Controlling the Degradation Rate of Biodegradable Mg–Zn-Mn Alloys for Orthopedic Applications by Electrophoretic Deposition of Hydroxyapatite Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Magnesium Alloys and Hydroxyapatite
- -
- obtaining Mg alloy specimens and evaluating their surface properties;
- -
- preparing HAP solution;
- -
- deposing HAP on the ZM21, ZMX410 alloys surface by EPD.
2.1.1. Preparation of Hydroxyapatite Solution
2.1.2. Deposition of Hydroxyapatite on the Magnesium Alloys Surface by Electrophorethic Method
2.2. Characterization Methods
2.2.1. Scanning Electron Microscopy coupled with Energy Dispersive Spectroscopy
2.2.2. X-ray Diffraction
2.2.3. Fourier-Transform Infrared Spectroscopy
2.2.4. Corrosion Resistance of the Magnesium Alloys in the Simulated Media
Immersion Test
Evaluation of Electrochemical Corrosion Behaviour of the Uncoated and Hydroxyapatite Coated Specimens
- open circuit potential (Eoc) measurement over 1 h;
- plotting the linear polarization curves from –250 mV (vs. OCP) to + 250 mV (vs. OCP) - Tafel curves, with a scan rate of 1 mV/s.
- open circuit potential (Eoc);
- corrosion potential (Ecorr);
- the density of the corrosion current (icorr);
- the slope of the cathodic curve (βc);
- the slope of the anodic curve (βa).
- corrosion rate (CR);
- polarization resistance (Rp);
- corrosive attack efficiency (Pe).
2.2.5. Contact Angle Determination
3. Results and Discussion
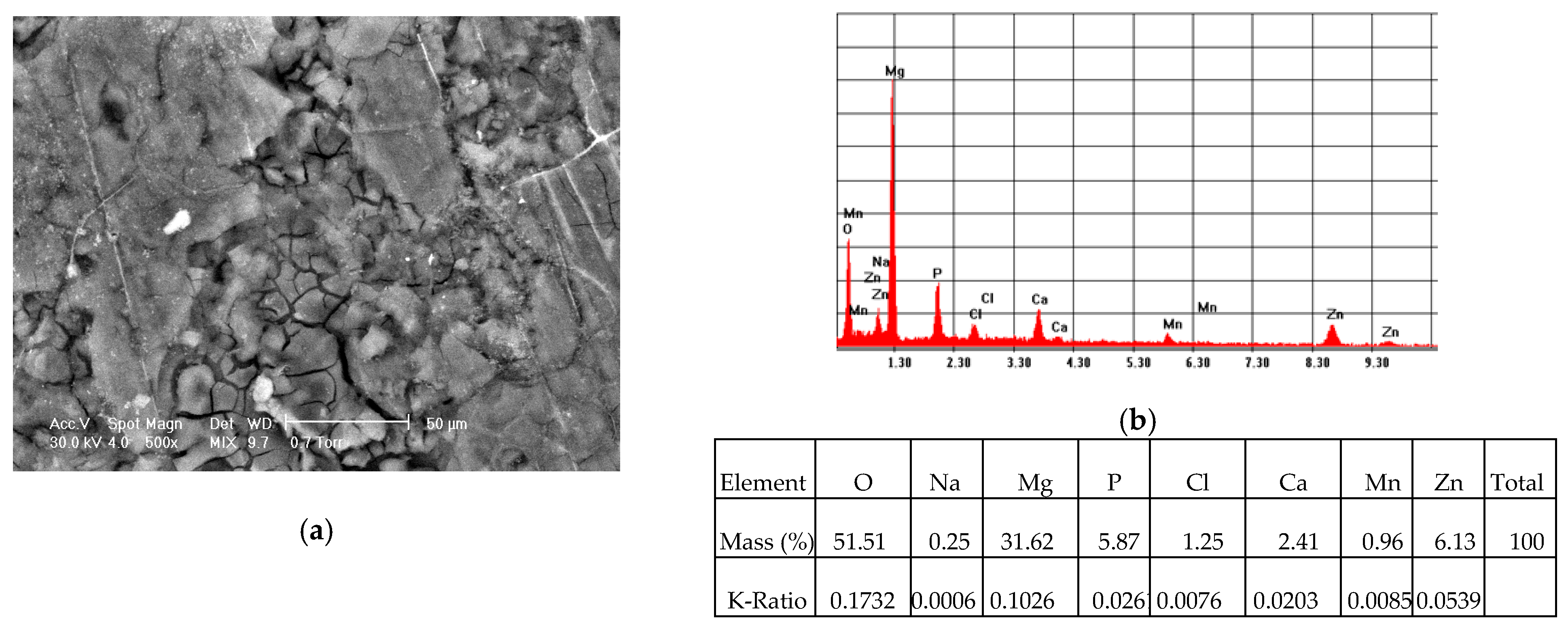
3.1. Scanning Electron Microscopy (SEM) Coupled with Energy-Dispersive Spectroscopy (EDS) Results
3.2. XRD Results
3.3. FTIR Results
3.4. Corrosion Behaviour of the Mg Alloys in the SBF
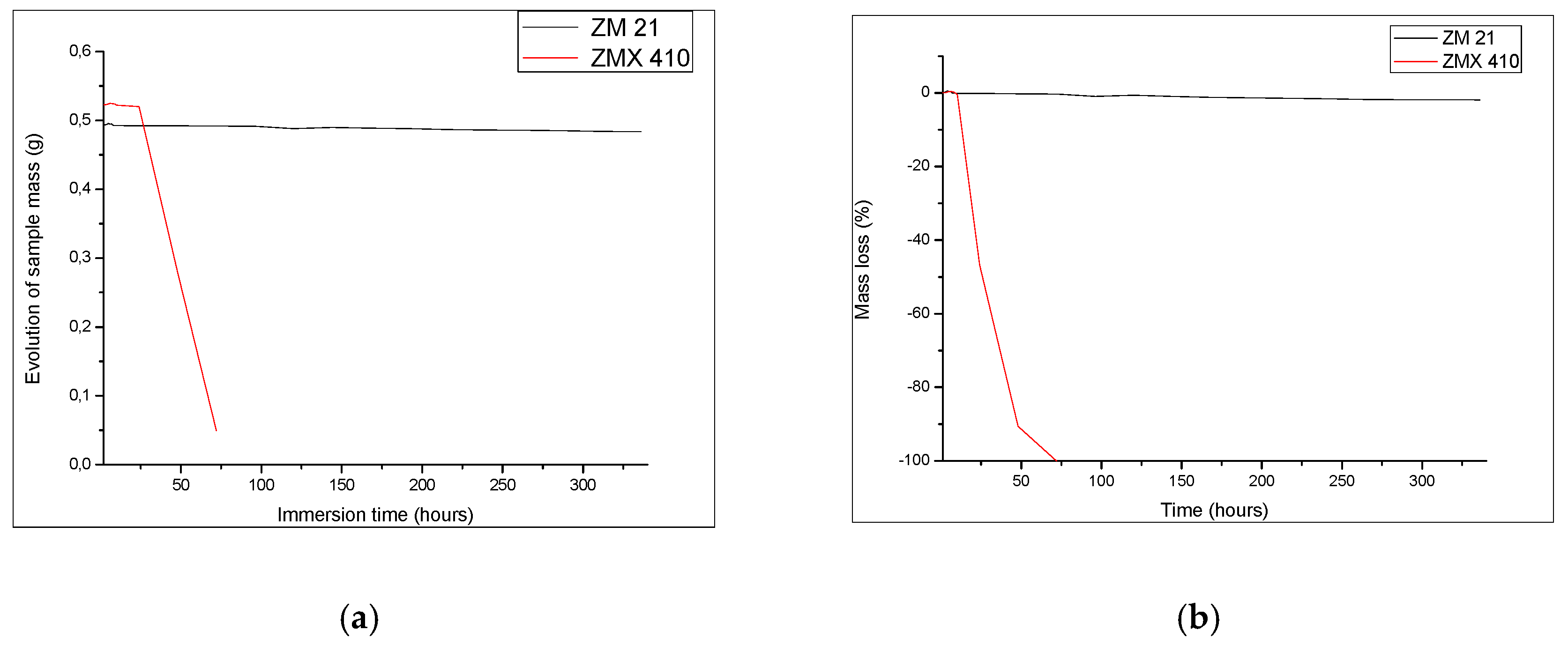
3.4.1. Immersion Test
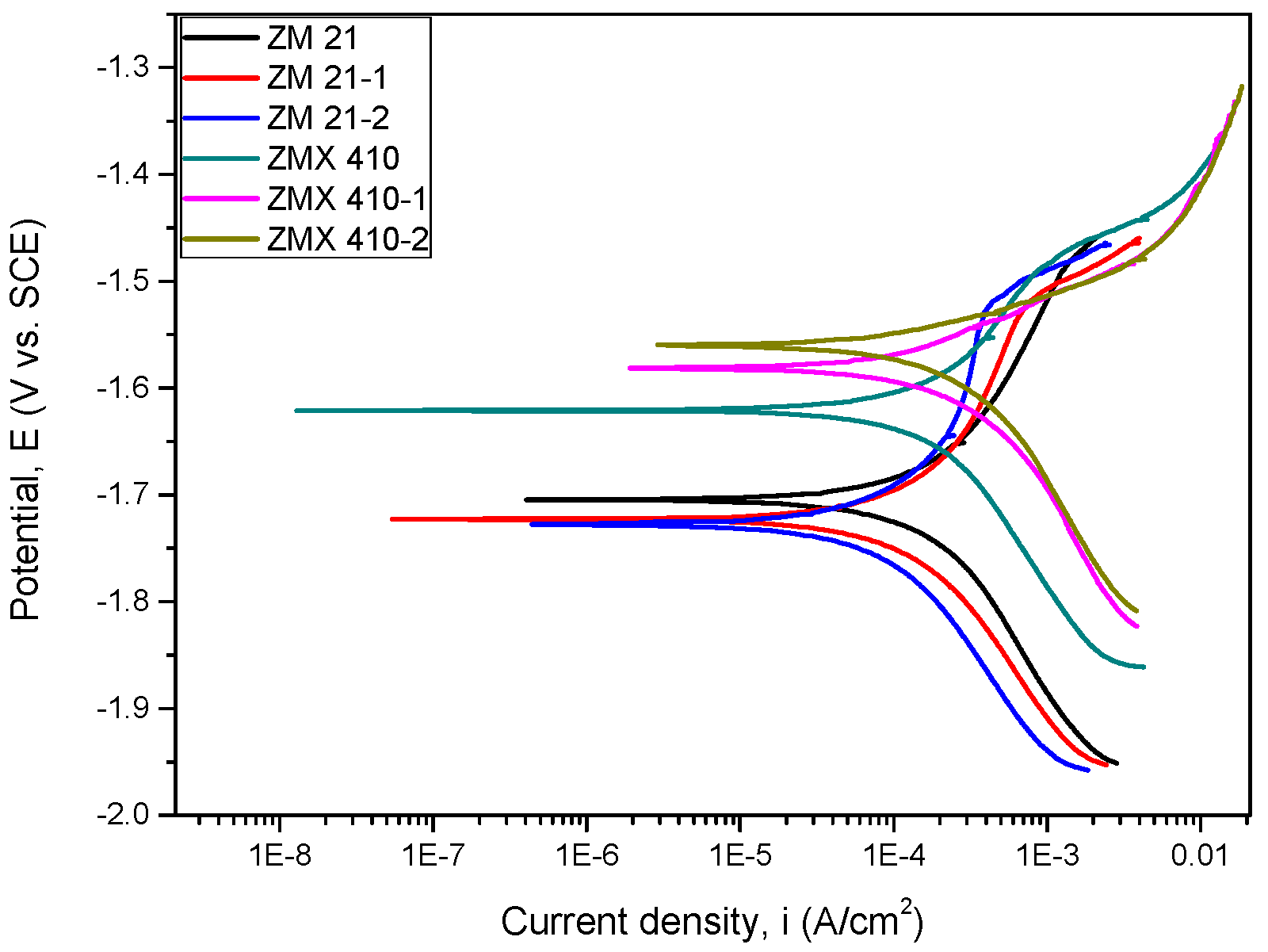
3.4.2. Evaluation of Electrochemical Corrosion Behavior of the Uncoated and HAP Coated Specimens
Corrosion Resistance
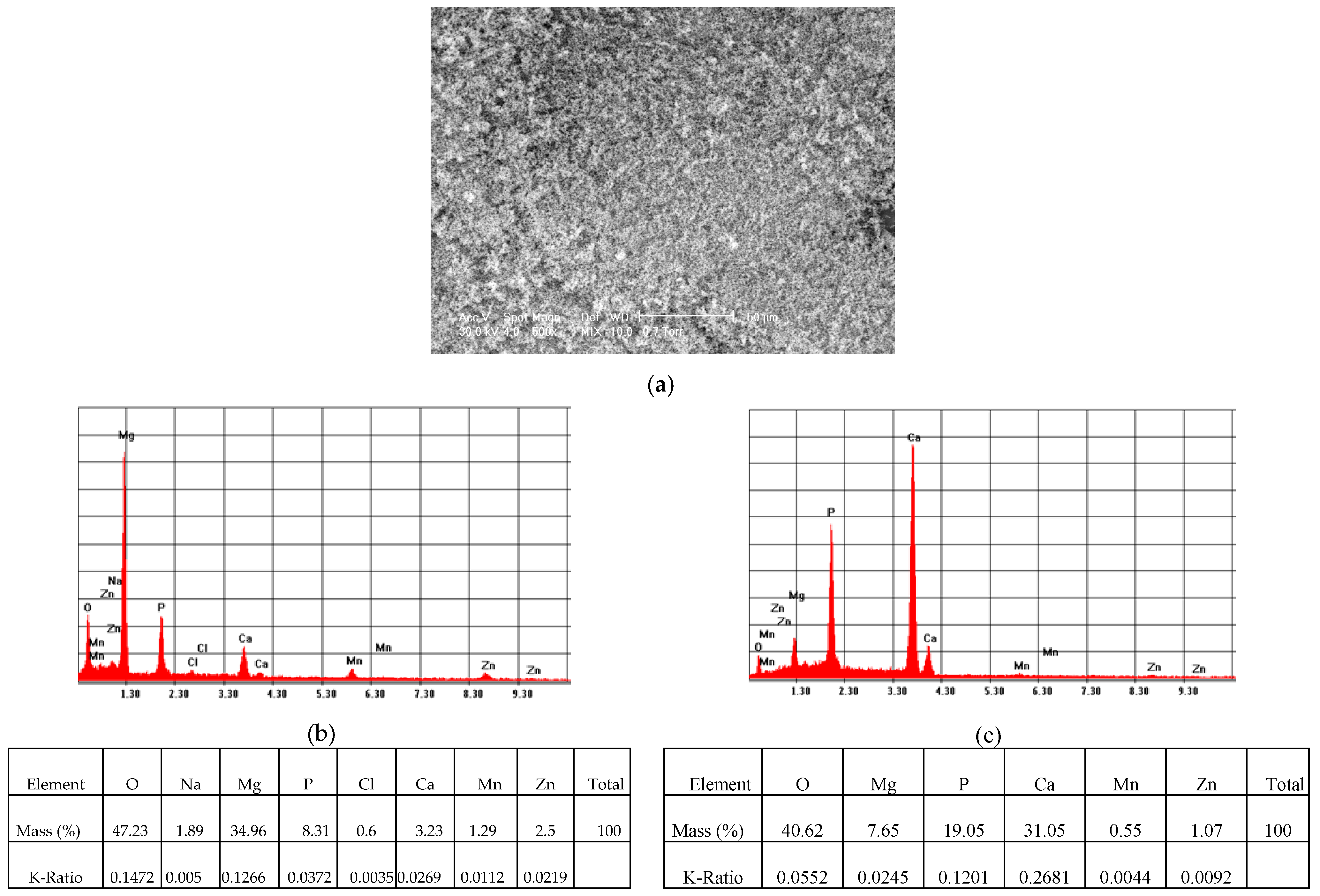
Surface Analysis by SEM- EDS of the Samples after Corrosion Experiments
XRD Analysis of the Samples after Corrosion Experiments
FTIR Analysis of the Samples after Corrosion Experiments
3.5. Contact Angle
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In vivo corrosion of magnesium alloys and the associated bone response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef] [PubMed]
