Material Analysis and Molecular Dynamics Simulation for Cavitation Erosion and Corrosion Suppression in Water Hydraulic Valves
Abstract
:1. Introduction
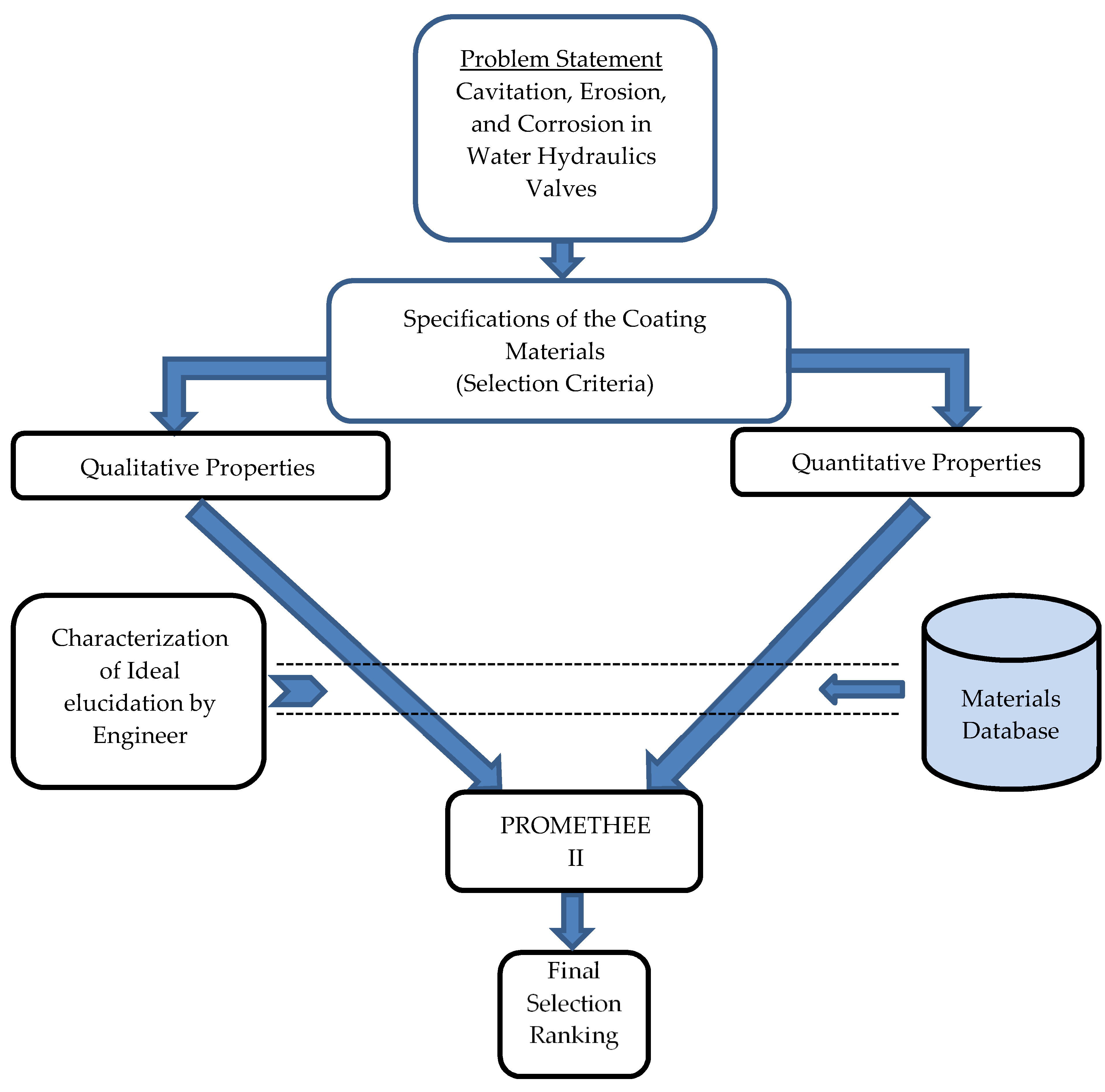
2. Materials and Methods
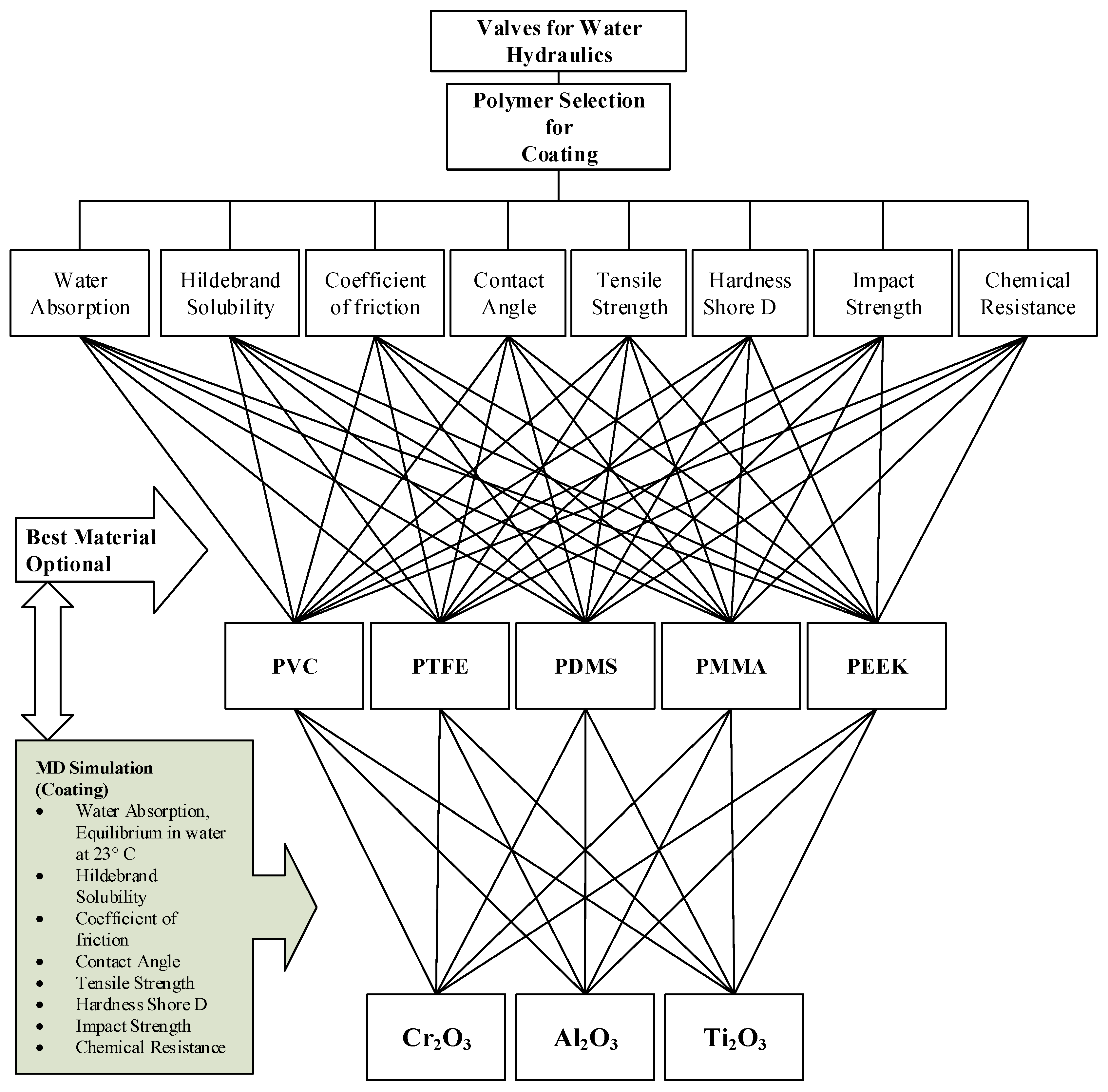
2.1. Polymer Selection for Water Hydraulics Valves
2.1.1. Polyvinylchloride (PVC)
2.1.2. Polytetrafluoroethylene (PTFE)
2.1.3. Polydimethylsiloxane (PDMS)
2.1.4. Polymethylmethacrylate (PMMA)
2.1.5. Polyaryletheretherketone (PEEK)
2.2. PROMETHEE (Preference Ranking Organization Method for Enrichment Evaluations) II
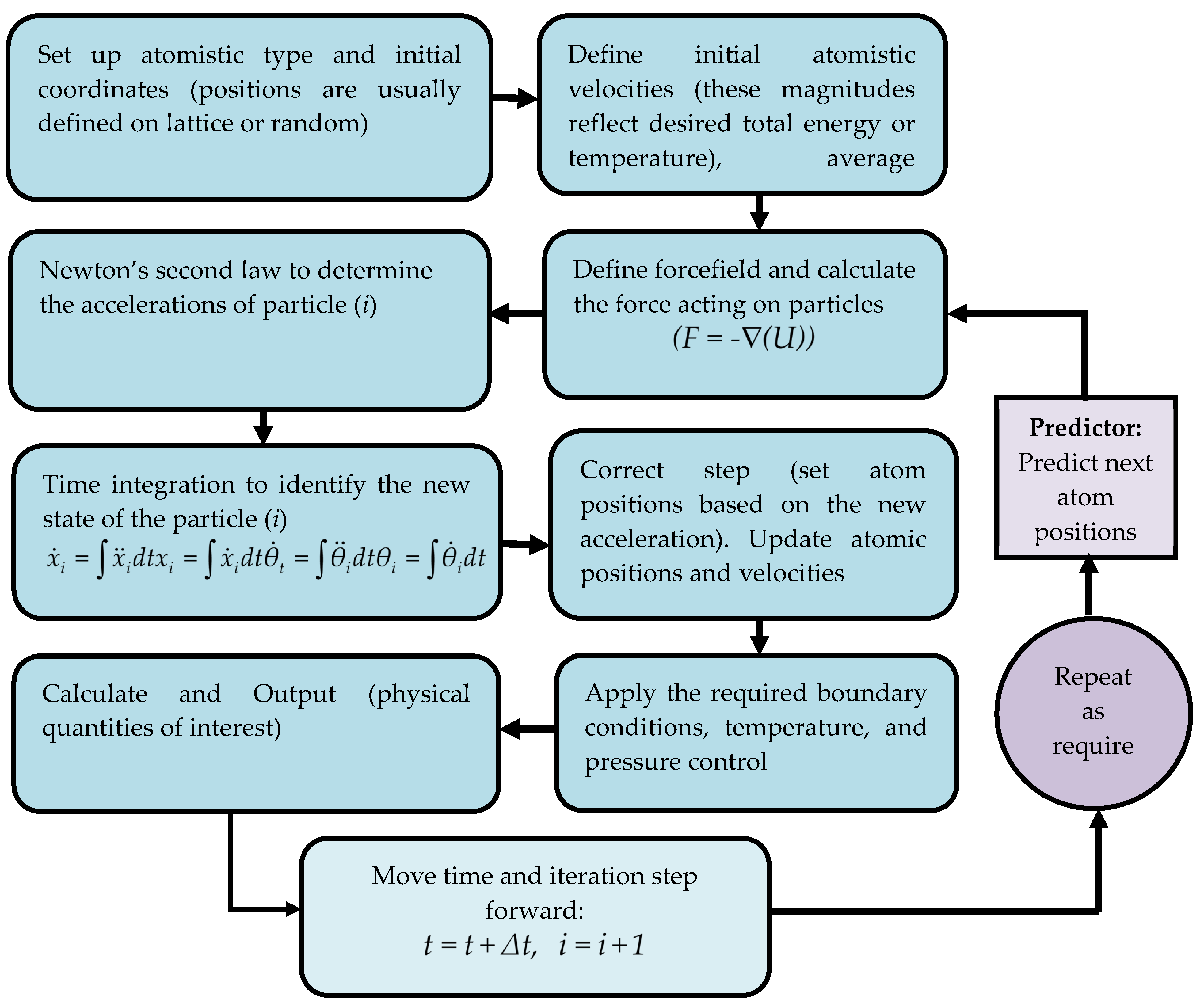
2.3. Molecular Dynamics Simulation to Predict the Substrate and Suitable Coating Plane
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pham, N.P.; Ito, K.; Ikeo, S. Energy Saving for Water Hydraulic Pushing Cylinder in Meat Slicer. JFPS Int. J. Fluid Power Syst. 2017, 10, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H. Cavitation Effect to the Hydraulic Piston Pump Flow Pulsation. Appl. Mech. Mater. 