [27] | |||||||||||
| 29.5 ± 1.2 ** | [27] | |||||||||||||
| Thyme —Thymus vulgaris L.—Lamiaceae | 3.36 ± 0.14 | [21] | 0.015–0.050 | [15] | _ | 0.0027 | [21] | L: 179.65 ± 8 *,a R: 67.00 ± 4 *,a F: 82.27 ± 12 * | [26] | L: 392.21 ± 1 *,a R: 104.20 ± 12 *,a F: 104.20 ± 3 * | [26] | _ | ||
| n.s. | [25] | 0.548 e | [19] | 6.81 e | [19] | |||||||||
| 0.0066 | [21] | 0.084 | [21] | |||||||||||
| 5.17 | [18] | 918 ± 27.5 ** | [27] | |||||||||||
| 117 ± 10.4 ** | [27] | |||||||||||||
| Oregano —Origanum vulgare L.—Lamiaceae | 2.23 ± 0.18 | [21] | ≤LOQ | [25] | _ | 0.0049 | [21] | 0.500 e | [19] | 0.0520 | [21] | |||
| 0.0106 | [21] | 25.63 e | [19] | |||||||||||
| 0.500 e | [19] | |||||||||||||
| 6.49 | [18] | |||||||||||||
| 39.56 ± 0.42 b 9.73 ± 0.07 c 56.83 ± 1.65 d 22.12 ± 0.30 e | [28] | 0.109 ± 0.002 b 0.054 ± 0.002 c 0.365 ± 1.050 d 0.065 ± 0.003 e | [28] | 0.172 ± 0.010 b 0.258 ± 0.019 c 0.367 ± 0.008 d 0.180 ± 0.008 e | [28] | 7.599 ± 0.115 b 4.303 ± 0.113 c 19.269 ± 1.035 d 6.958 ± 0.071 e | [28] | 0.323 ± 0.830 b 0.160 ± 0.004 c 0.910 ± 0.040 d 0.355 ± 0.007 e | [28] | |||||
| —Origanum acutidens L.—Lamiaceae | _ | _ | _ | 0.068 ± 0.003 b 0.002 ± 0.001 c 0.267 ± 0.011 d ND e | [28] | 0.024 ± 0.002 b 0.478 ± 0.015 c 0.092 ± 0.003 d 0.096 ± 0.012 e | [28] | 0.392 ± 0.012 b 4.858 ± 0.435 c 6.951 ± 0.539 d 0.525 ± 0.004 e | [28] | NDb 0.003 ± 0.001 c ND d ND e | [28] | |||
| Basil —Ocimum basilicum L.—Lamiaceae | L: ≤ LOQ –45.69 * S: ≤ LOQ * | [25] | _ | _ | 0.204 e | [19] | 10.86 e | [19] | ||||||
| fresh | L: 6.516 R: 2.234 | [24] | _ | _ | _ | _ | L: 1.386 R: 0.376 | [24] | L: 0.370 R: 0.075 | [24] | ||||
| dry | 4.236 | [24] | _ | _ | _ | _ | 0.557 | [24] | 0.107 | [24] | ||||
| Caraway —Carum carvi L.—Apiaceae | _ | ≤LOQ * | [25] | _ | 0.0106 | [29] | 0.164 | [19] | _ | _ | ||||
| 0.010 | [29] | |||||||||||||
| Turmeric —Curcuma longa L.—Zingiberaceae | _ | 0.003–0.005 | [15] | _ | 0.0011 | [29] | _ | _ | _ | |||||
| ≤LOQ–71.47 * | [25] | |||||||||||||
| Marjoram —Origanum majorana L.—Lamiaceae | _ | _ | _ | _ | 0.0002 | [29] | _ | _ | ||||||
| —Origanum x majoricum—Lamiaceae | _ | _ | 104 ± 2.7 ** | [27] | 1546 ± 32.9 ** | [27] | _ | _ | ||||||
| Pepper —Piper nigrum L. —Piperaceae | _ | 0.003–0.005 | [15] | _ | _ | _ | _ | _ | ||||||
| ≤LOQ–25.90 * | [25] | |||||||||||||
| Cumin —Cuminum cyminum L.—Apiaceae | 4.98 ± 0.31 | [21] | _ | _ | 0.00074 | [21] | 0.0031 | [21] | 0.0033 | [21] | _ | |||
| Bay —Laurus nobilis L.—Lauraceae | 1.12 ± 0.08 | [21] | _ | _ | 0.0096 | [21] | 0.0004 | [21] | 0.00039 | [21] | _ | |||
| Sage —Salvia officinalis L.—Lamiaceae | _ | _ | _ | 1.215 e | [19] | 21.86 e | [19] | _ | ||||||
| 2.96 | [18] | 1178 ± 10.1 ** | [27] | |||||||||||
| 74.2 ± 3.5 ** | [27] | |||||||||||||
| Melisa —Melisa officinalis L.—Lamiaceae | _ | _ | _ | _ | 8.580 | [18] | _ | _ | ||||||
| Mint —Mentha canadensis L.—Lamiaceae | _ | _ | _ | _ | 0.271 e | [19] | 19.085 e | [19] | _ | |||||
| Echinacea purpurea L.—Asteraceae fresh | 4.441 | [24] | _ | _ | _ | _ | _ | 2.423 | [24] | |||||
| extract | 1640 * | [24] | _ | _ | _ | _ | _ | 323 * | [24] | |||||