- Winzer, N.; Atrens, A.; Song, G.; Ghali, E.; Dietzel, W.; Kainer, K.U.; Hort, N.; Blawert, C. A critical review of the stress corrosion cracking (SCC) of magnesium alloys. Adv. Eng. Mater. 2005, 7, 659–693. [Google Scholar] [CrossRef]
- Liu, L.J.; Schlesinger, M. Corrosion of magnesium and its alloys. Corros. Sci. 2009, 51, 1733–1737. [Google Scholar] [CrossRef]
- Waizy, H.; Seitz, J.-M.; Reifenrath, J.; Weizbauer, A.; Bach, F.-W.; Meyer-Lindenberg, A.; Denkena, B.; Windhagen, H. Biodegradable Magnesium Implants for Orthopedic Applications. J. Mater. Sci. 2012, 48, 39–50. [Google Scholar] [CrossRef]
- Staigeret, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.N.; Zheng, Y.F. A Review on Magnesium Alloys as Biodegradable Materials. Front. Mater. Sci. China 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Shi, P.; Ng, W.F.; Wong, M.H.; Cheng, F.T. Improvement of Corrosion Resistance of Pure Magnesium in Hanks’ Solution by MicroarcOxidation with Sol–Gel TiO2 Sealing. J. Alloys Compd. 2009, 469, 286–292. [Google Scholar] [CrossRef]
- Vilcioiu, J.D.; Zamfirescu, D.G.; Cristescu, I.; Ursache, A.; Popescu, S.A.; Creanga, C.A.; Lascar, I. The interdisciplinary approach of an aggressive giant cell tumor of bone complicated with a fracture of the distal femur. Rom. J. Morphol. Embryol. 2016, 57, 567–572. [Google Scholar]
- Rau, J.V.; Antoniac, I.; Cama, G.; Komlev, V.S.; Ravaglioli, A. Bioactive Materials for Bone Tissue Engineering. BioMed Res. Int. 2016, 2016, 3. [Google Scholar] [CrossRef]
- Fathi, M.; Meratian, M.; Ravazi, M. Novel magnesium-nanofluorapatite metal matrix nanocomposite with improved biodegradation behavior. J. BioMed Nanotechnol. 2011, 7, 441–445. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Filipescu, M.; Cotrut, C.; Fosca, M.; Nistor, L.C.; Birjega, R.; Dinescu, M. Hydroxyapatite coatings on Mg-Ca alloy prepared by Pulsed Laser Deposition: Properties and corrosion resistance in Simulated Body Fluid. Ceram. Int. 2018, 44, 16678–16687. [Google Scholar] [CrossRef]
- Miculescu, F.; Bojin, D.; Ciocan, L.T.; Antoniac, I.; Miculescu, M.; Miculescu, N. Experimental researches on biomaterial-tissue interface interactions. J. Optoelectron. Adv. Mater. 2007, 9, 3303–3306. [Google Scholar]
- Ionescu, R.; Cristescu, I.; Dinu, M.; Saban, R.; Antoniac, I.; Vilcioiu, D. Clinical, Biomechanical and Biomaterials Approach in the Case of Fracture Repair Using Different Systems Type Plate-Screw. Key Eng. Mater. 2014, 583, 150–154. [Google Scholar] [CrossRef]
