A Review of Non-Soil Biochar Applications
Abstract
:1. Introduction
2. Biochar Production Strategies
3. Non-Soil Biochar Applications
3.1. Environmental Remediation Applications
3.1.1. Inorganic Pollutants Removal
3.1.2. Organic Pollutants Removal
3.1.3. Gaseous Pollutants Removal
3.2. Energy Storage Applications
3.2.1. Biochar Used for Supercapacitor Production
3.2.2. Biochar Used for Batteries Production
3.2.3. Biochar Used for Fuel Cell Production
3.3. Biochar-Based Composites Production and Properties
3.3.1. Biochar–Inorganic-Based Composites
3.3.2. Biochar-Containing Reinforced Plastics
3.4. Other Uses of Biochar
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burchell, T.D. Carbon Materials for Advanced Technologies; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Holmes, M. Global carbon fibre market remains on upward trend. Reinf. Plast. 2014, 58, 38–45. [Google Scholar] [CrossRef]
- Association, I.C.B. What is Carbon Black? Available online: http://www.carbon-black.org/ (accessed on 17 December 2019).
- Endo, M. Carbon nanotube research: Past and future. Jpn. J. Appl. Phys. 2012, 51, 040001. [Google Scholar]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Segal, M. Selling graphene by the ton. Nat. Nanotechnol. 2009, 4, 612. [Google Scholar] [CrossRef]
- Tubes, C. Welcome To Cheap Tubes. Available online: https://www.cheaptubes.com/ (accessed on 9 October 2019).
- Alibaba.com. Market Price for Carbon Black n550. Available online: https://www.alibaba.com/product-detail/Market-price-for-carbon-black-n550_62080154763.html?spm=a2700.7724857.discountZoneStyleB_top.2.708e4ca9xSBebz (accessed on 14 November 2019).
- Bezama, A.; Agamuthu, P. Addressing the Big Issues in Waste Management; SAGE Publications Sage UK: London, UK, 2019. [Google Scholar]
- Mui, E.L.; Ko, D.C.; McKay, G. Production of active carbons from waste tyres––A review. Carbon 2004, 42, 2789–2805. [Google Scholar] [CrossRef]
- Couth, R.; Trois, C. Carbon emissions reduction strategies in Africa from improved waste management: A review. Waste Manag. 2010, 30, 2336–2346. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Damartzis, T.; Zabaniotou, A. Thermochemical conversion of biomass to second generation biofuels through integrated process design—A review. Renew. Sustain. Energy Rev. 2011, 15, 366–378. [Google Scholar] [CrossRef]
- Amen-Chen, C.; Pakdel, H.; Roy, C. Production of monomeric phenols by thermochemical conversion of biomass: A review. Bioresour. Technol. 2001, 79, 277–299. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Nieto, A.; Méndez, A.; Askeland, M.P.J.; Gascó, G. Biochar from Biosolids Pyrolysis: A Review. Int. J. Environ. Res. Public Health 2018, 15, 956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Energy, F. Biochar: Prospects of Commercialization. Available online: https://farm-energy.extension.org/ (accessed on 14 November 2019).
- Vochozka, M.; Maroušková, A.; Váchal, J.; Straková, J. Biochar pricing hampers biochar farming. Clean Technol. Environ. Policy 2016, 18, 1225–1231. [Google Scholar] [CrossRef]
- Maroušek, J. Significant breakthrough in biochar cost reduction. Clean Technol. Envrion. Policy 2014, 16, 1821–1825. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef]
- Maroušek, J.; Strunecký, O.; Stehel, V. Biochar farming: Defining economically perspective applications. Clean Technol. Environ. Policy 2019, 1389–1395. [Google Scholar] [CrossRef]
- Maroušek, J.; Kolář, L.; Vochozka, M.; Stehel, V.; Maroušková, A. Biochar reduces nitrate level in red beet. Environ. Sci. Pollut. Res. 2018, 25, 18200–18203. [Google Scholar] [CrossRef]
- Abdullah, H.; Wu, H. Biochar as a fuel: 1. Properties and grindability of biochars produced from the pyrolysis of mallee wood under slow-heating conditions. Energy Fuels 2009, 23, 4174–4181. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Gronnow, M.J.; Budarin, V.L.; Mašek, O.; Crombie, K.N.; Brownsort, P.A.; Shuttleworth, P.S.; Hurst, P.R.; Clark, J.H. Torrefaction/biochar production by microwave and conventional slow pyrolysis–comparison of energy properties. Gcb Bioenergy 2013, 5, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Bach, Q.-V.; Chen, W.-H.; Chu, Y.-S.; Skreiberg, Ø. Predictions of biochar yield and elemental composition during torrefaction of forest residues. Bioresour. Technol. 2016, 215, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, M.; Rosi, L.; Giovannelli, A.; Frediani, P.; Frediani, M. Characterization of bio-oil and bio-char produced by low-temperature microwave-assisted pyrolysis of olive pruning residue using various absorbers. Waste Manag. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, M.; Rosi, L.; Giovannelli, A.; Frediani, P.; Frediani, M. Bio-oil from residues of short rotation coppice of poplar using a microwave assisted pyrolysis. J. Anal. Appl. Pyrolysis 2016, 119, 224–232. [Google Scholar] [CrossRef]
- Bartoli, M.; Rosi, L.; Giovannelli, A.; Frediani, P.; Frediani, M. Pyrolysis of α-cellulose in a microwave multimode batch reactor. J. Anal. Appl. Pyrolysis 2016, 120, 284–296. [Google Scholar] [CrossRef]
- Bartoli, M.; Rosi, L.; Giovannelli, A.; Frediani, P.; Frediani, M. Production of bio-oils and bio-char from Arundo donax through microwave assisted pyrolysis in a multimode batch reactor. J. Anal. Appl. Pyrolysis 2016, 122, 479–489. [Google Scholar] [CrossRef]
- Wampler, T.P. Applied Pyrolysis Handbook; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Bridgwater, A.; Peacocke, G. Fast pyrolysis processes for biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Ragucci, R.; Giudicianni, P.; Cavaliere, A. Cellulose slow pyrolysis products in a pressurized steam flow reactor. Fuel 2013, 107, 122–130. [Google Scholar] [CrossRef]
- Tsai, W.; Lee, M.; Chang, Y. Fast pyrolysis of rice straw, sugarcane bagasse and coconut shell in an induction-heating reactor. J. Anal. Appl. Pyrolysis 2006, 76, 230–237. [Google Scholar] [CrossRef]
- Karaduman, A.; Şimşek, E.H.; Çiçek, B.; Bilgesü, A.Y. Flash pyrolysis of polystyrene wastes in a free-fall reactor under vacuum. J. Anal. Appl. Pyrolysis 2001, 60, 179–186. [Google Scholar] [CrossRef]
- Li, D.; Briens, C.; Berruti, F. Improved lignin pyrolysis for phenolics production in a bubbling bed reactor—Effect of bed materials. Bioresour. Technol. 2015, 189, 7–14. [Google Scholar] [CrossRef]
- Horne, P.A.; Williams, P.T. Influence of temperature on the products from the flash pyrolysis of biomass. Fuel 1996, 75, 1051–1059. [Google Scholar] [CrossRef]
- Behrendt, F.; Neubauer, Y.; Oevermann, M.; Wilmes, B.; Zobel, N. Direct Liquefaction of Biomass. Chem. Eng. Technol. 2008, 31, 667–677. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S. Effects of various solvents on the liquefaction of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2008, 49, 3498–3504. [Google Scholar] [CrossRef]
- Zou, S.; Wu, Y.; Yang, M.; Li, C.; Tong, J. Thermochemical catalytic liquefaction of the marine microalgae Dunaliella tertiolecta and characterization of bio-oils. Energy Fuels 2009, 23, 3753–3758. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub-and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Mok, W.S.L.; Antal, M.J., Jr. Uncatalyzed solvolysis of whole biomass hemicellulose by hot compressed liquid water. Ind. Eng. Chem. Res. 1992, 31, 1157–1161. [Google Scholar] [CrossRef]
- Zhong, C.; Wei, X. A comparative experimental study on the liquefaction of wood. Energy 2004, 29, 1731–1741. [Google Scholar] [CrossRef]
- Guizani, C.; Sanz, F.E.; Salvador, S. Influence of temperature and particle size on the single and mixed atmosphere gasification of biomass char with H 2 O and CO 2. Fuel Process. Technol. 2015, 134, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Barisano, D.; Canneto, G.; Nanna, F.; Alvino, E.; Pinto, G.; Villone, A.; Carnevale, M.; Valerio, V.; Battafarano, A.; Braccio, G. Steam/oxygen biomass gasification at pilot scale in an internally circulating bubbling fluidized bed reactor. Fuel Process. Technol. 2016, 141, 74–81. [Google Scholar] [CrossRef]
- Cheah, S.; Jablonski, W.S.; Olstad, J.L.; Carpenter, D.L.; Barthelemy, K.D.; Robichaud, D.J.; Andrews, J.C.; Black, S.K.; Oddo, M.D.; Westover, T.L. Effects of thermal pretreatment and catalyst on biomass gasification efficiency and syngas composition. Green Chem. 2016, 18, 6291–6304. [Google Scholar] [CrossRef]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. Energy 2009, 28, 386–396. [Google Scholar] [CrossRef]
- Lv, G.; Wu, S.; Yang, G.; Chen, J.; Liu, Y.; Kong, F. Comparative study of pyrolysis behaviors of corn stalk and its three components. J. Anal. Appl. Pyrolysis 2013, 104, 185–193. [Google Scholar] [CrossRef]
- Truong, H.B.; Ike, I.A.; Ok, Y.S.; Hur, J. Polyethyleneimine modification of activated fly ash and biochar for enhanced removal of natural organic matter from water via adsorption. Chemosphere 2020, 243, 125454–125483. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Sui, Z.; Zhang, Y.; Sun, B.; Pan, W.-P. Enhanced mercury removal by transplanting sulfur-containing functional groups to biochar through plasma. Fuel 2019, 253, 703–712. [Google Scholar] [CrossRef]
- Seckler, D.W. World Water Demand and Supply, 1990 to 2025: Scenarios and Issues; Iwmi: Colombo, Sri Lanka, 1998; Volume 19. [Google Scholar]
- Connor, R. Wastewater: The Untapped Resource; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2017. [Google Scholar]
- Ramalho, R. Introduction to Wastewater Treatment Processes; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Cassivi, A.; Guilherme, S.; Bain, R.; Tilley, E.; Waygood, E.O.D.; Dorea, C. Drinking water accessibility and quantity in low and middle-income countries: A systematic review. Int. J. Hyg. Environ. Health 2019, 222, 1011–1020. [Google Scholar] [CrossRef]
- Goel, P. Water Pollution: Causes, Effects and Control; New Age International: Delhi, India, 2006. [Google Scholar]
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Hillie, T.; Hlophe, M. Nanotechnology and the challenge of clean water. Nat. Nanotechnol. 2007, 2, 663. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Gowd, S.S.; Govil, P.K. Distribution of heavy metals in surface water of Ranipet industrial area in Tamil Nadu, India. Environ. Monit. Assess. 2008, 136, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, K.-H.; Yan, X.; Chen, S.-J.; Hu, G.-C.; Peng, X.-W.; Yuan, J.-G.; Mai, B.-X.; Yang, Z.-Y. Heavy metals in food, house dust, and water from an e-waste recycling area in South China and the potential risk to human health. Ecotoxicol. Environ. Saf. 2013, 96, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Szymanowska, A.; Samecka-Cymerman, A.; Kempers, A. Heavy metals in three lakes in West Poland. Ecotoxicol. Environ. Saf. 1999, 43, 21–29. [Google Scholar] [CrossRef]
- Karadede, H.; Ünlü, E. Concentrations of some heavy metals in water, sediment and fish species from the Atatürk Dam Lake (Euphrates), Turkey. Chemosphere 2000, 41, 1371–1376. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Wong, H.K.; Lawson, G.; Daniel, P. Saturation of ecosystems with toxic metals in Sudbury basin, Ontario, Canada. Sci. Total Environ. 1998, 223, 99–117. [Google Scholar] [CrossRef]