| Nutmeg —Myristica fragrans Houtt.—Myristicaceae | _ | 0.217 | [15] | _ | _ | 0.163 e | [19] | _ | _ | |||||
| ≤LOQ * | [25] | |||||||||||||
| Parsley —Petroselinum crispum L.—Apiaceae | _ | <0.001 | [15] | _ | _ | 1.037 e | [19] | _ | _ | |||||
| ≤LOQ–4.11 * | [25] | |||||||||||||
| White pepper —Piper nigrum L.—Piperaceae | _ | 0.001–0.003 | [15] | _ | _ | _ | _ | _ | ||||||
| ≤LOQ | [25] | |||||||||||||
| Acid | BA | CinA | p-CA | CFA | RA | ChA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mw | 122.12 | [30] | 148.16 | [30] | 164.16 | [30] | 180.16 | [30] | 360.31 | [30] | 474.37 | [30] | ||||||
| pKa | 4.19 | 1 | [31] | 4.44 | 1 | [32,33] | 4.64 | 1 | [34] | 4.36 | 1 | [35] | 3.57 | 1 | [36] | |||
| 4.21 | 1 | [33] | 4.70 | 1 | [37] | 4.41 | 1 | [38] | 3.62 | 1 | [39] | |||||||
| 9.15 | 2 | [40] | 4.49 | 1 | [41] | |||||||||||||
| 9.50 | 2 | [34] | 8.48 | 2 | [35] | |||||||||||||
| 8.72 | 2 | [41] | ||||||||||||||||
| 8.85 | 2 | [40] | ||||||||||||||||
| >10 | 3 | [40] | ||||||||||||||||
| 11.17 | 3 | [35] | ||||||||||||||||
| 11.38 | 3 | [35] | ||||||||||||||||
| pKa calculated | 4.2 | 1 | [42] | 4.3 | 1 | [42] | 4.6 | 1 | [42] | 4.6 | 1 | [42] | 2.8 | 1 | [42] | 2.72 | 1 | [43] |
| 4.65 | 1 | [44] | 9.8 | 2 | [42] | 2.78 | 1 | [45] | ||||||||||
| 10.2 | 2 | [42] | 12.8 | 3 | [42] | 9.3 | 2 | [42] | ||||||||||
| 9.92 | 2 | [44] | 9.33 | 2 | [45] | |||||||||||||
| 9.8 | 3 | [42] | ||||||||||||||||
| 9.77 | 3 | [45] | ||||||||||||||||
| 12.3 | 4 | [42] | ||||||||||||||||
| 12.33 | 4 | [45] | ||||||||||||||||
| 12.6 | 5 | [42] | ||||||||||||||||
| 12.65 | 5 | [45] | ||||||||||||||||
| Log p | 1.87 | [46] | 2.13 | [46,47] | 1.46 | [48] | 1.15 | [48] | 1.60 | [49] | 0.72 | [49] | ||||||
| 2.03 | [50] | 2.08 | [51] | 1.79 | [47] | 1.63 | [52] | 1.23 | [43] | |||||||||
| Log p calculated | 1.89 | [42] | 2.41 | [42] | 1.88 | [42] | 1.42 | [42] | 1.70 | [42] | 3.02 | [43] | ||||||
| 3.48 | [43] | |||||||||||||||||
| HBA | 2 | [53] | 2 | [54] | 3 | [54] | 4 | [54,55] | 8 | [52] | 11 | [56] | ||||||
| 4 | [49] | 10 | [43] | |||||||||||||||
| 8 | [49] | |||||||||||||||||
| HBD | 1 | [53] | 1 | [54] | 2 | [54] | 3 | [54,55] | 5 | [52] | 6 | [56] | ||||||
| 4 | [49] | 4 | [49,43] |
| Method | BA | CinA | p-CA | CFA | RA | ChA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH, IC50 [µmol/L] | n.d. | >160 (CDPPH = 150 µmol/L; t = 30 min) | [74] | 3.96 ± 0.06 (CDPPH = 50 µmol/L; t = 30 min) | [75] | 0.17 (CDPPH = 304.3 µmol/L; t = 30 min) | [77] | 0.5 ± 0.03 (CDPPH = 1000 μmol/L, t = 30 min) | [79] | 8.6 ± 0.9 (CDPPH = 1000 µmol/L; t = 15 min) | [81] | |
| >250 (CDPPH = 250 µmol/L; t = 30 min) | [82] | 6.65 (CDPPH = n.d.; t = n.d.) | [83] | 0.97 (CDPPH = n.d.; t = n.d.) | [83] | 1.83 ± 0.08 (CDPPH = 100 μmol/L, t = 30 min) | [96] | 140 (CDPPH = 553 µmol/L; t = 15 min) | [97] | |||
| n.s. (CDPPH = 101 µmol/L; t = 30 min) | [87] | 163.1 (CDPPH = 304.3 µmol/L; t = 30 min) | [77] | 1.55 ± 0.22 (CDPPH = 500 µmol/L; t = 30 min) | [75] | 4.19 ± 0.19 (CDPPH = 100 μmol/L, t = 30 min) | [98] | |||||
| >250 (CDPPH = 250 µmol/L; t = 30 min) | [82] | 4.72 (CDPPH = 101 µmol/L; t = 30 min) | [5] | 6.33 (CDPPH = 55 μmol/L, t = 30 min) | [94] | |||||||