- Bita, A.I.; Antoniac, A.; Cotrut, C.; Vasile, E.; Ciuca, I.; Niculescu, M.; Antoniac, I. In vitro Degradation and Corrosion Evaluation of Mg-Ca Alloys for Biomedical Applications. J. Optoelectron. Adv. Mater. 2016, 18, 394–398. [Google Scholar]
- Wong, H.M.; Yeung, K.W.K.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.K.; Cheung, K.M.C. A biodegradable polymer-based coatings to control the performance of magnesium alloy orthopaedic implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar] [CrossRef]
- Kainer, K.U.; Srinivasan, P.B.; Blawert, C.; Dietzel, W. Chapter 3.09. Corrosion of magnesium and its alloys. In Shreir’s Corrosion; Elsevier Science: Amsterdam, The Netherlands, 2009; Volume 3, pp. 2011–2041. [Google Scholar]
- Zeng, R.C.; Zhang, J.; Huang, W.; Dietzel, W.; Kainer, K.U.; Blawert, C.; Ke, W. Review of studies on corrosion of magnesium alloys. Trans. Nonferr. Met. Soc. China 2006, 16, 763–771. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Fosca, M.; De Bonis, A.; Blajan, A.I.; Cotrut, C.; Graziani, V.; Curcio, M.; Cricenti, A.; Niculescu, M.; et al. Glass-ceramic coated Mg-Ca alloys for biomedical implant applications. Mater. Sci. Eng. C 2016, 64, 362–369. [Google Scholar] [CrossRef]
- Yu, W.; Sun, R.; Guo, Z.; Wang, Z.; He, Y.; Lu, G.; Chen, P.; Chen, K. Novel fluoridated hydroxyapatite/MAO composite coating on AZ31B, magnesium alloy for biomedical application. Appl. Surf. Sci. 2019, 464, 708–715. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, Y.; Wang, Y.; Ma, L.; Li, F. Preparation and thermal stability analysis of hydroxyapatite derived from the precipitation process and microwave irradiation method. Mater. Lett. 2005, 58, 3586–3590. [Google Scholar] [CrossRef]
- Tecu, C.; Antoniac, I.; Goller, G.; Yavas, B.; Gheorghe, D.; Antoniac, A.; Ciuca, I.; Semenescu, A.; Raiciu, A.D.; Cristescu, I. The Sintering Behaviour and Mechanical Properties of Hydroxyapatite—Based Composites for Bone Tissue Regeneration. Mater. Plast. 2019, 56, 644–648. [Google Scholar]
- Majumdara, J.D.; Galun, R.; Mordike, B.L.; Manna, I. Effect of laser melting on corrosion and wear resistance of a commercial magnesium alloy. Mater. Sci. Eng. 2003, 361, 119–129. [Google Scholar] [CrossRef]