- Ikem, A.; Egiebor, N.; Nyavor, K. Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern USA. Waterairsoil Pollut. 2003, 149, 51–75. [Google Scholar] [CrossRef]
- Huggins, T.M.; Haeger, A.; Biffinger, J.C.; Ren, Z.J. Granular biochar compared with activated carbon for wastewater treatment and resource recovery. Water Res. 2016, 94, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Arán, D.; Antelo, J.; Fiol, S.; Macías, F. Influence of feedstock on the copper removal capacity of waste-derived biochars. Bioresour. Technol. 2016, 212, 199–206. [Google Scholar] [CrossRef]
- Towill, L.E.; Shriner, C.; Drury, J.; Hammons, A.; Holleman, J. Reviews of the Environmental Effects of Pollutants. III. Chromium; Oak Ridge National Lab.: Oak Ridge, TN, USA, 1978. [Google Scholar]
- Rengaraj, S.; Yeon, K.-H.; Moon, S.-H. Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. 2001, 87, 273–287. [Google Scholar] [CrossRef]
- Brum, M.C.; Capitaneo, J.L.; Oliveira, J.F. Removal of hexavalent chromium from water by adsorption onto surfactant modified montmorillonite. Miner. Eng. 2010, 23, 270–272. [Google Scholar] [CrossRef]
- Gifford, M.; Hristovski, K.; Westerhoff, P. Ranking traditional and nano-enabled sorbents for simultaneous removal of arsenic and chromium from simulated groundwater. Sci. Total Environ. 2017, 601, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Joshi, S.R.; Mandal, T.; Halder, G. Application of zirconium caged activated biochar alginate beads towards deionization of Cr(VI) laden water in a fixed bed column reactor. J. Environ. Chem. Eng. 2018, 6, 4018–4029. [Google Scholar] [CrossRef]
- Zhou, L.; Deng, H.; Wan, J.; Shi, J.; Su, T. A solvothermal method to produce RGO-Fe3O4 hybrid composite for fast chromium removal from aqueous solution. Appl. Surf. Sci. 2013, 283, 1024–1031. [Google Scholar] [CrossRef]
- Liang, S.; Shi, S.; Zhang, H.; Qiu, J.; Yu, W.; Li, M.; Gan, Q.; Yu, W.; Xiao, K.; Liu, B.; et al. One-pot solvothermal synthesis of magnetic biochar from waste biomass: Formation mechanism and efficient adsorption of Cr(VI) in an aqueous solution. Sci. Total Environ. 2019, 695, 133886. [Google Scholar] [CrossRef]
- Shi, S.; Yang, J.; Liang, S.; Li, M.; Gan, Q.; Xiao, K.; Hu, J. Enhanced Cr(VI) removal from acidic solutions using biochar modified by Fe3O4@SiO2-NH2 particles. Sci. Total Environ. 2018, 628, 499–508. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Y.; Tang, J.; He, J.; Sun, H. Aqueous Cr(VI) removal by a novel ball milled Fe0-biochar composite: Role of biochar electron transfer capacity under high pyrolysis temperature. Chemosphere 2019. [Google Scholar] [CrossRef]
- Xu, X.; Huang, H.; Zhang, Y.; Xu, Z.; Cao, X. Biochar as both electron donor and electron shuttle for the reduction transformation of Cr(VI) during its sorption. Environ. Pollut. 2019, 244, 423–430. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Liu, J.; Liu, J.; Xia, F.; Wang, C.; Dahlgren, R.A.; Liu, W. Mechanism of Cr(VI) removal by magnetic greigite/biochar composites. Sci. Total Environ. 2019. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef]
- Álvarez-Rogel, J.; Tercero Gómez, M.d.C.; Conesa, H.M.; Párraga-Aguado, I.; González-Alcaraz, M.N. Biochar from sewage sludge and pruning trees reduced porewater Cd, Pb and Zn concentrations in acidic, but not basic, mine soils under hydric conditions. J. Environ. Manag. 2018, 223, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, S.; Chiodo, V.; Crea, F.; Maisano, S.; Milea, D.; Pettignano, A. Biochar from byproduct to high value added material—A new adsorbent for toxic metal ions removal from aqueous solutions. J. Mol. Liq. 2018, 271, 481–489. [Google Scholar] [CrossRef]
- Chen, Z.-L.; Zhang, J.-Q.; Huang, L.; Yuan, Z.-H.; Li, Z.-J.; Liu, M.-C. Removal of Cd and Pb with biochar made from dairy manure at low temperature. J. Integr. Agric. 2019, 18, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Huang, S.; Laird, D.A.; Wang, X.; Meng, Z. Adsorption behaviour and mechanisms of cadmium and nickel on rice straw biochars in single- and binary-metal systems. Chemosphere 2019, 218, 308–318. [Google Scholar] [CrossRef]
- Hadjittofi, L.; Charalambous, S.; Pashalidis, I. Removal of trivalent samarium from aqueous solutions by activated biochar derived from cactus fibres. J. Rare Earths 2016, 34, 99–104. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Wnętrzak, R.; Leahy, J.J.; Hayes, M.H.B.; Kwapiński, W.; Hubicki, Z. Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 2012, 197, 295–305. [Google Scholar] [CrossRef]
- Pal, D.; Maiti, S.K. Abatement of cadmium (Cd) contamination in sediment using tea waste biochar through meso-microcosm study. J. Clean. Prod. 2019, 212, 986–996. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Guo, L.; Zhang, Y.; Lou, Z.; Wang, Y.; Qian, G. Cu(II) removal from aqueous solution by Spartina alterniflora derived biochar. Bioresour. Technol. 2013, 141, 83–88. [Google Scholar] [CrossRef]
- Tong, X.; Xu, R. Removal of Cu(II) from acidic electroplating effluent by biochars generated from crop straws. J. Environ. Sci. 2013, 25, 652–658. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Lv, Q. Influence of Pyrolysis Temperature on Cadmium Removal Capacity and Mechanism by Maize Straw and Platanus Leaves Biochars. Int. J. Environ. Res. Public Health 2019, 16, 845. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Shan, B.; Tang, W.; Zhu, Y. Comparison of cadmium and lead sorption by Phyllostachys pubescens biochar produced under a low-oxygen pyrolysis atmosphere. Bioresour. Technol. 2017, 238, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Xiang, L.; Hu, H.; Fu, Q.; Zhu, J.; Liu, Y.; Huang, G. High-efficiency removal capacities and quantitative sorption mechanisms of Pb by oxidized rape straw biochars. Sci. Total Environ. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liatsou, I.; Pashalidis, I.; Oezaslan, M.; Dosche, C. Surface characterization of oxidized biochar fibers derived from Luffa Cylindrica and lanthanide binding. J. Environ. Chem. Eng. 2017, 5, 4069–4074. [Google Scholar] [CrossRef]
- Feng, Z.; Chen, N.; Feng, C.; Fan, C.; Wang, H.; Deng, Y.; Gao, Y. Roles of functional groups and irons on bromate removal by FeCl3 modified porous carbon. Appl. Surf. Sci. 2019, 488, 681–687. [Google Scholar] [CrossRef]
- Zhu, L.; Tong, L.; Zhao, N.; Li, J.; Lv, Y. Coupling interaction between porous biochar and nano zero valent iron/nano α-hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution. Chemosphere 2019, 219, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ji, Y.; Dang, J.; Zhao, J.; Chen, S. Magnetic apple pomace biochar: Simple preparation, characterization, and application for enriching Ag(I) in effluents. Sci. Total Environ. 2019, 668, 115–123. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Zhou, J.; Guo, J.; Ren, J.; Zhou, F. Efficient removal of lead from aqueous solution by urea-functionalized magnetic biochar: Preparation, characterization and mechanism study. J. Taiwan Inst. Chem. Eng. 2018, 91, 457–467. [Google Scholar] [CrossRef]
- Guo, J.; Yan, C.; Luo, Z.; Fang, H.; Hu, S.; Cao, Y. Synthesis of a novel ternary HA/Fe-Mn oxides-loaded biochar composite and its application in cadmium(II) and arsenic(V) adsorption. J. Environ. Sci. 2019, 85, 168–176. [Google Scholar] [CrossRef]
- Hu, R.; Xiao, J.; Wang, T.; Chen, G.; Chen, L.; Tian, X. Engineering of phosphate-functionalized biochars with highly developed surface area and porosity for efficient and selective extraction of uranium. Chem. Eng. J. 2020, 379, 122388. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Liu, T.; Liu, Y.; Yan, L.; Wei, Q.; Du, B.; Xu, W. EDTA functionalized magnetic biochar for Pb(II) removal: Adsorption performance, mechanism and SVM model prediction. Sep. Purif. Technol. 2019, 227, 115696. [Google Scholar] [CrossRef]
- Lyu, H.; Xia, S.; Tang, J.; Zhang, Y.; Gao, B.; Shen, B. Thiol-modified biochar synthesized by a facile ball-milling method for enhanced sorption of inorganic Hg2+ and organic CH3Hg+. J. Hazard. Mater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jellali, S.; Diamantopoulos, E.; Haddad, K.; Anane, M.; Durner, W.; Mlayah, A. Lead removal from aqueous solutions by raw sawdust and magnesium pretreated biochar: Experimental investigations and numerical modelling. J. Environ. Manag. 2016, 180, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-J.; Dong, C.-D.; Huang, Y.-H.; Huang, C.P. Electro-sorption of ammonium ion onto nickel foam supported highly microporous activated carbon prepared from agricultural residues (dried Luffa cylindrica). Sci. Total Environ. 2019, 673, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, C.; Zhang, M. Chapter 8-Biochar for Anionic Contaminants Removal From Water. In Biochar from Biomass and Waste; Ok, Y.S., Tsang, D.C.W., Bolan, N., Novak, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–160. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Gaston, L.A.; Zhou, B.; Li, M.; Xiao, R.; Wang, Q.; Zhang, Z.; Huang, H.; Liang, W.; et al. An overview of carbothermal synthesis of metal–biochar composites for the removal of oxyanion contaminants from aqueous solution. Carbon 2018, 129, 674–687. [Google Scholar] [CrossRef]
- Vikrant, K.; Kim, K.-H.; Ok, Y.S.; Tsang, D.C.W.; Tsang, Y.F.; Giri, B.S.; Singh, R.S. Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci. Total Environ. 2018, 616, 1242–1260. [Google Scholar] [CrossRef]
- Bacelo, H.; Pintor, A.M.A.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Golterman, H. Natural phosphate sources in relation to phosphate budgets: A contribution to the understanding of eutrophication. Phosphorus Fresh Water Mar. Environ. Prog. Water Technol. 1973, 2, 3–17. [Google Scholar]
- Smolders, A.; Lamers, L.; Lucassen, E.; Van der Velde, G.; Roelofs, J. Internal eutrophication: How it works and what to do about it—A review. Chem. Ecol. 2006, 22, 93–111. [Google Scholar] [CrossRef]
- Correll, D.L. The role of phosphorus in the eutrophication of receiving waters: A review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Dieguez-Alonso, A.; Anca-Couce, A.; Frišták, V.; Moreno-Jiménez, E.; Bacher, M.; Bucheli, T.D.; Cimò, G.; Conte, P.; Hagemann, N.; Haller, A.; et al. Designing biochar properties through the blending of biomass feedstock with metals: Impact on oxyanions adsorption behavior. Chemosphere 2019, 214, 743–753. [Google Scholar] [CrossRef]
- Trazzi, P.A.; Leahy, J.J.; Hayes, M.H.B.; Kwapinski, W. Adsorption and desorption of phosphate on biochars. J. Environ. Chem. Eng. 2016, 4, 37–46. [Google Scholar] [CrossRef]
- Jung, K.-W.; Jeong, T.-U.; Kang, H.-J.; Chang, J.-S.; Ahn, K.-H. Preparation of modified-biochar from Laminaria japonica: Simultaneous optimization of aluminum electrode-based electro-modification and pyrolysis processes and its application for phosphate removal. Bioresour. Technol. 2016, 214, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Ahn, K.-H. Fabrication of porosity-enhanced MgO/biochar for removal of phosphate from aqueous solution: Application of a novel combined electrochemical modification method. Bioresour. Technol. 2016, 200, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Jeong, T.-U.; Hwang, M.-J.; Kim, K.; Ahn, K.-H. Phosphate adsorption ability of biochar/Mg–Al assembled nanocomposites prepared by aluminum-electrode based electro-assisted modification method with MgCl2 as electrolyte. Bioresour. Technol. 2015, 198, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-W.; Jeong, T.-U.; Choi, J.-W.; Ahn, K.-H.; Lee, S.-H. Adsorption of phosphate from aqueous solution using electrochemically modified biochar calcium-alginate beads: Batch and fixed-bed column performance. Bioresour. Technol. 2017, 244, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Karydis, M. Eutrophication assessment of coastal waters based on indicators: A literature review. In Proceedings of the International Conference on Environmental Science and Technology, Mytilini, Greece, 4–7 September 2009. [Google Scholar]