| 7817 ± 77 (CDPPH = 75 µmol/L; t = 30 min) | [91] | 13.3 (CDPPH = 101 µmol/L; t = 30 min) | [99] | 9.16 ± 0.31 (CDPPH = 0.254 µmol/L, t = n.d.) | [100] | |||||||
| 12,800 ± 100 (CDPPH = 50 µmol/L; t = 30 min) | [95] | 21.7 ± 0.2 (CDPPH = 6.85 µmol/L, t = 1–45 min) | [93] | 72.3 ± 3.3 (CDPPH = 200 µmol/L, t = 30 min) | [101] | |||||||
| n.s. | [62] | 32.2 (CDPPH = 100 µmol/L; t = n.d.) | [102] | 230 (CDPPH = 101 µmol/L, t = 15 min) | [103] | |||||||
| 35.2 ± 2.1 (CDPPH = 75 µmol/L; t = 30 min) | [91] | 381 ± 11 (CDPPH = 1000 µmol/L, t = 30 min) | [104] | |||||||||
| 50.0 (CDPPH = 355 µmol/L; t = 30 min) | [105] | 1210 (CDPPH = 100 μmol/L, t = 30 min) | [106] | |||||||||
| 155.3 (CDPPH = 250 µmol/L; t = 30 min | [82] | |||||||||||
| 1110 ± 10 (CDPPH = 63.4 µmol/L; t = 2 min) | [62] | |||||||||||
| DPPH radical scavenging activity [%] | 0 (CBA = 1000 µmol/L, CDPPH = 60 µmol/L; t = 30 min) | [70] | 0.5 (CCinA = 169 µmol/L; CDPPH = 100 µmol/L; t = 20 min) | [85] | 3.6 (Cp-CA = 152 µmol/L; CDPPH = 100 µmol/L; t = 20 min) | [85] | 28.5 (CCFA = 167 µmol/L; CDPPH = 100 µmol/L; t = 30 min) | [84] | 88.4 (CRA = 69 µmol/L; CDPPH = 100 µmol/L; t = 20 min) | [85] | 55.6 (CChA = 25 µmol/L; CDPPH = 200 µmol/L; methanol, λ = 517 nm, t = 30 min | [80] |
| ~4 (CBA = 15 µmol/L; CDPPH = 60 µmol/L; t = 30 min) | [71] | 2.06 — 3.25 (CCinA = 17—135 µmol/L; CDPPH = 1000 µmol/L; t = 30 min) | [107] | 5.3 ± 0.50 (Cp-CA = 167 µmol/L, CDPPH = 80 µmol/L; t = 10 min;) | [84] | 46.1 — 75.8 (CCFA = 1—5 µmol/L, CDPPH = n.d.; t = n.d.) | [83] | |||||
| ~35(CCinA = 1000 µmol/L, CDPPH = 60 µmol/L; t = 30 min) | [70] | ~27 (Cp-CA = 1000 µmol/L, CDPPH = 60 µmol/L; t = 30 min) | [70] | 47.8 (CCFA = 50 µmol/L; CDPPH = 0.25 µmol/L; t = 30 min) | [108] | |||||||
| 60.3 —62.5 (CCinA = 675 i 1350 µmol/L; CDPPH = 1000 µmol/L; t = 30 and 60 min) | [72] | 30.1—43.9 (Cp-CA = 1—5 µmol/L, CDPPH = n.d.; t = n.d.) | [83] | 17.5 ± 0.2 (CABTS = 2.45 mmol/L; t = 15 min) | [93] | |||||||
| 43.9 ± 9.2 (Cp-CA = 5000 µmol/L; CDPPH = n.d.; t = n.d.) | [83] | 100 ± 1 (CABTS = 7 mmol/L, t = 30 min) | [91] | |||||||||
| 55.6 (Cp-CA = 167 µmol/L CDPPH = 100 µmol/L; t = 30 min;) | [73] | 1010 ± 0 (CABTS = 30 µmol/L; t = 2 min) | [62] | |||||||||
| n.s. | [62] | 51.5 (CCFA = 20 µmol/L; CDPPH = 0.1 µmol/L; t = 30 min) | [109] | |||||||||
| 76.6 (CCFA = 25 µmol/L; CDPPH = 100 µmol/L; t = 20 min | [85] | |||||||||||
| 93.9 (CCFA = 111 µmol/L, CDPPH = 100 µmol/L, t = 30 min) | [76] | |||||||||||
| ABTS, IC50 [µmol/L] | n.d. | n.s. | [82] [87] [110] [111] | 50.0 ± 3.3 (CABTS = 7 mmol/L, t = 30 min) | [91] | 10.9 (CABTS = 7 mmol/L, t = 6 min) | [99] | 2.91 (CABTS = 7 mmol/L, t = 6 min) | [94] | n.d. | ||
| ABTS radical scavenging activity [%] | 0 (CBA = 5 mmol/L; CABTS = 0.15 mmol/L; t = n.d.) | n.d. | 51.7±0.41 (Cp-CA = 183 µmol/L; CABTS = 6 mmol/L; t = 10 min;) | [84] | 32.1% (CCFA = 167 µmol/L; CABTS = 6 mmol/L; t = 2 min) | [84] | n.d. | 49.1% (CChA = 105 µmol/L; CABTS = 7 mmol/L; t = 6 min) | [80] | |||
| <1% (CBA = 0.01 mmol/L; CABTS = 2.45-mmol/L; t = 7 min) | [90] | 47.98% (CCFA = 111 µmol/L; CABTS = 6 mmol/L, t = 2 min) | [92] | |||||||||