- Antoniac, I.V.; Dinu, M.; Cotrut, C. The potential of magnesium alloys for hard tissue regeneration. J. Tissue Eng. Regen. Med. 2012, 6, 234. [Google Scholar]
- Dearnly, P.A. A brief review of test methodologies for surface-engineered biomedical implant alloy. Surf. Coat. Technol. 2005, 198, 483–490. [Google Scholar] [CrossRef]
- Carboneras, M.; Hernández, L.S.; del Valle, J.A.; García-Alonso, M.C.; Escudero, M.L. Corrosion protection of different environmentally friendly coatings on powder metallurgy magnesium. J. Alloys Compd. 2010, 496, 442–448. [Google Scholar] [CrossRef]
- Liu, M.; Uggowitzer, P.J.; Nagasekhar, A.V.; Schmutz, P.; Easton, M.; Song, G.; Atrens, A. Calculated phase diagrams and the corrosion of die-cast Mg–Al alloys. Corros. Sci. 2009, 51, 602–619. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. ActaBiomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Coy, A.E.; Viejo, F.; Garcia-Garcia, F.J.; Liu, Z.; Skeldon, P.; Thompson, G.E. Effect of eximer laser surface melting on the microstructure and corrosion performance of die cast AZ91D magnesium alloy. Corros. Sci. 2010, 52, 387–397. [Google Scholar] [CrossRef]
- Lhotka, C.; Szekeres, T.; Steffan, I.; Zhuber, K.; Zweymuller, K. Four-year study of cobalt and chromium blood levels in patients managed with two different metal-on-metal total hip replacements. J. Orthop. Res. 2003, 21, 189–195. [Google Scholar] [CrossRef]
- Mareci, D.; Bolat, G.; Izquierdo, J.; Crimu, C.; Munteanu, C.; Antoniac, I.; Souto, R.M. Electrochemical characteristics of bioresorbable binary MgCa alloys in Ringer’s solution: Revealing the impact of local pH distributions during in-vitro dissolution. Mater. Sci. Eng. C 2016, 60, 402–410. [Google Scholar] [CrossRef]
- Niu, L.Y.; Jiang, Z.H.; Li, G.Y.; Gu, C.D.; Lian, J.S. A study and application of zinc phosphate coating on AZ91D magnesium alloy. Surf. Coat. Techol. 2006, 200, 3021–3026. [Google Scholar] [CrossRef]
- Bita, A.I.; Stan, G.E.; Niculescu, M.; Ciuca, I.; Vasile, E.; Antoniac, I. Adhesion evaluation of different bioceramic coatings on Mg-Ca alloys for biomedical applications. J. Adhes. Sci. Technol. 2016, 30, 1968–1983. [Google Scholar] [CrossRef]
- Gray, J.E. Protective coatings on magnesiumand its alloys—A critical review. J. Alloys Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Ghasemi, A.; Raja, V.S.; Blawert, C.; Dietzel, W.; Kainer, K.U. Study of the structure and corrosion behavior of PEO coatings on AM50 magnesium alloy by electrochemical impedance spectroscopy. Surf. Coat. Techol. 2008, 202, 3513–3518. [Google Scholar] [CrossRef]