- Divband Hafshejani, L.; Hooshmand, A.; Naseri, A.A.; Mohammadi, A.S.; Abbasi, F.; Bhatnagar, A. Removal of nitrate from aqueous solution by modified sugarcane bagasse biochar. Ecol. Eng. 2016, 95, 101–111. [Google Scholar] [CrossRef]
- Goswami, R.; Kumar, M. Removal of fluoride from aqueous solution using nanoscale rice husk biochar. Groundw. Sustain. Dev. 2018, 7, 446–451. [Google Scholar] [CrossRef]
- Jin, J.; Li, S.; Peng, X.; Liu, W.; Zhang, C.; Yang, Y.; Han, L.; Du, Z.; Sun, K.; Wang, X. HNO3 modified biochars for uranium (VI) removal from aqueous solution. Bioresour. Technol. 2018, 256, 247–253. [Google Scholar] [CrossRef]
- Li, N.; Yin, M.; Tsang, D.C.W.; Yang, S.; Liu, J.; Li, X.; Song, G.; Wang, J. Mechanisms of U(VI) removal by biochar derived from Ficus microcarpa aerial root: A comparison between raw and modified biochar. Sci. Total Environ. 2019, 697, 134115. [Google Scholar] [CrossRef]
- Harikishore Kumar Reddy, D.; Vijayaraghavan, K.; Kim, J.A.; Yun, Y.-S. Valorisation of post-sorption materials: Opportunities, strategies, and challenges. Adv. Colloid Interface Sci. 2017, 242, 35–58. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Ma, F.; Tankpa, V.; Bai, S.; Guo, X.; Wang, X. Mechanisms and reutilization of modified biochar used for removal of heavy metals from wastewater: A review. Sci. Total Environ. 2019, 668, 1298–1309. [Google Scholar] [CrossRef] [PubMed]
- Hai, A.; Bharath, G.; Babu, K.R.; Taher, H.; Naushad, M.; Banat, F. Date seeds biomass-derived activated carbon for efficient removal of NaCl from saline solution. Process Saf. Environ. Prot. 2019, 129, 103–111. [Google Scholar] [CrossRef]
- Welgemoed, T.; Schutte, C. Capacitive deionization technology™: An alternative desalination solution. Desalination 2005, 183, 327–340. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Liu, S.-H.; Tsang, D.C.W. Microwave-assisted production of CO2-activated biochar from sugarcane bagasse for electrochemical desalination. J. Hazard. Mater. 2020, 383, 121192. [Google Scholar] [CrossRef] [PubMed]
- Dehkhoda, A.M.; Ellis, N.; Gyenge, E. Effect of activated biochar porous structure on the capacitive deionization of NaCl and ZnCl2 solutions. Microporous Mesoporous Mater. 2016, 224, 217–228. [Google Scholar] [CrossRef]
- Han, B.; Cheng, G.; Wang, Y.; Wang, X. Structure and functionality design of novel carbon and faradaic electrode materials for high-performance capacitive deionization. Chem. Eng. J. 2019, 360, 364–384. [Google Scholar] [CrossRef]
- Meakins, N.; Bubb, J.; Lester, J. The fate and behaviour of organic micropollutants during wastewater treatment processes: A review. Int. J. Environ. Pollut. 1994, 4, 27–58. [Google Scholar]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of removal by low-cost adsorbents: A review. Chemosphere 2019. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Singh, P.; Raizada, P.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Thakur, V.K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Clean. Prod. 2019. [Google Scholar] [CrossRef]
- Ahmad, J.; Naeem, S.; Ahmad, M.; Usman, A.R.; Al-Wabel, M.I. A critical review on organic micropollutants contamination in wastewater and removal through carbon nanotubes. J. Environ. Manag. 2019, 246, 214–228. [Google Scholar] [CrossRef]
- Baig, N.; Sajid, M.; Saleh, T.A. Graphene-based adsorbents for the removal of toxic organic pollutants: A review. J. Envron. Manag. 2019, 244, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Yang, Y.; Tsang, Y.F.; Sarkar, B.; Hou, D.; Cao, X.; Meers, E.; Rinklebe, J.; Kim, K.-H.; Ok, Y.S. Occurrence of contaminants in drinking water sources and the potential of biochar for water quality improvement: A review. Crit. Rev. Environ. Sci. Technol. 2019, 1–63. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and Water Quality. J. Environ. Qual. 2019, 48, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; McNamara, P.J.; Mayer, B.K. Adsorption of organic micropollutants onto biochar: A review of relevant kinetics, mechanisms and equilibrium. Environ. Sci. Water Res. Technol. 2019, 5, 821–838. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef]
- Premarathna, K.; Rajapaksha, A.U.; Sarkar, B.; Kwon, E.E.; Bhatnagar, A.; Ok, Y.S.; Vithanage, M. Biochar-based engineered composites for sorptive decontamination of water: A review. Chem. Eng. J. 2019, 372, 536–550. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. A review on the synthesis and characterization of biomass-derived carbons for adsorption of emerging contaminants from water. C—J. Carbon Res. 2018, 4, 63. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Deng, S.; Yu, G. Selective removal of perfluorooctane sulfonate from aqueous solution using chitosan-based molecularly imprinted polymer adsorbents. Water Res. 2008, 42, 3089–3097. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: A review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Jayawardhana, Y.; Gunatilake, S.R.; Mahatantila, K.; Ginige, M.P.; Vithanage, M. Sorptive removal of toluene and m-xylene by municipal solid waste biochar: Simultaneous municipal solid waste management and remediation of volatile organic compounds. J. Environ. Manag. 2019, 238, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Jung, J.; Choe, J.K.; Ok, Y.S.; Choi, Y. Effect of biochar particle size on hydrophobic organic compound sorption kinetics: Applicability of using representative size. Sci. Total Environ. 2018, 619, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Lingamdinne, L.P.; Roh, H.; Choi, Y.-L.; Koduru, J.R.; Yang, J.-K.; Chang, Y.-Y. Influencing factors on sorption of TNT and RDX using rice husk biochar. J. Ind. Eng. Chem. 2015, 32, 178–186. [Google Scholar] [CrossRef]
- Mandal, S.; Sarkar, B.; Igalavithana, A.D.; Ok, Y.S.; Yang, X.; Lombi, E.; Bolan, N. Mechanistic insights of 2,4-D sorption onto biochar: Influence of feedstock materials and biochar properties. Bioresour. Technol. 2017, 246, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, N.; Tong, L.; Lv, Y.; Li, G. Characterization and evaluation of surface modified materials based on porous biochar and its adsorption properties for 2,4-dichlorophenoxyacetic acid. Chemosphere 2018, 210, 734–744. [Google Scholar] [CrossRef]
- Silvani, L.; Cornelissen, G.; Hale, S.E. Sorption of α-, β-, γ- and δ-hexachlorocyclohexane isomers to three widely different biochars: Sorption mechanisms and application. Chemosphere 2019, 219, 1044–1051. [Google Scholar] [CrossRef] [Green Version]
- AlAmeri, K.; Giwa, A.; Yousef, L.; Alraeesi, A.; Taher, H. Sorption and removal of crude oil spills from seawater using peat-derived biochar: An optimization study. J. Environ. Manag. 2019, 250, 109465. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- He, J.; Cui, A.; Deng, S.; Chen, J.P. Treatment of methylene blue containing wastewater by a cost-effective micro-scale biochar/polysulfone mixed matrix hollow fiber membrane: Performance and mechanism studies. J. Colloid Interface Sci. 2018, 512, 190–197. [Google Scholar] [CrossRef]
- Hou, Y.; Huang, G.; Li, J.; Yang, Q.; Huang, S.; Cai, J. Hydrothermal conversion of bamboo shoot shell to biochar: Preliminary studies of adsorption equilibrium and kinetics for rhodamine B removal. J. Anal. Appl. Pyrolysis 2019, 143, 104694. [Google Scholar] [CrossRef]
- Zhang, Z.; O’Hara, I.M.; Kent, G.A.; Doherty, W.O.S. Comparative study on adsorption of two cationic dyes by milled sugarcane bagasse. Ind. Crop. Prod. 2013, 42, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Zazycki, M.A.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. New biochar from pecan nutshells as an alternative adsorbent for removing reactive red 141 from aqueous solutions. J. Clean. Prod. 2018, 171, 57–65. [Google Scholar] [CrossRef]
- Netpradit, S.; Thiravetyan, P.; Towprayoon, S. Application of ‘waste’ metal hydroxide sludge for adsorption of azo reactive dyes. Water Res. 2003, 37, 763–772. [Google Scholar] [CrossRef]
- Jung, K.-W.; Choi, B.H.; Jeong, T.-U.; Ahn, K.-H. Facile synthesis of magnetic biochar/Fe3O4 nanocomposites using electro-magnetization technique and its application on the removal of acid orange 7 from aqueous media. Bioresour. Technol. 2016, 220, 672–676. [Google Scholar] [CrossRef]
- Yoon, S.; Calvo, J.; So, M. Removal of Acid Orange 7 from Aqueous Solution by Metal-Organic Frameworks. Crystals 2019, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.; Yoon, Y.; Lee, G.; Kim, Y.; Han, J.; Park, C.M. Enhanced adsorption of bisphenol A and sulfamethoxazole by a novel magnetic CuZnFe2O4–biochar composite. Bioresour. Technol. 2019, 281, 179–187. [Google Scholar] [CrossRef]
- Wang, D.; Sui, Q.; Zhao, W.; Shuguang, L.; Qiu, Z.; Yu, G. Pharmaceutical and personal care products in the surface water of China: A review. Chin. Sci. Bull. 2014, 59, 743–751. [Google Scholar]
- Derksen, J.; Rijs, G.; Jongbloed, R. Diffuse pollution of surface water by pharmaceutical products. Water Sci. Technol. 2004, 49, 213–221. [Google Scholar] [CrossRef]
- Larsson, D.J. Pollution from drug manufacturing: Review and perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130571. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Park, C.M.; Jang, A.; Jang, M.; Hernández-Maldonado, A.J.; Yu, M.; Heo, J.; Yoon, Y. Removal of selected pharmaceuticals in an ultrafiltration-activated biochar hybrid system. J. Membr. Sci. 2019, 570, 77–84. [Google Scholar] [CrossRef]
- Li, F.; Feng, D.; Deng, H.; Yu, H.; Ge, C. Effects of Biochars Prepared from Cassava Dregs on Sorption Behavior of Ciprofloxacin. Procedia Environ. Sci. 2016, 31, 795–803. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.M.; Kan, E. Engineered biochar from agricultural waste for removal of tetracycline in water. Bioresour. Technol. 2019, 284, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Singh, N. Optimization of atrazine and imidacloprid removal from water using biochars: Designing single or multi-staged batch adsorption systems. Int. J. Hyg. Environ. Health 2017, 220, 637–645. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Wang, Y.; Zhang, Y.; Gao, X.; Ahmed, M.B.; Zhang, J.; Yang, Y.; Zhou, J.L.; Lowry, G.V. Distributing sulfidized nanoscale zerovalent iron onto phosphorus-functionalized biochar for enhanced removal of antibiotic florfenicol. Chem. Eng. J. 2019, 359, 713–722. [Google Scholar] [CrossRef]
- Zhang, M.-h.; Dong, H.; Zhao, L.; Wang, D.-X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- He, J.; Xiao, Y.; Tang, J.; Chen, H.; Sun, H. Persulfate activation with sawdust biochar in aqueous solution by enhanced electron donor-transfer effect. Sci. Total Environ. 2019, 690, 768–777. [Google Scholar] [CrossRef]