| 92.9% (CCFA = 111 µmol/L; CABTS = 7 mmol/L; t = 30 min) | [76] | |||||||||||
| FRAP [μmol Fe2+/L] | n.d. | n.d. | n.d. | 42 (C = 10 μmol/L; λ = 593 nm, t = 30 min) | [80] | 37.813 (λ = 596 nm, t = 15 min) | [104] | 82 (C = 10 μmol/L; λ = 593 nm, t = 30 min) | [80] | |||
| 180 (C = 500 µmol/L; λ = 593 nm, t = 30 min) | [80] | 145 (C = 500 µmol/L; λ = 593 nm, t = 30 min) | [80] | |||||||||
| FRAP, IC50 [µmol/L] | n.d. | >200,000 | [95] | 420 | [95] | 60 | [112] | 19.6 | [113] | n.d. | ||
| 120 | [95] | |||||||||||
| FRAP [%] | n.d. | n.d. | 4.6 (C = 167 µmol/L) | [84] | 30.8 (C = 167 µmol/L) | [84] | n.d. | n.d. | ||||
| CUPRAC [µmol Trolox equ./mg of pure compound] | n.d. | n.d. | 0.55 | [66] | 2.6 | [114] | 4.88 | [114] | ||||
| 0.55 | [115] | 2.89 | [66] | 4.95 | [116] | |||||||
| 2.89 | [115] | 5.65 | [115] | |||||||||
| 1.12 | [116] | 3.4 | [116] | n.d. | ||||||||
| CUPRAC [%] | n.d. | n.d. | 1.37 (C = 167 µmol/L) | [84] | 3.3 (C = 167 µmol/L) | [84] | n.d. | n.d. | ||||
| ORAC [µmol Trolox equ./mg of pure compound] | n.d. | n.d. | 1.67 | [62] | 2.75 | [62] | n.d. | n.d. | ||||
| 4.51 | [117] | 6.63 | [117] | |||||||||
| Nitric Oxide Radical Scavenging Assay, IC50 [µmol/L] | n.d. | n.d. | 17 | [118] | 0.5 | [118] | 43.49 | [96] | n.d. |
| Compound | Relation | Value [mmol/L] | Standard | Microorganism Strain | Source |
|---|---|---|---|---|---|
| BA | >= | 0.52 | MIC | Staphylococcus aureus | [144] |
| > | 1.05 | MIC | Staphylococcus aureus | [144] | |
| = | 6.55 | MIC | Saccharomyces cerevisiae | [145] | |
| = | 6.55 | MIC | Saccharomyces cerevisiae | [145] | |
| = | 6.55 | MIC | Saccharomyces cerevisiae | [145] | |
| = | 6.55 | MIC | Phellinus tremulae | [146] | |
| > | 13.10 | MIC | Saccharomyces cerevisiae | [145] | |
| > | 13.10 | MIC | Saccharomyces cerevisiae | [145] | |
| = | 0.33 | MIC | Mycobacterium tuberculosis | [147] | |
| = | 5.00 | MIC | Cochliobolus lunatus | [148] | |
| = | 5.00 | MIC | Lasiodiplodia theobromae | [146] | |
| = | 5.00 | MIC | Neofusicoccum ribis | [146] | |
| = | 5.00 | MIC | Diplodia seriata | [146] | |
| = | 5.00 | MIC | Botryosphaeria dothidea | [146] | |
| CinA | = | 3.37 | MIC | Aspergillus parasiticus | [149] |
| > | 0.86 | MIC | Streptococcus pyogenes | [150] | |
| > | 0.86 | MIC | Staphylococcus aureus | [150] | |
| = | 0.27 | MIC | Mycobacterium tuberculosis H37Rv | [151] | |
| = | 1.68 | MIC | Aspergillus niger | [149] | |
| = | 3.37 | MIC | Staphylococcus aureus | [149] | |
| > | 0.86 | MIC | Staphylococcus epidermidis | [150] | |
| = | 6.75 | MIC | Klebsiella pneumoniae | [149] | |
| = | 6.75 | MIC | Bacillus subtilis | [149] | |
| > | 0.86 | MIC | Pseudomonas aeruginosa | [150] | |
| > | 0.86 | MIC | Staphylococcus aureus | [150] | |
| = | 0.42 | MIC | Trichophyton rubrum | [149] | |
| > | 0.86 | MIC | Escherichia coli | [150] | |
| = | 0.84 | MIC | Issatchenkia orientalis | [149] | |
| = | 6.75 | MIC | Burkholderia cepacia | [149] | |
| = | 6.75 | MIC | Micrococcus luteus | [149] | |
| = | 6.75 | MIC | Enterobacter cloacae | [149] | |
| > | 0.86 | MIC | Bacillus subtilis | [150] | |
| > | 0.60 | MIC | Mycobacterium smegmatis str. MC2 155 | [151] | |
| = | 13.50 | MIC | Pseudomonas aeruginosa | [149] | |