- Yan, L. Characterization of chemical in homogeneity in plasma-sprayed hydroxyapatite coatings. Biomaterials 2003, 24, 2585–2592. [Google Scholar] [CrossRef]
- Ding, S.J. Properties and immersion behavior of magnetron-sputtered multi-layered hydroxyapatite/titanium composite coatings. Biomaterials 2003, 24, 4233–4238. [Google Scholar] [CrossRef]
- Song, W.H. Biomimetic apatite coatings on micro-arc oxidized titania. Biomaterials 2004, 25, 3341–3349. [Google Scholar] [CrossRef]
- Meng, X. Hydroxyapatite coating by electrophoretic deposition at dynamic voltage. Dent. Mater. J. 2008, 27, 666–671. [Google Scholar] [CrossRef]
- Chiesa, R. Osteointegration of titanium and its alloys by anodic spark deposition and other electrochemical techniques: A review. J. Appl. Biomater. Biomech. 2003, 1, 91–107. [Google Scholar]
- Iafisco, M. Electrostatic spray deposition of biomimetic nanocrystalline apatite coatings onto titanium. Adv. Eng. Mater. 2012, 14, B13–B20. [Google Scholar] [CrossRef]
- Antoniac, I.; Sinescu, C.; Antoniac, A. Adhesion aspects in biomaterials and medical devices. J. Adhes. Sci. Technol. 2016, 30, 1711–1715. [Google Scholar]
- Zeng, R.; Han, E.; Ke, W. Corrosion of artificial aged magnesium alloy AZ80 in 3.5 wtpctNaCl solutions. J. Mater. Sci. Technol. 2007, 23, 353–358. [Google Scholar]
- Waksman, R.; Pakala, R.; Baffour, R.; Seabron, R.; Hellinga, D.; Tio, F.O. Short-term effects of biocorrodible iron stents in porcine coronary arteries. J. Interv. Cardiol. 2008, 21, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Paun, M.A.; Frunza, A.; Stanciulescu, E.L.; Munteanu, T.C.; Cristescu, I.; Grama, S.; Chiotoroiu, A.; Ene, A.; Mihai, C. The use of collagen-coated polypropylene meshes for nasal reconstructive surgery. Ind. Textila 2019, 70, 242–247. [Google Scholar] [CrossRef]
- An, J.; Li, R.G.; Lu, Y.; Chen, C.M.; Xu, Y.; Chen, X.; Wang, L.M. Dry Sliding Wear Behavior of Magnesium Alloys. Wear 2008, 265, 97–104. [Google Scholar] [CrossRef]
- Aung, N.N.; Zhou, W.; Lim, L.E.N. Wear Behaviour of AZ91D Alloy at Low Sliding Speeds. Wear 2008, 265, 780–786. [Google Scholar] [CrossRef]
- Hu, M.; Wang, Q.; Cheng, L.I.; Ding, W. Dry Sliding Wear Behavior of Cast Mg–11Y–5Gd–2Zn Magnesium Alloy. Trans. Nonferr. Met. Soc. China 2012, 22, 1918–1923. [Google Scholar] [CrossRef]