- Ho, S.-H.; Chen, Y.-d.; Li, R.; Zhang, C.; Ge, Y.; Cao, G.; Ma, M.; Duan, X.; Wang, S.; Ren, N.-Q. N-doped graphitic biochars from C-phycocyanin extracted Spirulina residue for catalytic persulfate activation toward nonradical disinfection and organic oxidation. Water Res. 2019, 159, 77–86. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism. Chem. Eng. J. 2019, 375, 121980. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Tsai, M.-L.; Chang, J.-H.; Lyu, S.-Y.; Hung, C.-M. Degradation of 4-nonylphenol in marine sediments by persulfate over magnetically modified biochars. Bioresour. Technol. 2019, 281, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, L.; Ding, D.; Cai, T. From rice straw to magnetically recoverable nitrogen doped biochar: Efficient activation of peroxymonosulfate for the degradation of metolachlor. Appl. Catal. B: Environ. 2019, 254, 312–320. [Google Scholar] [CrossRef]
- Jiang, S.-F.; Ling, L.-L.; Chen, W.-J.; Liu, W.-J.; Li, D.-C.; Jiang, H. High efficient removal of bisphenol A in a peroxymonosulfate/iron functionalized biochar system: Mechanistic elucidation and quantification of the contributors. Chem. Eng. J. 2019, 359, 572–583. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.Y.; Lee, Y.J.; Choi, J.-W. Ultrasound-assisted heterogeneous Fenton-like process for bisphenol A removal at neutral pH using hierarchically structured manganese dioxide/biochar nanocomposites as catalysts. Ultrason. Sonochem. 2019, 57, 22–28. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Dong, F.-X.; Yan, L.; Chen, Z.-L.; Qian, W.; Kong, L.-J.; Zhang, Z.-W.; Zhang, T.; Tao, X.-Q.; Du, J.-J.; et al. Synergistic oxidation of Bisphenol A in a heterogeneous ultrasound-enhanced sludge biochar catalyst/persulfate process: Reactivity and mechanism. J. Hazard. Mater. 2019. [Google Scholar] [CrossRef]
- Gan, L.; Zhong, Q.; Geng, A.; Wang, L.; Song, C.; Han, S.; Cui, J.; Xu, L. Cellulose derived carbon nanofiber: A promising biochar support to enhance the catalytic performance of CoFe2O4 in activating peroxymonosulfate for recycled dimethyl phthalate degradation. Sci. Total Environ. 2019, 694, 133705. [Google Scholar] [CrossRef]
- Moussavi, G.; Khosravi, R. Preparation and characterization of a biochar from pistachio hull biomass and its catalytic potential for ozonation of water recalcitrant contaminants. Bioresour. Technol. 2012, 119, 66–71. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Seo, Y.-D.; Ryu, K.-S. Reductive removal of 2,4-dinitrotoluene and 2,4-dichlorophenol with zero-valent iron-included biochar. Bioresour. Technol. 2016, 216, 1014–1021. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Sun, A.; Tong, S.; Su, G.; Jiang, X.; Li, J.; Han, W.; Sun, X.; Wang, L.; et al. Enhanced nitrobenzene reduction by modified biochar supported sulfidated nano zerovalent iron: Comparison of surface modification methods. Sci. Total Environ. 2019, 694, 133701. [Google Scholar] [CrossRef]
- Lyu, H.; Tang, J.; Shen, B.; Siddique, T. Development of a novel chem-bio hybrid process using biochar supported nanoscale iron sulfide composite and Corynebacterium variabile HRJ4 for enhanced trichloroethylene dechlorination. Water Res. 2018, 147, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Sophia Ayyappan, C.; Bhalambaal, V.M.; Kumar, S. Effect of biochar on bio-electrochemical dye degradation and energy production. Bioresour. Technol. 2018, 251, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Shirvanimoghaddam, K.; Czech, B.; Wójcik, G.; Naebe, M. The light enhanced removal of Bisphenol A from wastewater using cotton waste derived carbon microtubes. J. Colloid Interface Sci. 2019, 539, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, Z.; Zhao, X.; Yang, X.; Liang, G.; Xie, X. Insight into enhanced carbamazepine photodegradation over biochar-based magnetic photocatalyst Fe3O4/BiOBr/BC under visible LED light irradiation. Chem. Eng. J. 2019, 360, 600–611. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.a.H.; Naushad, M.; Ghfar, A.A.; Guo, C.; Stadler, F.J. Biochar-templated g-C3N4/Bi2O2CO3/CoFe2O4 nano-assembly for visible and solar assisted photo-degradation of paraquat, nitrophenol reduction and CO2 conversion. Chem. Eng. J. 2018, 339, 393–410. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Al-Muhtaseb, A.a.H.; Kumar, A.; Hira, I.; Ahamad, T.; Ghfar, A.A.; Stadler, F.J. Visible photodegradation of ibuprofen and 2,4-D in simulated waste water using sustainable metal free-hybrids based on carbon nitride and biochar. J. Environiron. Manag. 2019, 231, 1164–1175. [Google Scholar] [CrossRef]
- Xie, X.; Li, S.; Zhang, H.; Wang, Z.; Huang, H. Promoting charge separation of biochar-based Zn-TiO2/pBC in the presence of ZnO for efficient sulfamethoxazole photodegradation under visible light irradiation. Sci. Total Environ. 2019, 659, 529–539. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R. Gas Purification; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Eddaoudi, M.; Almaythalony, B.A.; Shekhah, O.; Belmabkhout, Y. Zeolite-Like Metal-Organic Framework Membrane. U.S. Patent US10130908B2, 20 Novemebr 2018. [Google Scholar]
- Kimura, S.; Walmet, G. Fuel gas purification with permselective membranes. Sep. Sci. Technol. 1980, 15, 1115–1133. [Google Scholar] [CrossRef]
- Dumée, L.; Scholes, C.; Stevens, G.; Kentish, S. Purification of aqueous amine solvents used in post combustion CO2 capture: A review. Int. J. Greenh. Gas Control 2012, 10, 443–455. [Google Scholar] [CrossRef]
- Das, J.; Rene, E.R.; Dupont, C.; Dufourny, A.; Blin, J.; van Hullebusch, E.D. Performance of a compost and biochar packed biofilter for gas-phase hydrogen sulfide removal. Bioresour. Technol. 2019, 273, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Braghiroli, F.L.; Bouafif, H.; Koubaa, A. Enhanced SO2 adsorption and desorption on chemically and physically activated biochar made from wood residues. Ind. Crop. Prod. 2019, 138, 111456. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, J.; Zhang, X.; Feng, Y.; Zhang, H.; Zhang, S.; Chen, H. Enhance SO2 adsorption performance of biochar modified by CO2 activation and amine impregnation. Fuel 2018, 224, 138–146. [Google Scholar] [CrossRef]
- Rind, D.; Suozzo, R.; Balachandran, N.; Prather, M. Climate change and the middle atmosphere. Part I: The doubled CO2 climate. J. Atmos. Sci. 1990, 47, 475–494. [Google Scholar] [CrossRef] [Green Version]
- Feijoo, F.; Mignone, B.K.; Kheshgi, H.S.; Hartin, C.; McJeon, H.; Edmonds, J. Climate and carbon budget implications of linked future changes in CO2 and non-CO2 forcing. Environ. Res. Lett. 2019, 14, 044007. [Google Scholar] [CrossRef]
- Kellogg, W.W. Climate Change and Society: Consequences of Increasing Atmospheric Carbon Dioxide; Routledge: Abingdon upon Thames, UK, 2019. [Google Scholar]
- Jung, S.; Park, Y.-K.; Kwon, E.E. Strategic use of biochar for CO2 capture and sequestration. J. CO2 Util. 2019, 32, 128–139. [Google Scholar] [CrossRef]
- Liu, S.-H.; Huang, Y.-Y. Valorization of coffee grounds to biochar-derived adsorbents for CO2 adsorption. J. Clean. Prod. 2018, 175, 354–360. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Choi, S.W.; Dissanayake, P.D.; Shang, J.; Wang, C.-H.; Yang, X.; Kim, S.; Tsang, D.C.; Lee, K.B.; Ok, Y.S. Gasification biochar from biowaste (food waste and wood waste) for effective CO2 adsorption. J. Hazard. Mater. 2019, 121147. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. CO2 adsorption on biochar from co-torrefaction of sewage sludge and leucaena wood using microwave heating. Energy Procedia 2019, 158, 4435–4440. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Juang, R.-S. Surface modifications of carbonaceous materials for carbon dioxide adsorption: A review. J. Taiwan Inst. Chem. Eng. 2017, 71, 214–234. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, H.; Shao, J.; Chen, Y.; Feng, Y.; Wang, X.; Chen, H. Effects of hydrofluoric acid pre-deashing of rice husk on physicochemical properties and CO2 adsorption performance of nitrogen-enriched biochar. Energy 2015, 91, 903–910. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Shih, C.-H.; Lo, S.-L.; Sun, L.; Zhong, Y.; Qiu, C. Microwave pyrolysis of rice straw to produce biochar as an adsorbent for CO2 capture. Energy 2015, 84, 75–82. [Google Scholar] [CrossRef]
- Shen, Y.; Fu, Y. KOH-activated rice husk char via CO2 pyrolysis for phenol adsorption. Mater. Today Energy 2018, 9, 397–405. [Google Scholar] [CrossRef]
- Przepiórski, J.; Skrodzewicz, M.; Morawski, A. High temperature ammonia treatment of activated carbon for enhancement of CO2 adsorption. Appl. Surf. Sci. 2004, 225, 235–242. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Arami-Niya, A. Ammonia modification of activated carbon to enhance carbon dioxide adsorption: Effect of pre-oxidation. Appl. Surf. Sci. 2011, 257, 3936–3942. [Google Scholar] [CrossRef]
- Xie, P.; Sun, F.; Wang, L.; Liu, P. A review on China’s Energy Storage Industry under the “Internet Plus” initiative. Int. J. Energy Res. 2019, 43, 717–741. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What are Batteries, Fuel Cells, and Supercapacitors; ACS Publications: Washington, DC, USA, 2004. [Google Scholar]
- Fonash, S. Solar Cell Device Physics; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Blomen, L.J.; Mugerwa, M.N. Fuel Cell Systems; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Yoshio, M.; Brodd, R.J.; Kozawa, A. Lithium-Ion Batteries; Springer: Berlin, Germany, 2009; Volume 1. [Google Scholar]
- Lu, M. Supercapacitors: Materials, Systems and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [Green Version]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Kubota, K.; Komaba, S. practical issues and future perspective for Na-ion batteries. J. Electrochem. Soc. 2015, 162, A2538–A2550. [Google Scholar] [CrossRef]
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrog. Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef] [Green Version]
- Giddey, S.; Badwal, S.; Kulkarni, A.; Munnings, C. A comprehensive review of direct carbon fuel cell technology. Prog. Energy Combust. Sci. 2012, 38, 360–399. [Google Scholar] [CrossRef]
- Munjewar, S.S.; Thombre, S.B.; Mallick, R.K. Approaches to overcome the barrier issues of passive direct methanol fuel cell–Review. Renew. Sustain. Energy Rev. 2017, 67, 1087–1104. [Google Scholar] [CrossRef]
- Bartoli, M.; Rosi, L.; Frediani, M.; Frediani, P. Challenges and Opportunities in the Field of Energy Storage: Supercapacitors and Activated Biochar; Nova Publisher: Hauppauge, NY, USA, 2018. [Google Scholar]
- Jin, H.; Wang, X.; Gu, Z.; Polin, J. Carbon materials from high ash biochar for supercapacitor and improvement of capacitance with HNO 3 surface oxidation. J. Power Sources 2013, 236, 285–292. [Google Scholar] [CrossRef]
- Gabhi, R.S.; Kirk, D.W.; Jia, C.Q. Preliminary investigation of electrical conductivity of monolithic biochar. Carbon 2017, 116, 435–442. [Google Scholar] [CrossRef]
- Luo, W.; Wang, B.; Heron, C.G.; Allen, M.J.; Morre, J.; Maier, C.S.; Stickle, W.F.; Ji, X. Pyrolysis of cellulose under ammonia leads to nitrogen-doped nanoporous carbon generated through methane formation. Nano Lett. 2014, 14, 2225–2229. [Google Scholar] [CrossRef]