| p-CA | > | 0.60 | MIC | Mycobacterium smegmatis str. MC2 155 | [151] |
| = | 5.00 | MIC | Diplodia seriata | [146] | |
| > | 305 | MIC | Bacillus subtilis | [152] | |
| = | 0.24 | MIC | Mycobacterium tuberculosis H37Rv | [151] | |
| = | 5.00 | MIC | Neofusicoccum ribis | [146] | |
| > | 305 | MIC | Candida albicans | [152] | |
| > | 305 | MIC | Staphylococcus aureus | [152] | |
| = | 5.00 | MIC | Botryosphaeria dothidea | [146] | |
| > | 305 | MIC | Pseudomonas fluorescens | [152] | |
| = | 0.37 | MIC | Mycobacterium bovis BCG | [151] | |
| CFA | > | 0.28 | MIC | Bacillus subtilis | [152] |
| > | 0.28 | MIC | Staphylococcus aureus | [152] | |
| = | 0.71 | MIC50 | Candida albicans | [153] | |
| = | 0.69 | MIC | Streptococcus pyogenes | [154] | |
| = | 0.69 | MIC | Staphylococcus aureus | [154] | |
| > | 0.30 | IC50 | Saccharomyces cerevisiae | [155] | |
| > | 0.28 | MIC | Pseudomonas fluorescens | [152] | |
| > | 0.35 | IC50 | Agaricus bisporus | [156] | |
| = | 1.47 | MIC50 | Candida albicans | [153] | |
| = | 0.28 | MIC50 | Candida albicans | [153] | |
| > | 0.28 | MIC | Candida albicans | [152] | |
| = | 0.71 | MIC50 | Candida albicans | [153] | |
| RA | > | 5556 | MIC | Aspergillus niger | [157] |
| = | 11111 | MIC | Aspergillus niger | [158] | |
| = | 333 | MBC | Bacillus subtilis subsp. spizizenii | [157] | |
| = | 11111 | MBC | Bacillus subtilis subsp. spizizenii | [158] | |
| = | 11111 | MIC | Bacillus subtilis subsp. spizizenii | [158] | |
| > | 5556 | MIC | Candida albicans | [157] | |
| = | 5556 | MIC | Candida albicans | [158] | |
| > | 5556 | MIC | Escherichia coli | [157] | |
| = | 333 | MBC | Escherichia coli | [157] | |
| > | 5556 | MIC | Pseudomonas aeruginosa | [157] | |
| = | 333 | MBC | Pseudomonas aeruginosa | [157] | |
| = | 11111 | MBC | Pseudomonas aeruginosa | [158] | |
| = | 5556 | MIC | Pseudomonas aeruginosa | [158] | |
| = | 333 | MIC | Staphylococcus aureus | [157] | |
| = | 333 | MBC | Staphylococcus aureus | [157] | |
| = | 333 | MBC | Staphylococcus aureus | [158] | |
| = | 333 | MIC | Staphylococcus aureus | [158] | |
| = | 333 | MIC | Staphylococcus epidermidis | [157] | |
| = | 333 | MBC | Staphylococcus epidermidis | [157] | |
| = | 2778 | MBC | Staphylococcus epidermidis | [158] | |
| = | 333 | MIC | Staphylococcus epidermidis | [158] | |
| D-ChA | = | 0.0039 | Ki | Clostridium botulinum * | [159] |
| = | 0.0067 | Ki | Clostridium botulinum # | [159] | |
| = | 0.0016 | Ki | Clostridium botulinum ** | [159] | |
| = | 0.0014 | Ki | Clostridium botulinum ## | [159] | |
| L-ChA | = | 0.0158 | Potency | Bacillus anthracis str. A2012 | [160] |
| Compound | Relation | Value | Unit | Standard | Assay | Cell Line | Source |
|---|---|---|---|---|---|---|---|
| BA | > | 10 | µmol/L | IC50 | Cytotoxicity against human cells after 48 h by SRB assay | BT-549 | [164] |
| > | 10 | µmol/L | IC50 | A549 | [164] | ||
| > | 10 | µmol/L | IC50 | SK-MEL-2 | [164] | ||
| > | 50 | µmol/L | IC50 | Antineuroinflammatory activity in mouse BV2 cells assessed as inhibition of LPS-induced nitric oxide production after 24 h by Griess assay | BV-2 | [164] | |