- Selvan, S.; Ramanathan, S. A Comparative Study of the Wear Behavior of As-Cast and Hot Extruded ZE41A Magnesium Alloy. J. Alloys Compd. 2010, 502, 495–502. [Google Scholar] [CrossRef]
- El-Morsy, A.W. Dry Sliding Wear Behavior of Hot Deformed Magnesium AZ61 Alloy as Influenced by the Sliding Conditions. Mater. Sci. Eng. A 2008, 473, 330–335. [Google Scholar] [CrossRef]
- Hort, N.; Huang, Y.; Fechner, D.; Störmer, M.; Blawert, C.; Witte, F.; Vogt, C.; Drücker, H.; Willumeit, R.; Kainer, K.U.; et al. Magnesium Alloys as Implant Materials—Principles of Property Design for Mg-RE Alloys. Acta Biomater. 2010, 6, 1714–1725. [Google Scholar] [CrossRef]
- Witecka, A.; Bogucka, A.; Yamamoto, A.; Máthis, K.; Krajňák, T.; Jaroszewicz, J.; Święszkowski, W. In vitro degradation of ZM21 magnesium alloy in simulated body fluids. Mater. Sci. Eng. C 2016, 65, 59–69. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Hindawi Scanning Vol. 2018, 2018, 15. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Heldbrant, D.; Pan, N. Preparation and preliminary property study of carbon nanotubes films by electrophoretic deposition. Mater. Lett. 2002, 57, 434–438. [Google Scholar] [CrossRef]
- Boccaccini, A.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface 2010, 7, 581–613. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Song, L.; Atrens, A. Corrosion mechanisms of magnesium alloys. Adv. Eng. Mater. 1999, 1, 11–33. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Mass Loss and Residue Measurement Validation of Thermogravimetric Analyzers; ASTM E2402—11; American Society for Testing and Materials: Philadelphia, PA, USA, 2017. [Google Scholar]
- American Society for Testing and Materials. ASTM-G31-72: Standard practice for laboratory immersion corrosion testing of metals. In Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 2004. [Google Scholar]
- Su, Y.; Li, G.; Lian, J. A Chemical Conversion Hydroxyapatite Coating on AZ60 Magnesium Alloy and Its Electrochemical Corrosion Behaviour. Int. J. Electrochem. Sci. 2012, 7, 11497–11511. [Google Scholar]
- Drop Shape Analyzer–DSA 100. Available online: https://www.kruss-scientific.com/products/drop-shape/dsa100/drop-shape-analyzer-dsa100/?gclid=EAIaIQobChMI4d2Ap67o4QIVD-R3Ch3TWgMWEAAYASAAEgKcN_D_BwE (accessed on 4 November 2019).