- Qu, W.-H.; Xu, Y.-Y.; Lu, A.-H.; Zhang, X.-Q.; Li, W.-C. Converting biowaste corncob residue into high value added porous carbon for supercapacitor electrodes. Bioresour. Technol. 2015, 189, 285–291. [Google Scholar] [CrossRef]
- Cheng, B.-H.; Tian, K.; Zeng, R.J.; Jiang, H. Preparation of high performance supercapacitor materials by fast pyrolysis of corn gluten meal waste. Sustain. Energy Fuels 2017, 1, 891–898. [Google Scholar] [CrossRef]
- Sun, W.; Lipka, S.M.; Swartz, C.; Williams, D.; Yang, F. Hemp-derived activated carbons for supercapacitors. Carbon 2016, 103, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Sivachidambaram, M.; Vijaya, J.J.; Kennedy, L.J.; Jothiramalingam, R.; Al-Lohedan, H.A.; Munusamy, M.A.; Elanthamilan, E.; Merlin, J.P. Preparation and characterization of activated carbon derived from the Borassus flabellifer flower as an electrode material for supercapacitor applications. New J. Chem. 2017, 41, 3939–3949. [Google Scholar] [CrossRef]
- Zou, R.; Quan, H.; Wang, W.; Gao, W.; Dong, Y.; Chen, D. Porous carbon with interpenetrating framework from Osmanthus flower as electrode materials for high-performance supercapacitor. J. Environ. Chem. Eng. 2018, 6, 258–265. [Google Scholar] [CrossRef]
- Song, M.; Zhou, Y.; Ren, X.; Wan, J.; Du, Y.; Wu, G.; Ma, F. Biowaste-based porous carbon for supercapacitor: The influence of preparation processes on structure and performance. J. Colloid Interface Sci. 2019, 535, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, C.; Tang, Z. High specific capacitance and high energy density supercapacitor electrodes enabled by porous carbon with multilevel pores and self-doped heteroatoms derived from Chinese date. Diam. Relat. Mater. 2019, 97, 107455. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, S.-Y.; Tang, Z.-S.; Song, Z.-X.; Sun, J. Xanthoceras sorbifolia seed coats derived porous carbon with unique architecture for high rate performance supercapacitors. Diam. Relat. Mater. 2019, 91, 119–126. [Google Scholar] [CrossRef]
- Jiao, C.; Xu, J.L.; Chen, X.Y.; Zhang, Z.J. Design and synthesis of phosphomolybdic acid/silver dual-modified microporous carbon composite for high performance supercapacitors. J. Alloy. Compd. 2019, 791, 1005–1014. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dubey, M.; Kharel, P.; Gu, Z.; Fan, Q.H. Biochar activated by oxygen plasma for supercapacitors. J. Power Sources 2015, 274, 1300–1305. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Xu, S.; Yue, Q.; Ortaboy, S.; Gao, B.; Sun, Y. Synthesis and characterization of heteroatom-enriched biochar from keratin-based and algous-based wastes. Adv. Powder Technol. 2016, 27, 1280–1286. [Google Scholar] [CrossRef]
- Pontiroli, D.; Scaravonati, S.; Magnani, G.; Fornasini, L.; Bersani, D.; Bertoni, G.; Milanese, C.; Girella, A.; Ridi, F.; Verucchi, R.; et al. Super-activated biochar from poultry litter for high-performance supercapacitors. Microporous Mesoporous Mater. 2019, 285, 161–169. [Google Scholar] [CrossRef]
- Dai, X.-H.; Fan, H.-X.; Zhang, J.-J.; Yuan, S.-J. Sewage sludge-derived porous hollow carbon nanospheres as high-performance anode material for lithium ion batteries. Electrochim. Acta 2019, 319, 277–285. [Google Scholar] [CrossRef]
- Luna-Lama, F.; Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Muñoz-Batista, M.J.; Caballero, A.; Balu, A.M.; Romero, A.A.; Luque, R. Non-porous carbonaceous materials derived from coffee waste grounds as highly sustainable anodes for lithium-ion batteries. J. Clean. Prod. 2019, 207, 411–417. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, F.; Kang, W.; Shen, Q. Encapsulating selenium into macro-/micro-porous biochar-based framework for high-performance lithium-selenium batteries. Carbon 2015, 95, 354–363. [Google Scholar] [CrossRef]
- Benítez, A.; González-Tejero, M.; Caballero, Á.; Morales, J. Almond Shell as a Microporous Carbon Source for Sustainable Cathodes in Lithium–Sulfur Batteries. Materials 2018, 11, 1428. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Du, G.; Zhang, M.; Kalam, A.; Su, Q.; Ding, S.; Xu, B. Nitrogen-doped hierarchical porous carbon derived from low-cost biomass pomegranate residues for high performance lithium-sulfur batteries. J. Electroanal. Chem. 2019, 848, 113316. [Google Scholar] [CrossRef]
- Chen, F.; Ma, L.; Ren, J.; Zhang, M.; Luo, X.; Li, B.; Song, Z.; Zhou, X. Wheat Straw-Derived N-, O-, and S-Tri-doped Porous Carbon with Ultrahigh Specific Surface Area for Lithium-Sulfur Batteries. Materials 2018, 11, 989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnacca, G.; Guerretta, F.; Vizintin, A.; Benzi, P.; Valsania, M.C.; Nisticò, R. Preparation, characterization and environmental/electrochemical energy storage testing of low-cost biochar from natural chitin obtained via pyrolysis at mild conditions. Appl. Surf. Sci. 2018, 427, 883–893. [Google Scholar] [CrossRef]
- Pan, P.; Hu, Y.; Wu, K.; Cheng, Z.; Shen, Z.; Jiang, L.; Mao, J.; Ni, C.; Ge, Y.; Wang, Z. Growth of ZnCo2O4 nanocubes on flexible biochar substrate derived from natural silk waste fabric for lithium-ion battery anode. J. Alloy. Compd. 2020, 814, 152306. [Google Scholar] [CrossRef]
- Li, T.; Bai, X.; Qi, Y.-X.; Lun, N.; Bai, Y.-J. Fe3O4 nanoparticles decorated on the biochar derived from pomelo pericarp as excellent anode materials for Li-ion batteries. Electrochim. Acta 2016, 222, 1562–1568. [Google Scholar] [CrossRef]
- Salimi, P.; Norouzi, O.; Pourhoseini, S.E.M.; Bartocci, P.; Tavasoli, A.; Di Maria, F.; Pirbazari, S.M.; Bidini, G.; Fantozzi, F. Magnetic biochar obtained through catalytic pyrolysis of macroalgae: A promising anode material for Li-ion batteries. Renew. Energy 2019, 140, 704–714. [Google Scholar] [CrossRef]
- Salimi, P.; Norouzi, O.; Pourhosseini, S.E.M. Two-step synthesis of nanohusk Fe3O4 embedded in 3D network pyrolytic marine biochar for a new generation of anode materials for Lithium-Ion batteries. J. Alloy. Compd. 2019, 786, 930–937. [Google Scholar] [CrossRef]
- Saavedra Rios, C.d.M.; Simone, V.; Simonin, L.; Martinet, S.; Dupont, C. Biochars from various biomass types as precursors for hard carbon anodes in sodium-ion batteries. Biomass Bioenergy 2018, 117, 32–37. [Google Scholar] [CrossRef]
- Jafri, N.; Wong, W.Y.; Doshi, V.; Yoon, L.W.; Cheah, K.H. A review on production and characterization of biochars for application in direct carbon fuel cells. Process Saf. Environ. Prot. 2018, 118, 152–166. [Google Scholar] [CrossRef]
- Konsolakis, M.; Kaklidis, N.; Marnellos, G.E.; Zaharaki, D.; Komnitsas, K. Assessment of biochar as feedstock in a direct carbon solid oxide fuel cell. RSC Adv. 2015, 5, 73399–73409. [Google Scholar] [CrossRef]
- Elleuch, A.; Halouani, K.; Li, Y. Investigation of chemical and electrochemical reactions mechanisms in a direct carbon fuel cell using olive wood charcoal as sustainable fuel. J. Power Sources 2015, 281, 350–361. [Google Scholar] [CrossRef]
- Xu, K.; Dong, J.; Li, X.; Wang, J.; Hu, Z.; Li, A.; Yao, H. Evaluation of biomass and its thermal decomposition products as fuels for direct carbon fuel cells. Biomass Bioenergy 2019, 130, 105359. [Google Scholar] [CrossRef]
- Qiu, Q.; Zhou, M.; Cai, W.; Zhou, Q.; Zhang, Y.; Wang, W.; Liu, M.; Liu, J. A comparative investigation on direct carbon solid oxide fuel cells operated with fuels of biochar derived from wheat straw, corncob, and bagasse. Biomass Bioenergy 2019, 121, 56–63. [Google Scholar] [CrossRef]
- Kacprzak, A.; Kobyłecki, R.; Włodarczyk, R.; Bis, Z. The effect of fuel type on the performance of a direct carbon fuel cell with molten alkaline electrolyte. J. Power Sources 2014, 255, 179–186. [Google Scholar] [CrossRef]
- Kacprzak, A.; Kobyłecki, R.; Włodarczyk, R.; Bis, Z. Efficiency of non-optimized direct carbon fuel cell with molten alkaline electrolyte fueled by carbonized biomass. J. Power Sources 2016, 321, 233–240. [Google Scholar] [CrossRef]
- Kacprzak, A.; Kobylecki, R.; Bis, Z. Influence of temperature and composition of NaOH–KOH and NaOH–LiOH electrolytes on the performance of a direct carbon fuel cell. J. Power Sources 2013, 239, 409–414. [Google Scholar] [CrossRef]
- Ali, A.; Raza, R.; Shakir, M.I.; Iftikhar, A.; Alvi, F.; Ullah, M.K.; Hamid, A.; Kim, J.-S. Promising electrochemical study of titanate based anodes in direct carbon fuel cell using walnut and almond shells biochar fuel. J. Power Sources 2019, 434, 126679. [Google Scholar] [CrossRef]
- Elleuch, A.; Boussetta, A.; Yu, J.; Halouani, K.; Li, Y. Experimental investigation of direct carbon fuel cell fueled by almond shell biochar: Part I. Physico-chemical characterization of the biochar fuel and cell performance examination. Int. J. Hydrog. Energy 2013, 38, 16590–16604. [Google Scholar] [CrossRef]
- Huggins, T.; Wang, H.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol. 2014, 157, 114–119. [Google Scholar] [CrossRef]
- Huggins, T.M.; Pietron, J.J.; Wang, H.; Ren, Z.J.; Biffinger, J.C. Graphitic biochar as a cathode electrocatalyst support for microbial fuel cells. Bioresour. Technol. 2015, 195, 147–153. [Google Scholar] [CrossRef]
- Md Khudzari, J.; Gariépy, Y.; Kurian, J.; Tartakovsky, B.; Raghavan, G.S.V. Effects of biochar anodes in rice plant microbial fuel cells on the production of bioelectricity, biomass, and methane. Biochem. Eng. J. 2019, 141, 190–199. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Xiao, T.; Wang, S.; Zhang, B.; Chen, D.; Su, M.; Tang, J. Low-cost biochar derived from corncob as oxygen reduction catalyst in air cathode microbial fuel cells. Electrochim. Acta 2018, 283, 780–788. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, T.; Wang, D.; Tang, J.; Zhou, S. Sewage sludge biochar as an efficient catalyst for oxygen reduction reaction in an microbial fuel cell. Bioresour. Technol. 2013, 144, 115–120. [Google Scholar] [CrossRef]
- Chakraborty, I.; Das, S.; Dubey, B.K.; Ghangrekar, M.M. Novel low cost proton exchange membrane made from sulphonated biochar for application in microbial fuel cells. Mater. Chem. Phys. 2020, 239, 122025. [Google Scholar] [CrossRef]
- Research, G.V. Market Value of Composite Materials Worldwide from 2015 to 2024 (in Billion U.S. Dollars). Available online: https://www.statista.com/statistics/944471/global-market-value-of-composites/ (accessed on 3 November 2019).
- Sauer, M.; Kühnel, M.; Witten, E. Composites Market Report 2018–Market Developments, Trends, Outlook and Challenges; AVK-TV Industrievereinigung verstrkte Kunststoffe Carbon Composite: Augsburg, Germany, 2018. [Google Scholar]
- Chand, S. Review carbon fibers for composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Samal, S.S.; Bal, S. Carbon nanotube reinforced ceramic matrix composites-a review. J. Miner. Mater. Charact. Eng. 2008, 7, 355. [Google Scholar] [CrossRef]
- Afzal, A.; Kausar, A.; Siddiq, M. A review on polymer/cement composite with carbon nanofiller and inorganic filler. Polym. Plast. Technol. Eng. 2016, 55, 1299–1323. [Google Scholar] [CrossRef]
- Downie, A.; Crosky, A.; Munroe, P. Physical properties of biochar. Biochar Environ. Manag. Sci. Technol. 2009, 2, 13–32. [Google Scholar]
- Bernhardt, D.; Reilly, J., II. Mineral Commodity Summaries 2019; Department of the Interior (DOI), U.S. Geological Survey (USGS): Reston, VA, USA, 2019.