2014, 599–601, 230–236. [Google Scholar] [CrossRef]
- Han, M.; Liu, Y.; Wu, D.; Zhao, X.; Tan, H. A numerical investigation in characteristics of flow force under cavitation state inside the water hydraulic poppet valves. Int. J. Heat Mass Transf. 2017, 111, 1–16. [Google Scholar] [CrossRef]
- Xia, Q.; Pan, G. Flow Field Analysis and Structure Optimization of Water Hydraulic Poppet Valve Based on the CFD. Adv. Mater. Res. 2013, 791–793, 734–737. [Google Scholar] [CrossRef]
- Yang, Y.S.; Semini, C.; Tsagarakis, N.G.; Caldwell, D.G.; Zhu, Y. Water Hydraulics—A Novel Design of Spool-type valves for Enhanced Dynamic Performance. In Proceedings of the International Conference on Advanced Intelligent Mechatronics, Xi’an, China, 4–7 June 2008. [Google Scholar] [CrossRef]
- Majdič, F.; Velkavrh, I.; Kalin, M. Improving the performance of a proportional 4/3 water–hydraulic valve by using a diamond-like-carbon coating. Wear 2013, 297, 1016–1024. [Google Scholar] [CrossRef]
- Han, M.; Liu, Y.; Wu, D.; Tan, H.; Li, C. Numerical Analysis and Optimisation of the Flow Forces in a Water Hydraulic Proportional Cartridge Valve for Injection System. IEEE Access 2018, 6, 10392–10401. [Google Scholar] [CrossRef]
- Trostmann, E. Water Hydraulics Control Technology; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Kozák, J.; Rudolf, P.; Hudec, M.; Štefan, D.; Forman, M. Numerical and experimental investigation of the cavitating flow within venturi tube. J. Fluids Eng. Trans. ASME 2019, 141, 041101. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.S.; Huang, Y.; Li, Z.Y. Experimental investigation of flow and cavitation characteristics of a two-step throttle in water hydraulic valves. Proc. Inst. Mech. Eng. Part A 2002. [Google Scholar] [CrossRef]
- Herakovič, N. Flow-Force Analysis in a Hydraulic Sliding-Spool Valve. CODEN STJSAO 2009, 51, 555–564. [Google Scholar]
- Rahman, R.; Zhafer, S. Firdaus Syed Putra, 5—Tensile properties of natural and synthetic fiber-reinforced polymer composites. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Jawaid, M., Thariq, M., Saba, N., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 81–102. [Google Scholar] [CrossRef]
- Fairfield, C.A. Cavitation erosion resistance of sewer pipe materials. Proc. ICE Constr. Mater. 2015, 168, 1–15. [Google Scholar] [CrossRef]
- Jayadev, D. Chapter 5—Characterization of superhydrophobic polymer coating. In Superhydrophobic Polymer Coatings; Samal, S.K., Mohanty, S., Nayak, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–121. [Google Scholar] [CrossRef]
- Jensen, C.W. The Book and Paper Group Annual. Available online: https://cool.conservation-us.org/coolaic/sg/bpg/annual/v03/bp03-04.html (accessed on 28 November 2019).