| > | 10 | µmol/L | IC50 | Cytotoxicity against human SKOV3 cells after 48 h by SRB assay | SK-OV-3 | [164] | |
| CinA | = | 64 | µmol/L | IC50 | Anticancer activity against human cells after 48 h by MTT assay | A-375 | [165] |
| = | 108 | µmol/L | IC50 | MCF7 | [165] | ||
| = | 91 | µmol/L | IC50 | ACHN | [165] | ||
| = | 87 | µmol/L | IC50 | A549 | [165] | ||
| = | 114 | µmol/L | IC50 | HT-29 | [165] | ||
| > | 100 | µmol/L | IC50 | Antineuroinflammatory activity in mouse BV2 cells assessed as inhibition of LPS-induced NO production after 24 h in presence of LPS by Griess assay | BV-2 | [165] | |
| p-CA | > | 100 | µmol/L | IC50 | Cytotoxicity against human SK-MEL-28 cells after 72 h by MTT assay | SK-MEL-28 | [166] |
| > | 100 | µmol/L | IC50 | Cytotoxicity against human A549 cells after 72 h by MTT assay | A549 | [166] | |
| > | 10 | µmol/L | IC50 | Cytotoxicity against human SKOV3 cells after 48 h by SRB assay | SK-OV-3 | [164] | |
| = | 10 | µmol/L | IC50 | Cytotoxicity against human BT549 cells after 48 h by SRB assay | BT-549 | [164] | |
| > | 2000 | µmol/L | IC50 | Antiproliferative activity against human U937 cells assessed as incorporation of methyl-3H-thymidine after 12 h by scintillation counting | U-937 | [167] | |
| > | 200 | µmol/L | IC50 | Antitumor activity against KB cells by MTT assay | KB | [168] | |
| = | 82 | µmol/L | IC50 | Cytotoxicity against human LoVo cells after 72 h by MTT assay | LoVo | [166] | |
| > | 10 | µmol/L | IC50 | Cytotoxicity against human A549 cells after 48 h by SRB assay | A549 | [164] | |
| > | 100 | µmol/L | IC50 | Cytotoxicity against human PC3 cells after 72 h by MTT assay | PC-3 | [166] | |
| > | 10 | µmol/L | IC50 | Cytotoxicity against human SK-MEL-2 cells after 48 h by SRB assay | SK-MEL-2 | [164] | |
| CFA | = | 317 | µmol/L | IC50 | Antiproliferative activity against human U937 cells assessed as incorporation of methyl-3H-thymidine after 12 h by scintillation counting | U-937 | [167] |
| = | 700 | µmol/L | IC50 | Cytotoxicity against human A549 cells assessed as reduction in cell viability measured after 48 h by luminescence-based ATP assay | A549 | [167] | |
| = | 6.4 | µmol/L | IC50 | Antiproliferative activity against human MOLM13 cells by CellTiter-Glo assay | MOLM-13 | [169] | |
| = | 500 | µmol/L | IC50 | Cytotoxicity against human A549 cells assessed as reduction in cell viability measured after 48 h by FMCA assay | A549 | [167] | |
| = | 30 | µmol/L | IC50 | Cytotoxicity against human HCT116 cells after 96 h by MTT assay | HCT-116 | [170] | |
| > | 10 | µmol/L | IC50 | Antiproliferative activity against human MV4-11 cells by CellTiter-Glo assay | MV4-11 | [169] | |
| = | 76 | µmol/L | IC50 | Neuroprotection against amyloid beta (25 to 35)-induced cell death in rat PC12 cells pre-incubated for 3 h followed by amyloid beta addition measured after 24 h by MTT assay | PC-12 | [171] | |
| - | - | - | IC50 | Activity against hydrogen peroxide induced DNA damage in Jurkat T cells | Jurkat | [172] | |
| = | 27 | µmol/L | IC50 | Cytotoxicity against human HT-29 cells after 96 h by MTT assay | HT-29 | [170] | |