- Lewis, S.J.; Sodhi, T.S.; Agapiou, K.; Kolasnikov, A. Non-Aqueous Liquid Anti-Shrinkage Cement Additives. U.S. Patents US20190062213A1, 28 February 2019. [Google Scholar]
- Riley, V.; Razl, I. Polymer additives for cement composites: A review. Composites 1974, 5, 27–33. [Google Scholar] [CrossRef]
- Reales, O.A.M.; Toledo Filho, R.D. A review on the chemical, mechanical and microstructural characterization of carbon nanotubes-cement based composites. Constr. Build. Mater. 2017, 154, 697–710. [Google Scholar] [CrossRef]
- Yang, H.; Cui, H.; Tang, W.; Li, Z.; Han, N.; Xing, F. A critical review on research progress of graphene/cement based composites. Compos. Part A Appl. Sci. Manuf. 2017, 102, 273–296. [Google Scholar] [CrossRef]
- Toutanji, H.A.; El-Korchi, T.; Katz, R.N. Strength and reliability of carbon-fiber-reinforced cement composites. Cem. Concr. Compos. 1994, 16, 15–21. [Google Scholar] [CrossRef]
- Cosentino, I.; Restuccia, L.; Ferro, G.A.; Tulliani, J.-M. Type of materials, pyrolysis conditions, carbon content and size dimensions: The parameters that influence the mechanical properties of biochar cement-based composites. Theor. Appl. Fract. Mech. 2019, 103, 102261. [Google Scholar] [CrossRef]
- Mašek, O.; Buss, W.; Roy-Poirier, A.; Lowe, W.; Peters, C.; Brownsort, P.; Mignard, D.; Pritchard, C.; Sohi, S. Consistency of biochar properties over time and production scales: A characterisation of standard materials. J. Anal. Appl. Pyrolysis 2018, 132, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Musso, S.; Tulliani, J.-M.; Ferro, G.; Tagliaferro, A. Influence of carbon nanotubes structure on the mechanical behavior of cement composites. Compos. Sci. Technol. 2009, 69, 1985–1990. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W. Carbonaceous micro-filler for cement: Effect of particle size and dosage of biochar on fresh and hardened properties of cement mortar. Sci. Total Environ. 2019, 662, 952–962. [Google Scholar] [CrossRef]
- Mo, L.; Fang, J.; Huang, B.; Wang, A.; Deng, M. Combined effects of biochar and MgO expansive additive on the autogenous shrinkage, internal relative humidity and compressive strength of cement pastes. Constr. Build. Mater. 2019, 229, 116877. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Gupta, S.; Kua, H.W. Application of rice husk biochar and thermally treated low silica rice husk ash to improve physical properties of cement mortar. Theor. Appl. Fract. Mech. 2019, 104, 102376. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W. Effect of water entrainment by pre-soaked biochar particles on strength and permeability of cement mortar. Constr. Build. Mater. 2018, 159, 107–125. [Google Scholar] [CrossRef]
- Dixit, A.; Gupta, S.; Pang, S.D.; Kua, H.W. Waste Valorisation using biochar for cement replacement and internal curing in ultra-high performance concrete. J. Clean. Prod. 2019, 238, 117876. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Pang, S.D. Effect of biochar on mechanical and permeability properties of concrete exposed to elevated temperature. Constr. Build. Mater. 2020, 234, 117338. [Google Scholar] [CrossRef]
- Oancea, I.; Bujoreanu, C.; Budescu, M.; Benchea, M.; Grădinaru, C.M. Considerations on sound absorption coefficient of sustainable concrete with different waste replacements. J. Clean. Prod. 2018, 203, 301–312. [Google Scholar] [CrossRef]
- Cuthbertson, D.; Berardi, U.; Briens, C.; Berruti, F. Biochar from residual biomass as a concrete filler for improved thermal and acoustic properties. Biomass Bioenergy 2019, 120, 77–83. [Google Scholar] [CrossRef]
- Kua, H.W.; Gupta, S.; Aday, A.N.; Srubar, W.V. Biochar-immobilized bacteria and superabsorbent polymers enable self-healing of fiber-reinforced concrete after multiple damage cycles. Cem. Concr. Compos. 2019, 100, 35–52. [Google Scholar] [CrossRef]
- Mu, B.; Wang, A. 11—Fabrication and Applications of Carbon/Clay Mineral Nanocomposites. In Nanomaterials from Clay Minerals; Wang, A., Wang, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 537–587. [Google Scholar] [CrossRef]
- Lee, H.; Yang, S.; Wi, S.; Kim, S. Thermal transfer behavior of biochar-natural inorganic clay composite for building envelope insulation. Constr. Build. Mater. 2019, 223, 668–678. [Google Scholar] [CrossRef]
- Yang, S.; Wi, S.; Lee, J.; Lee, H.; Kim, S. Biochar-red clay composites for energy efficiency as eco-friendly building materials: Thermal and mechanical performance. J. Hazard. Mater. 2019, 373, 844–855. [Google Scholar] [CrossRef]
- Dahal, R.K.; Acharya, B.; Saha, G.; Bissessur, R.; Dutta, A.; Farooque, A. Biochar as a filler in glassfiber reinforced composites: Experimental study of thermal and mechanical properties. Compos. Part B Eng. 2019, 175, 107169. [Google Scholar] [CrossRef]
- Bartoli, M.; Rosi, L.; Frediani, M. Chapter 22—Synthesis and Applications of Unsaturated Polyester Composites. In Unsaturated Polyester Resins; Thomas, S., Hosur, M., Chirayil, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 579–598. [Google Scholar] [CrossRef]
- Rana, S.; Alagirusamy, R.; Joshi, M. A review on carbon epoxy nanocomposites. J. Reinf. Plast. Compos. 2009, 28, 461–487. [Google Scholar] [CrossRef]
- Kim, S.; Pechar, T.W.; Marand, E. Poly (imide siloxane) and carbon nanotube mixed matrix membranes for gas separation. Desalination 2006, 192, 330–339. [Google Scholar] [CrossRef]
- Khan, A.; Savi, P.; Quaranta, S.; Rovere, M.; Giorcelli, M.; Tagliaferro, A.; Rosso, C.; Jia, C. Low-cost carbon fillers to improve mechanical properties and conductivity of epoxy composites. Polymers 2017, 9, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartoli, M.; Giorcelli, M.; Rosso, C.; Rovere, M.; Jagdale, P.; Tagliaferro, A. Influence of Commercial Biochar Fillers on Brittleness/Ductility of Epoxy Resin Composites. Appl. Sci. 2019, 9, 3109. [Google Scholar] [CrossRef] [Green Version]
- Giorcelli, M.; Khan, A.; Pugno, N.M.; Rosso, C.; Tagliaferro, A. Biochar as a cheap and environmental friendly filler able to improve polymer mechanical properties. Biomass Bioenergy 2019, 120, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Giorcelli, M.; Savi, P.; Khan, A.; Tagliaferro, A. Analysis of biochar with different pyrolysis temperatures used as filler in epoxy resin composites. Biomass Bioenergy 2019, 122, 466–471. [Google Scholar] [CrossRef]
- Giorcelli, M.; Khan, A.; Tagliaferro, A.; Savi, P.; Berruti, F. Microwave characterization of polymer composite based on Biochar: A comparison of composite behaviour for Biochar and MWCNTs. In Proceedings of the 2016 IEEE International Nanoelectronics Conference (INEC), Chengdu, China, 9–11 May 2016; pp. 1–2. [Google Scholar]