- De Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Studies in Inorganic Chemistry; Elsevier Science: Amsterdam, The Netherlands, 1996; Volume 18, p. 389. [Google Scholar]
- Youness, R.A.; Taha, M.A.; El-Kheshen, A.A.; Ibrahim, M. Influence of the addition of carbonated hydroxyapatite and selenium dioxide on mechanical properties and in vitro bioactivity of borosilicate inert glass. Ceram. Int. 2018, 44, 20677–20685. [Google Scholar] [CrossRef]
- Zeng, R.C.; Zhou, W.Q.; Han, E.H.; Ke, W. Effect of pH values onas-extruded magnesium alloy AM60. Acta Metall. Sin. 2005, 41, 307–311. [Google Scholar]
- Zeng, R.C.; Yin, Z.Z.; Chen, X.B.; Xu, D.K. Corrosion Types of Magnesium Alloys. In Magnesium Alloys—Selected Issue; InTech Open: London, UK, 2018. [Google Scholar] [CrossRef]
- Yang, J.; Cui, F.; Lee, I.S. Surface Modifications of Magnesium Alloys for Biomedical Applications. Ann. Biomed. Eng. 2011, 39, 1857–1871. [Google Scholar] [CrossRef]
- Zhang, Y. The Effect of Surface Roughness Parameters on Contact and Wettability of Solid Surfaces. Ph.D. Thesis, Iowa State University Ames, Ames, IA, USA, 2007. [Google Scholar]
- Zhang, X.; Wu, G.; Peng, X.; Li, L.; Feng, H.; Gao, B.; Huo, K.; Chu, P.K. Mitigation of Corrosion on Magnesium Alloy by Predesigned Surface Corrosion. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Dunne, C.F.; Katarivas Levy, G.; Hakimi, O.; Aghion, E.; Twomey, B.; Stanton, K.T. Corrosion behaviour of biodegradable magnesium alloys with hydroxyapatite coatings. Surf. Coat. Technol. 2016, 289, 37–44. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Wu, G.S.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Electrochemical corrosion behavior of biodegradable Mg–Y–RE and Mg–Zn–Zr alloys in Ringer’s solution and simulated body fluid. Corros. Sci. 2015, 91, 160–184. [Google Scholar] [CrossRef]








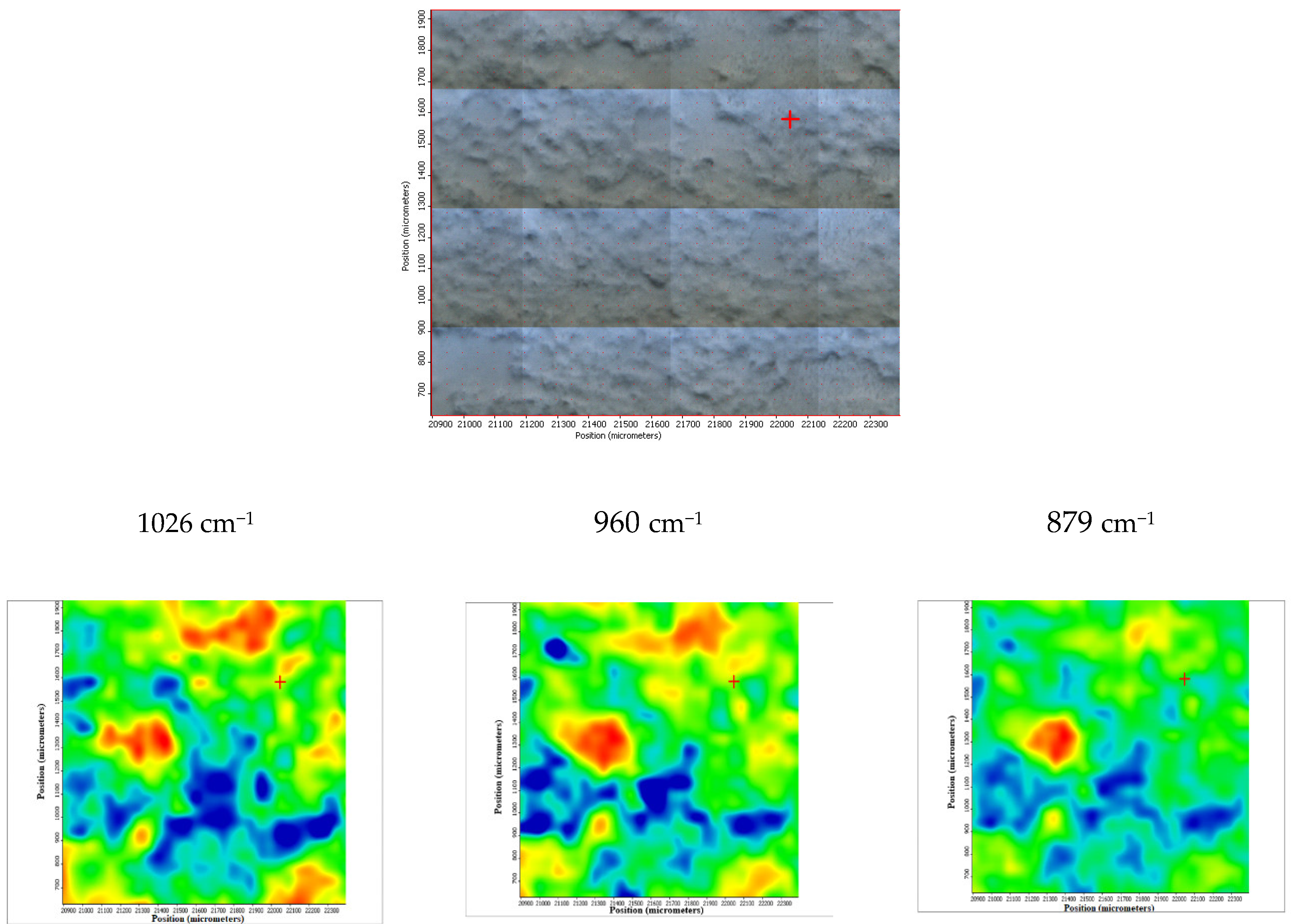









| Alloy | Chemical Element | ||||
|---|---|---|---|---|---|
| Mg (%wt) | Zn (%wt) | Y (%wt) | Mn (%wt) | Ca (%wt) | |
| ZM21 | 96.68 | 2.00 | 0.16 | 1.16 | – |
| ZMX410 | 94.78 | 4.30 | – | 0.62 | 0.30 |