| = | 700 | µmol/L | IC50 | Cytotoxicity against human A549 cells assessed as reduction in cell viability measured after 48 h by MTT assay | A549 | [167] | |
| > | 550 | µmol/L | IC50 | Cytotoxicity against African green monkey Vero cells assessed as [3H]-hypoxanthine incorporation after 48 h | Vero | [173] | |
| > | 550 | µmol/L | IC50 | Cytotoxicity against human MCF7 cells assessed as [3H]-hypoxanthine incorporation after 48 h | MCF7 | [173] | |
| = | 129 | µmol/L | IC50 | Cytotoxicity against human AGS cells after 96 h by MTT assay | AGS | [170] | |
| = | 940 | µmol/L | IC50 | Anticomplement activity in rabbit erythrocytes assessed as concentration required for 50% hemolytic inhibition by alternative pathway pre-treated for 10 min with normal human serum followed by erythrocyte addition measured after 30 min by spectrophotometric method | Erythrocyte | [174] | |
| = | 44.0 | µg/mL | IC50 | Antiallergic activity in Ca(2+)-stimulated differentiated human HeLa cells assessed as inhibition of cys-leukotriene release after 6 days by ELISA | HeLa | [175] | |
| = | 0.002 | µmol/L | IC50 | Antiproliferative activity against human T47D cells after 5 days by MTT assay | T47D | [176] | |
| = | 750 | µmol/L | IC50 | Anticomplement activity in sheep erythrocytes assessed as concentration required for 50% hemolytic inhibition by classic pathway pre-treated for 10 min with guinea pig serum followed by erythrocyte addition measured after 30 min by spectrophotometric method | Erythrocyte | [174] | |
| > | 100 | µmol/L | IC50 | Cytotoxicity against human LNCAP cells assessed as reduction in cell viability after 24 h by WST-1 assay | LNCaP | [177] | |
| > | 100 | µmol/L | IC50 | Antiproliferative activity against human A549 cells after 72 h by MTT assay | A549 | [178] | |
| > | 10 | µmol/L | IC50 | Antiproliferative activity against human MOLM14 cells by CellTiter-Glo assay | MOLM-14 | [169] | |
| RA | = | 40.4 | % | Inhibition | Inhibition of Jurkat cell activation assessed as blocking of T-cell antigen receptor-induced IL-2 expression at 10 µmol/L by luciferase assay | Jurkat | [179] |
| = | 50 | % | Inhibition | Inhibition of Jurkat cell activation assessed as blocking of T-cell antigen receptor-induced IL-2 expression at 30 µmol/L by luciferase assay | Jurkat | [179] | |
| - | - | - | Activity | Cytotoxicity against human HepG2 cells up to 20 µmol/L after 24 h by MTS assay | HepG2 | [180] | |
| = | 71 | % | Activity | Inhibition of cell proliferation of human U251 cells assessed as cell viability at 100 µmol/L after 72 h by SRB assay | U-251 | [181] | |
| = | 27 | % | Inhibition | Inhibition of Wnt/beta-catenin signaling pathway in human HEK293 cells at 20 µmol/L after 24 h by dual luciferase reporter gene assay relative to vehicle-treated control | HEK293 | [182] | |
| - | - | - | Activity | Cytoprotection against phototoxicity in human NHDF cells assessed as increase in cell viability at 3.9 to 31.3 µmol/L preincubated for 60 min followed by 7.5 J/cm2 UVA irradiation and measured after 24 h by neutral red uptake assay | NHDF | [183] | |