- Quaranta, S.; Savi, P.; Giorcelli, M.; Khan, A.A.; Tagliaferro, A.; Jia, C.Q. Biochar-polymer composites and thin films: Characterizations and applications. In Proceedings of the 2016 IEEE 2nd International Forum on Research and Technologies for Society and Industry Leveraging a better tomorrow (RTSI), Bologna, Italy, 7–9 September 2016; pp. 1–4. [Google Scholar]
- Jagdale, P.; Koumoulos, E.P.; Cannavaro, I.; Khan, A.; Castellino, M.; Dragatogiannis, D.A.; Tagliaferro, A.; Charitidis, C.A. Towards green carbon fibre manufacturing from waste cotton: A microstructural and physical property investigation. Manuf. Rev. 2017, 4, 10. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Jagdale, P.; Castellino, M.; Rovere, M.; Jehangir, Q.; Mandracci, P.; Rosso, C.; Tagliaferro, A. Innovative functionalized carbon fibers from waste: How to enhance polymer composites properties. Compos. Part B Eng. 2018, 139, 31–39. [Google Scholar] [CrossRef]
- Arrigo, R.; Jagdale, P.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Structure–Property Relationships in Polyethylene-Based Composites Filled with Biochar Derived from Waste Coffee Grounds. Polymers 2019, 11, 1336. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Khan, M.U.; Lin, X.; Cai, H.; Lei, H. Temperature varied biochar as a reinforcing filler for high-density polyethylene composites. Compos. Part B Eng. 2019, 175, 107151. [Google Scholar] [CrossRef]
- Li, S.; Huang, A.; Chen, Y.-J.; Li, D.; Turng, L.-S. Highly filled biochar/ultra-high molecular weight polyethylene/linear low density polyethylene composites for high-performance electromagnetic interference shielding. Compos. Part B Eng. 2018, 153, 277–284. [Google Scholar] [CrossRef]
- Bajwa, D.S.; Adhikari, S.; Shojaeiarani, J.; Bajwa, S.G.; Pandey, P.; Shanmugam, S.R. Characterization of bio-carbon and ligno-cellulosic fiber reinforced bio-composites with compatibilizer. Constr. Build. Mater. 2019, 204, 193–202. [Google Scholar] [CrossRef]
- Das, O.; Bhattacharyya, D.; Sarmah, A.K. Sustainable eco–composites obtained from waste derived biochar: A consideration in performance properties, production costs, and environmental impact. J. Clean. Prod. 2016, 129, 159–168. [Google Scholar] [CrossRef]
- Behazin, E.; Misra, M.; Mohanty, A.K. Sustainable biocarbon from pyrolyzed perennial grasses and their effects on impact modified polypropylene biocomposites. Compos. Part B Eng. 2017, 118, 116–124. [Google Scholar] [CrossRef]
- Das, O.; Kim, N.K.; Kalamkarov, A.L.; Sarmah, A.K.; Bhattacharyya, D. Biochar to the rescue: Balancing the fire performance and mechanical properties of polypropylene composites. Polym. Degrad. Stab. 2017, 144, 485–496. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K.; Bhattacharyya, D. Biocomposites from waste derived biochars: Mechanical, thermal, chemical, and morphological properties. Waste Manag. 2016, 49, 560–570. [Google Scholar] [CrossRef]
- Das, O.; Bhattacharyya, D.; Hui, D.; Lau, K.-T. Mechanical and flammability characterisations of biochar/polypropylene biocomposites. Compos. Part B Eng. 2016, 106, 120–128. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K.; Bhattacharyya, D. A sustainable and resilient approach through biochar addition in wood polymer composites. Sci. Total Environ. 2015, 512, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Poulose, A.M.; Elnour, A.Y.; Anis, A.; Shaikh, H.; Al-Zahrani, S.M.; George, J.; Al-Wabel, M.I.; Usman, A.R.; Ok, Y.S.; Tsang, D.C.W.; et al. Date palm biochar-polymer composites: An investigation of electrical, mechanical, thermal and rheological characteristics. Sci. Total Environ. 2018, 619, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Nan, N.; DeVallance, D.B.; Xie, X.; Wang, J. The effect of bio-carbon addition on the electrical, mechanical, and thermal properties of polyvinyl alcohol/biochar composites. J. Compos. Mater. 2016, 50, 1161–1168. [Google Scholar] [CrossRef]
- Nan, N.; DeVallance, D.B. Development of poly (vinyl alcohol)/wood-derived biochar composites for use in pressure sensor applications. J. Mater. Sci. 2017, 52, 8247–8257. [Google Scholar] [CrossRef]
- Nan, W.; Zhao, Y.; Ding, Y.; Shende, A.R.; Fong, H.; Shende, R.V. Mechanically flexible electrospun carbon nanofiber mats derived from biochar and polyacrylonitrile. Mater. Lett. 2017, 205, 206–210. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Misra, M.; Mohanty, A.K. Impact of interfacial adhesion on the microstructure and property variations of biocarbons reinforced nylon 6 biocomposites. Compos. Part A Appl. Sci. Manuf. 2017, 98, 32–44. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, S.; Qian, S.; Fontanillo Lopez, C.A. High-toughness PLA/Bamboo cellulose nanowhiskers bionanocomposite strengthened with silylated ultrafine bamboo-char. Compos. Part B Eng. 2019, 165, 174–182. [Google Scholar] [CrossRef]
- George, J.; Azad, L.B.; Poulose, A.M.; An, Y.; Sarmah, A.K. Nano-mechanical behaviour of biochar-starch polymer composite: Investigation through advanced dynamic atomic force microscopy. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105486. [Google Scholar] [CrossRef]
- Das, O.; Hedenqvist, M.S.; Johansson, E.; Olsson, R.T.; Loho, T.A.; Capezza, A.J.; Singh Raman, R.K.; Holder, S. An all-gluten biocomposite: Comparisons with carbon black and pine char composites. Compos. Part A Appl. Sci. Manuf. 2019, 120, 42–48. [Google Scholar] [CrossRef]
- Mardoyan, A.; Braun, P. Analysis of Czech subsidies for solid biofuels. Int. J. Green Energy 2015, 12, 405–408. [Google Scholar] [CrossRef]
- Maroušek, J.; Hašková, S.; Zeman, R.; Váchal, J.; Vaníčková, R. Processing of residues from biogas plants for energy purposes. Clean Technol. Environ. Policy 2015, 17, 797–801. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Lee, J.; Jung, J.-M.; Oh, J.-I.; Ok, Y.S.; Lee, S.-R.; Kwon, E.E. Evaluating the effectiveness of various biochars as porous media for biodiesel synthesis via pseudo-catalytic transesterification. Bioresour. Technol. 2017, 231, 59–64. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Luo, J.; Zhang, S.; Yang, X.; Igalavithana, A.D.; Ok, Y.S.; Tsang, D.C.W.; Lin, C.S.K. Efficient succinic acid production using a biochar-treated textile waste hydrolysate in an in situ fibrous bed bioreactor. Biochem. Eng. J. 2019, 149, 107249. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Impacts of acidity and textural properties of oxidized carbon materials on their catalytic activity for hydrolysis of cellobiose. Microporous Mesoporous Mater. 2016, 219, 317–321. [Google Scholar] [CrossRef]