| Time (h) | 24 | 48 | 72 | 96 | 120 | 192 | 336 |
|---|---|---|---|---|---|---|---|
| ZM21 CR (mm/year) | 0.28 | 0.42 | 0.47 | 0.52 | 0.61 | 0.73 | 1.01 |
| ZMX410 CR (mm/year) | 37.81 | 38.58 | 39.77 | – |
| Sample | Ecorr (V) | icorr (µA/cm2) | βc (mV) | βa (mV) | CR (mm/year) | Rp (Ωxcm2) | Pe (%) |
|---|---|---|---|---|---|---|---|
| ZM21 | −1.704 | 314.4 | 319.616 | 332.8 | 6.85 | 225.44 | − |
| ZM21-1 | −1.722 | 192.2 | 246.422 | 296.7 | − | 304.48 | 38.86 |
| ZM21-2 | −1.727 | 181.3 | 296.368 | 420.8 | − | 416.96 | 42.33 |
| ZMX410 | −1.620 | 295.7 | 290.676 | 236.2 | 6.34 | 191.56 | − |
| ZMX410-1 | −1.581 | 283.8 | 220.632 | 111.3 | − | 113.39 | 4.04 |
| ZMX410-2 | −1.560 | 282.6 | 233.624 | 90.8 | − | 100.62 | 4.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniac, I.; Miculescu, F.; Cotrut, C.; Ficai, A.; Rau, J.V.; Grosu, E.; Antoniac, A.; Tecu, C.; Cristescu, I. Controlling the Degradation Rate of Biodegradable Mg–Zn-Mn Alloys for Orthopedic Applications by Electrophoretic Deposition of Hydroxyapatite Coating. Materials 2020, 13, 263. https://doi.org/10.3390/ma13020263
Antoniac I, Miculescu F, Cotrut C, Ficai A, Rau JV, Grosu E, Antoniac A, Tecu C, Cristescu I. Controlling the Degradation Rate of Biodegradable Mg–Zn-Mn Alloys for Orthopedic Applications by Electrophoretic Deposition of Hydroxyapatite Coating. Materials. 2020; 13(2):263. https://doi.org/10.3390/ma13020263
Chicago/Turabian StyleAntoniac, Iulian, Florin Miculescu, Cosmin Cotrut, Anton Ficai, Julietta V. Rau, Elena Grosu, Aurora Antoniac, Camelia Tecu, and Ioan Cristescu. 2020. "Controlling the Degradation Rate of Biodegradable Mg–Zn-Mn Alloys for Orthopedic Applications by Electrophoretic Deposition of Hydroxyapatite Coating" Materials 13, no. 2: 263. https://doi.org/10.3390/ma13020263
APA StyleAntoniac, I., Miculescu, F., Cotrut, C., Ficai, A., Rau, J. V., Grosu, E., Antoniac, A., Tecu, C., & Cristescu, I. (2020). Controlling the Degradation Rate of Biodegradable Mg–Zn-Mn Alloys for Orthopedic Applications by Electrophoretic Deposition of Hydroxyapatite Coating. Materials, 13(2), 263. https://doi.org/10.3390/ma13020263