| - | - | - | Activity | Cytoprotection against phototoxicity in human HaCaT cells assessed as increase in cell viability at 3.9 µmol/L preincubated for 60 min followed by 10 J/cm2 UVA irradiation and measured after 24 h by neutral red uptake assay | HaCaT | [183] | |
| - | - | - | Activity | Cytoprotection against phototoxicity in human NHDF cells assessed as increase in cell viability at 3.9 to 31.3 µmol/L preincubated for 60 min followed by 150 mJ/cm2 UVB irradiation and measured after 24 h by neutral red uptake assay | NHDF | [183] | |
| - | - | - | Activity | Cytoprotection against phototoxicity in human HaCaT cells assessed as increase in cell viability at 3.9 to 31.3 µmol/L preincubated for 60 min followed by 10 J/cm2 UVA irradiation and measured after 24 h by neutral red uptake assay | HaCaT | [183] | |
| = | 2.9 | µmol/L | IC50 | Antiproliferative activity against human cells by CellTiter-Glo assay | MOLM-13 | [169] | |
| > | 10 | µmol/L | IC50 | MV4-11 | [169] | ||
| = | 7.1 | µmol/L | IC50 | MOLM-14 | [169] | ||
| = | 55 | µmol/L | CC50 | Cytotoxicity against human MT4 cells by MTT method | MT4 | [184] | |
| D-ChA | = | 39.7 | µmol/L | IC50 | Concentration of compound required to reduce MT-4 cell viability by 50% | MT4 | [185] |
| = | 35.5 | µmol/L | IC50 | Compound was evaluated for the cytoprotection of CEM-SS cells by XTT cytoprotection assay through the NCI AIDS Screen | CEM-SS | [185] | |
| L-ChA | = | 20.1 | µmol/L | IC50 | CEM-SS | [185] | |
| = | 45 | µmol/L | IC50 | Concentration of compound required to reduce MT-4 cell viability by 50% | MT4 | [185] | |
| D-ChA | = | 111 | µmol/L | CC50 | Cytotoxicity against human MT4 cells by MTT assay | MT4 | [186] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godlewska-Żyłkiewicz, B.; Świsłocka, R.; Kalinowska, M.; Golonko, A.; Świderski, G.; Arciszewska, Ż.; Nalewajko-Sieliwoniuk, E.; Naumowicz, M.; Lewandowski, W. Biologically Active Compounds of Plants: Structure-Related Antioxidant, Microbiological and Cytotoxic Activity of Selected Carboxylic Acids. Materials 2020, 13, 4454. https://doi.org/10.3390/ma13194454
Godlewska-Żyłkiewicz B, Świsłocka R, Kalinowska M, Golonko A, Świderski G, Arciszewska Ż, Nalewajko-Sieliwoniuk E, Naumowicz M, Lewandowski W. Biologically Active Compounds of Plants: Structure-Related Antioxidant, Microbiological and Cytotoxic Activity of Selected Carboxylic Acids. Materials. 2020; 13(19):4454. https://doi.org/10.3390/ma13194454
Chicago/Turabian StyleGodlewska-Żyłkiewicz, Beata, Renata Świsłocka, Monika Kalinowska, Aleksandra Golonko, Grzegorz Świderski, Żaneta Arciszewska, Edyta Nalewajko-Sieliwoniuk, Monika Naumowicz, and Włodzimierz Lewandowski. 2020. "Biologically Active Compounds of Plants: Structure-Related Antioxidant, Microbiological and Cytotoxic Activity of Selected Carboxylic Acids" Materials 13, no. 19: 4454. https://doi.org/10.3390/ma13194454
APA StyleGodlewska-Żyłkiewicz, B., Świsłocka, R., Kalinowska, M., Golonko, A., Świderski, G., Arciszewska, Ż., Nalewajko-Sieliwoniuk, E., Naumowicz, M., & Lewandowski, W. (2020). Biologically Active Compounds of Plants: Structure-Related Antioxidant, Microbiological and Cytotoxic Activity of Selected Carboxylic Acids. Materials, 13(19), 4454. https://doi.org/10.3390/ma13194454