- Kastner, J.R.; Miller, J.; Geller, D.P.; Locklin, J.; Keith, L.H.; Johnson, T. Catalytic esterification of fatty acids using solid acid catalysts generated from biochar and activated carbon. Catal. Today 2012, 190, 122–132. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, P.; Zhu, X.; Li, H.; Deng, Q.; Wang, J.; Zeng, Z.; Zou, J.-J.; Deng, S. Highly Efficient Alkylation Using Hydrophobic Sulfonic Acid-Functionalized Biochar as a Catalyst for Synthesis of High-Density Biofuels. Acs Sustain. Chem. Eng. 2019, 7, 14973–14981. [Google Scholar] [CrossRef]
- Zhong, Y.; Deng, Q.; Zhang, P.; Wang, J.; Wang, R.; Zeng, Z.; Deng, S. Sulfonic acid functionalized hydrophobic mesoporous biochar: Design, preparation and acid-catalytic properties. Fuel 2019, 240, 270–277. [Google Scholar] [CrossRef]
- Vidal, J.L.; Andrea, V.P.; MacQuarrie, S.L.; Kerton, F.M. Oxidized Biochar as a Simple, Renewable Catalyst for the Production of Cyclic Carbonates from Carbon Dioxide and Epoxides. ChemCatChem 2019, 11, 4089–4095. [Google Scholar] [CrossRef]
- Areeprasert, C.; Khaobang, C. Pyrolysis and catalytic reforming of ABS/PC and PCB using biochar and e-waste char as alternative green catalysts for oil and metal recovery. Fuel Process. Technol. 2018, 182, 26–36. [Google Scholar] [CrossRef]
- Yuan, Y.; Bolan, N.; Prévoteau, A.; Vithanage, M.; Biswas, J.K.; Ok, Y.S.; Wang, H. Applications of biochar in redox-mediated reactions. Bioresour. Technol. 2017, 246, 271–281. [Google Scholar] [CrossRef]
- Cao, X.; Huang, Y.; Tang, C.; Wang, J.; Jonson, D.; Fang, Y. Preliminary study on the electrocatalytic performance of an iron biochar catalyst prepared from iron-enriched plants. J. Environ. Sci. 2020, 88, 81–89. [Google Scholar] [CrossRef]
- Noman, M.; Sanginario, A.; Jagdale, P.; Castellino, M.; Demarchi, D.; Tagliaferro, A. Pyrolyzed bamboo electrode for electrogenerated chemiluminescence of Ru (bpy) 32+. Electrochim. Acta 2014, 133, 169–173. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, H.; Yang, J.; Shi, Z.; Tan, Y.; Jin, J.; Wang, R.; Zhang, S.; Wang, J. Biochar decorated with gold nanoparticles for electrochemical sensing application. Electrochim. Acta 2018, 261, 464–473. [Google Scholar] [CrossRef]
- Ziegler, D.; Palmero, P.; Giorcelli, M.; Tagliaferro, A.; Tulliani, J.-M. Biochars as Innovative Humidity Sensing Materials. Chemosensors 2017, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Jagdale, P.; Ziegler, D.; Rovere, M.; Tulliani, J.M.; Tagliaferro, A. Waste Coffee Ground Biochar: A Material for Humidity Sensors. Sensors 2019, 19, 801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agustini, D.; Mangrich, A.S.; Bergamini, M.F.; Marcolino-Junior, L.H. Sensitive voltammetric determination of lead released from ceramic dishes by using of bismuth nanostructures anchored on biochar. Talanta 2015, 142, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.R.; Lamy-Mendes, A.C.; Rezende, E.I.P.; Mangrich, A.S.; Marcolino Junior, L.H.; Bergamini, M.F. Electrochemical determination of copper ions in spirit drinks using carbon paste electrode modified with biochar. Food Chem. 2015, 171, 426–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, P.R.d.; Lamy-Mendes, A.C.; Gogola, J.L.; Mangrich, A.S.; Marcolino Junior, L.H.; Bergamini, M.F. Mercury nanodroplets supported at biochar for electrochemical determination of zinc ions using a carbon paste electrode. Electrochim. Acta 2015, 151, 525–530. [Google Scholar] [CrossRef]
- Oliveira, P.R.; Kalinke, C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Copper hexacyanoferrate nanoparticles supported on biochar for amperometric determination of isoniazid. Electrochim. Acta 2018, 285, 373–380. [Google Scholar] [CrossRef]
- de Oliveira, P.R.; Kalinke, C.; Gogola, J.L.; Mangrich, A.S.; Junior, L.H.M.; Bergamini, M.F. The use of activated biochar for development of a sensitive electrochemical sensor for determination of methyl parathion. J. Electroanal. Chem. 2017, 799, 602–608. [Google Scholar] [CrossRef]
- Kalinke, C.; Wosgrau, V.; Oliveira, P.R.; Oliveira, G.A.; Martins, G.; Mangrich, A.S.; Bergamini, M.F.; Marcolino-Junior, L.H. Green method for glucose determination using microfluidic device with a non-enzymatic sensor based on nickel oxyhydroxide supported at activated biochar. Talanta 2019, 200, 518–525. [Google Scholar] [CrossRef]
- Martins, G.; Gogola, J.L.; Caetano, F.R.; Kalinke, C.; Jorge, T.R.; Santos, C.N.D.; Bergamini, M.F.; Marcolino-Junior, L.H. Quick electrochemical immunoassay for hantavirus detection based on biochar platform. Talanta 2019, 204, 163–171. [Google Scholar] [CrossRef]
- Huang, J.-F.; Shi, Q.-S.; Feng, J.; Chen, M.-J.; Li, W.-R.; Li, L.-Q. Facile pyrolysis preparation of rosin-derived biochar for supporting silver nanoparticles with antibacterial activity. Compos. Sci. Technol. 2017, 145, 89–95. [Google Scholar] [CrossRef]
- Bock, E.M.; Coleman, B.S.L.; Easton, Z.M. Effect of biochar, hydraulic residence time, and nutrient loading on greenhouse gas emission in laboratory-scale denitrifying bioreactors. Ecol. Eng. 2018, 120, 375–383. [Google Scholar] [CrossRef]
- Duan, X.; Chen, Y.; Yan, Y.; Feng, L.; Chen, Y.; Zhou, Q. New method for algae comprehensive utilization: Algae-derived biochar enhances algae anaerobic fermentation for short-chain fatty acids production. Bioresour. Technol. 2019, 289, 121637. [Google Scholar] [CrossRef] [PubMed]
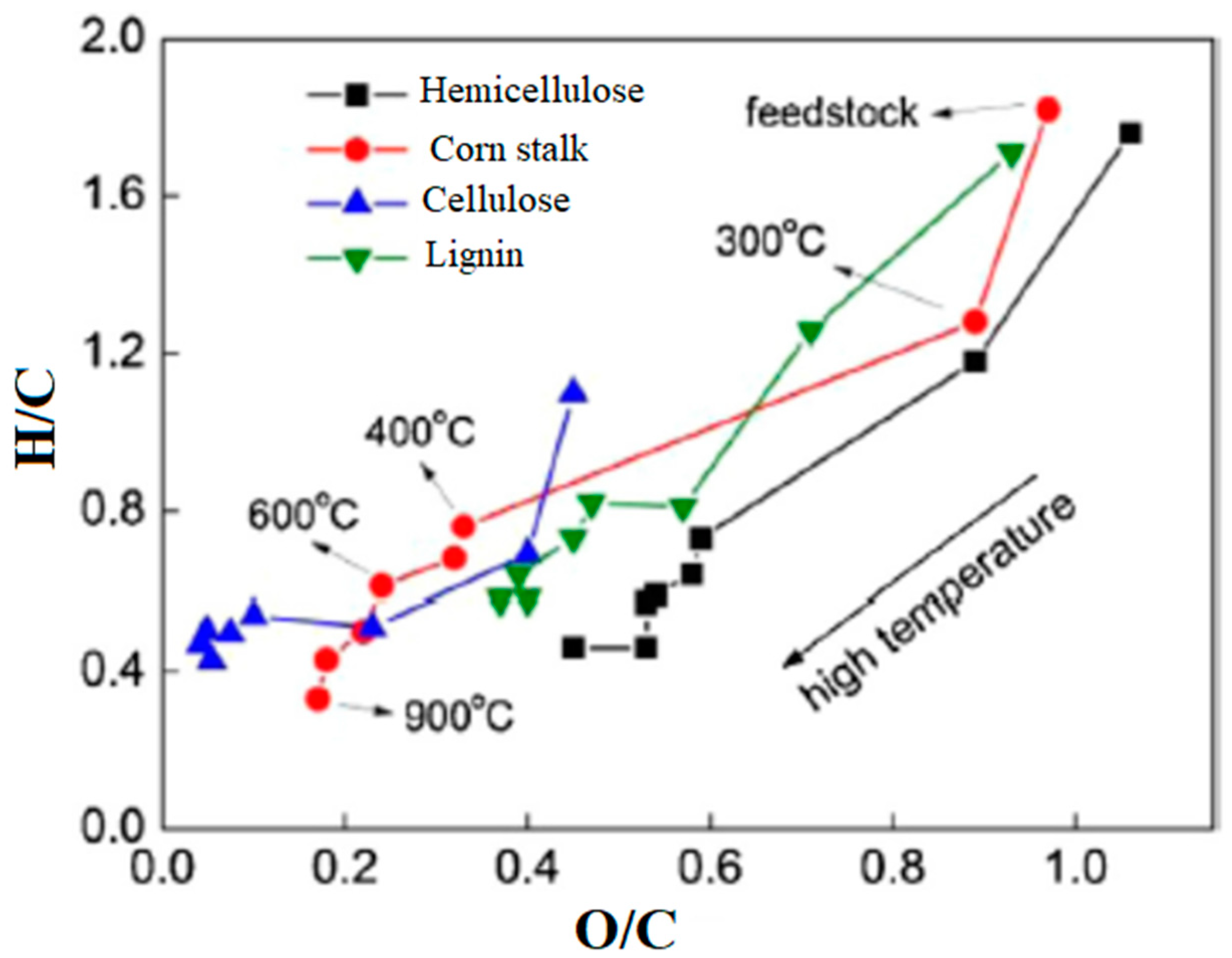
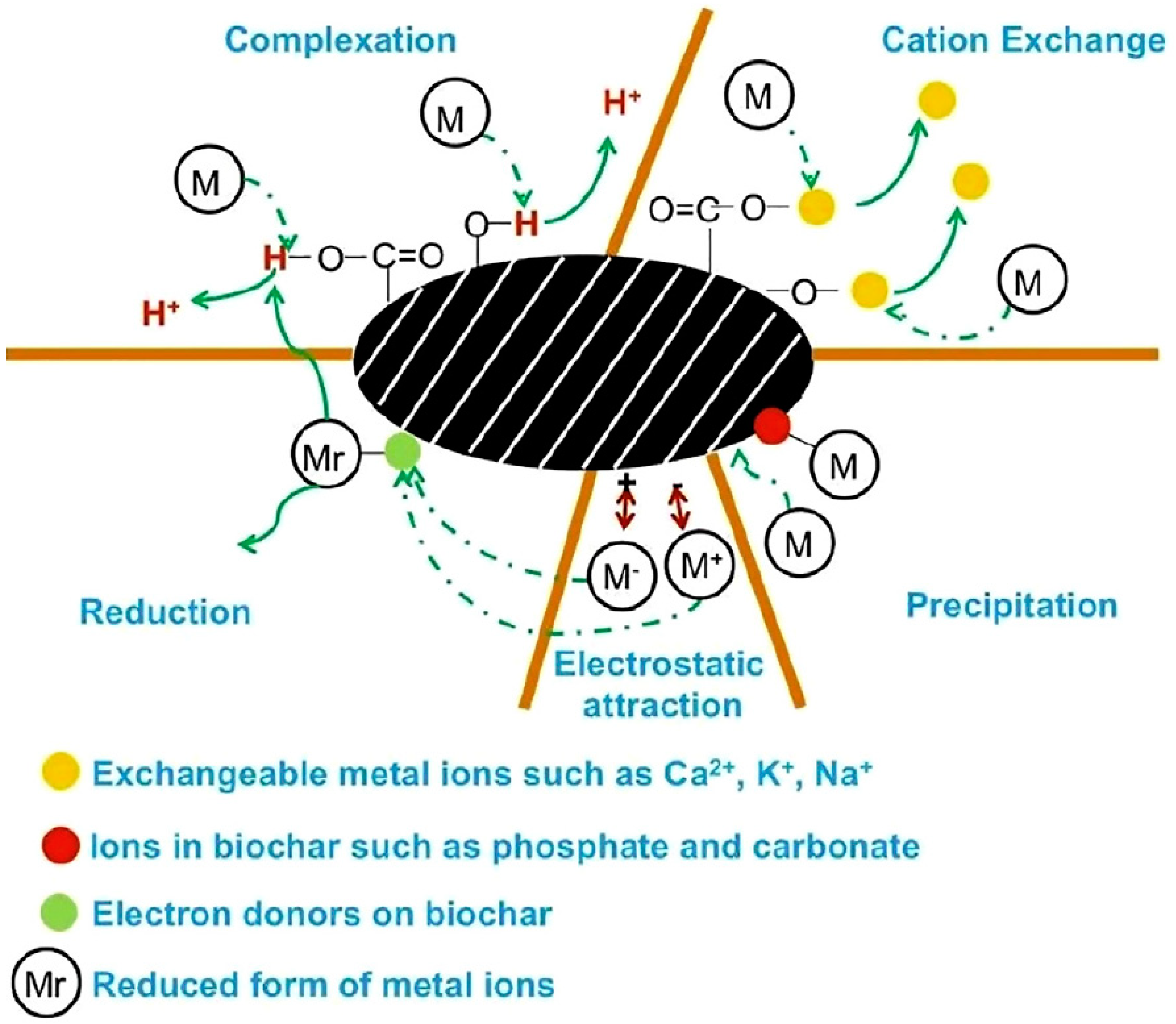
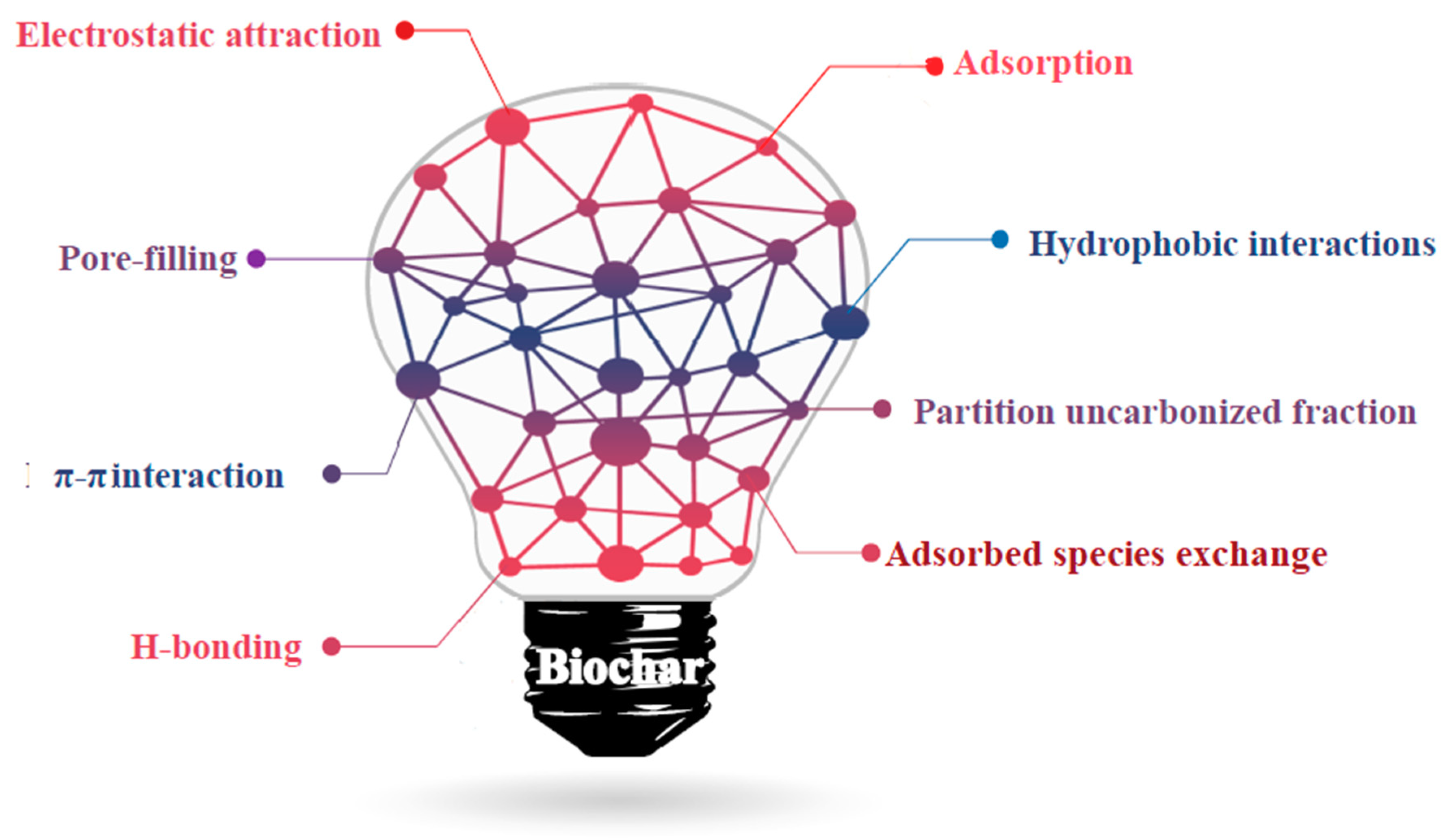

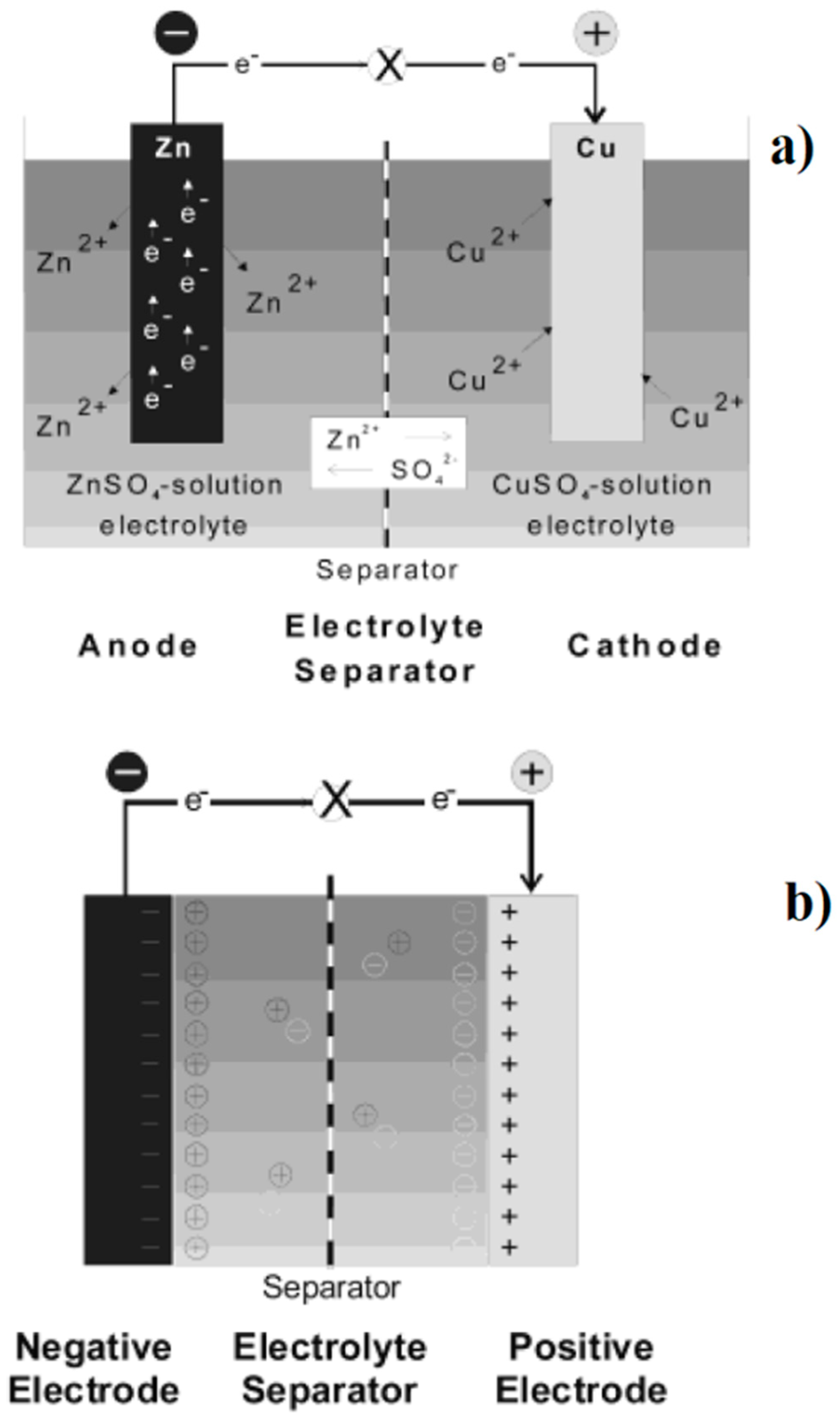

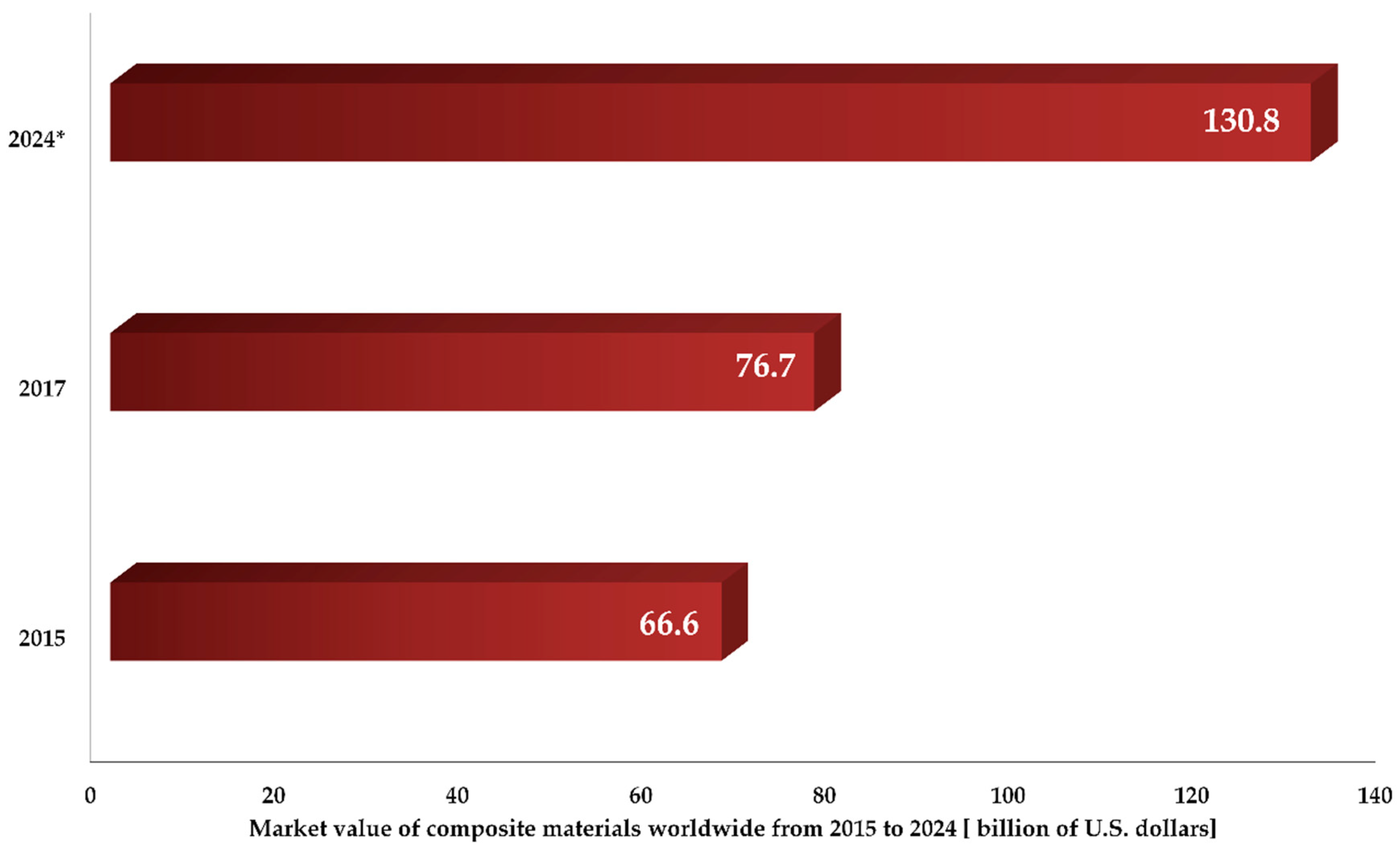

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A Review of Non-Soil Biochar Applications. Materials 2020, 13, 261. https://doi.org/10.3390/ma13020261
Bartoli M, Giorcelli M, Jagdale P, Rovere M, Tagliaferro A. A Review of Non-Soil Biochar Applications. Materials. 2020; 13(2):261. https://doi.org/10.3390/ma13020261
Chicago/Turabian StyleBartoli, Mattia, Mauro Giorcelli, Pravin Jagdale, Massimo Rovere, and Alberto Tagliaferro. 2020. "A Review of Non-Soil Biochar Applications" Materials 13, no. 2: 261. https://doi.org/10.3390/ma13020261
APA StyleBartoli, M., Giorcelli, M., Jagdale, P., Rovere, M., & Tagliaferro, A. (2020). A Review of Non-Soil Biochar Applications. Materials, 13(2), 261. https://doi.org/10.3390/ma13020261