- Hildebrand, J.H. Dipole attraction and hydrogen bond formation in their relation to solubility. Science 1936, 83, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Mark, J.E. Physical Properties of Polymers Handbook, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007; p. 1038. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; He, X.; Zhao, T. Friction and wear characteristics of natural bovine bone lubricated with water. Wear 2015, 322–323, 91–100. [Google Scholar] [CrossRef]
- Ji, X. Erosive wear resistance evaluation with the hardness after strain-hardening and its application for a high-entropy alloy. Wear 2018, 398–399, 178–182. [Google Scholar] [CrossRef]
- Kumar, R.; Jagadish; Ray, A. Selection of Material for Optimal Design Using Multi-criteria Decision Making. Procedia Mater. Sci. 2014, 6, 590–596. [Google Scholar] [CrossRef] [Green Version]
- Brans, J.P. L’ingénièrie de la décision; Elaboration d’instruments d’aide à la décision. La méthode PROMETHEE. In L’aide à la décision: Nature, Instruments et Perspectives d’Avenir; Presses de l’Université Laval: Quebec City, QC, Canada, 1982; pp. 183–213. [Google Scholar]
- Brans, J.P.; Vincke, P.; Mareschal, B. How to select and how to rank projects: The Promethee method. Eur. J. Oper. Res. 1986, 24, 228–238. [Google Scholar] [CrossRef]
- Behzadian, M.; Kazemzadeh, R.B.; Albadvi, A.; Aghdasi, M. PROMETHEE: A comprehensive literature review on methodologies and applications. Eur. J. Oper. Res. 2010, 200, 198–215. [Google Scholar] [CrossRef]
- Yong, M.; Zhang, Y.; Sun, S.; Liu, W. Properties of polyvinyl chloride (PVC) ultrafiltration membrane improved by lignin: Hydrophilicity and antifouling. J. Membr. Sci. 2019, 575, 50–59. [Google Scholar] [CrossRef]
- Wypych, G. 2-PVC PROPERTIES. In PVC Formulary, 2nd ed.; Wypych, G., Ed.; ChemTec Publishing: San Diego, CA, USA, 2015; pp. 5–44. [Google Scholar] [CrossRef]
- Ebewele, R.O. Polymer Science and Technology; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar] [CrossRef] [Green Version]
- Jia, P.; Hu, L.; Feng, G.; Bo, C.; Zhang, M.; Zhou, Y. PVC materials without migration obtained by chemical modification of azide-functionalized PVC and triethyl citrate plasticizer. Mater. Chem. Phys. 2017, 190, 25–30. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Polymers, 2nd ed.; ChemTec Publishing: Toronto, ON, Canada, 2016. [Google Scholar]
- Stanton, M.M. Super-hydrophobic, highly adhesive, polydimethylsiloxane (PDMS) surfaces. J. Colloid Interface Sci. 2012, 367, 502–508. [Google Scholar] [CrossRef]
- Penskiy, I.; Gerratt, P.A.; Bergbreiter, S. Friction, adhesion, and wear properties of PDMS coatings in MEMS devices. In Proceedings of the 2011 IEEE 24th International Conference on Micro Electro Mechanical Systems, Cancun, Mexico, 23–27 January 2011. [Google Scholar] [CrossRef]
- Win, K.; Loong, P.Y.; Liu, E.; Li, L. Enhancing electrical and tribological properties of poly (methyl methacrylate) matrix nanocomposite films by co-incorporation of multiwalled carbon nanotubes and silicon dioxide microparticles. J. Polym. Eng. 2016, 36, 23–30. [Google Scholar] [CrossRef]
- William, D.; Callister, D.G.R., Jr. Materials Science and Engineering: An Introduction, 9th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Van Krevelen, D.W.; Nijenhuis, K.T. Properties of Polymers, 4th ed.; van Krevelen, D.W., Nijenhuis, K.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Gilbert, M.; Patrick, S. Chapter 13—Poly (Vinyl Chloride). In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 329–388. [Google Scholar] [CrossRef]
- Handbook, P. Teflon PTFE. Available online: http://www.rjchase.com/ptfe_handbook.pdf (accessed on 28 November 2019).
- Kumar, M.; Arun, S.; Upadhyaya, P.; Pugazhenthi, G. Properties of PMMA/clay nanocomposites prepared using various compatibilizers. Int. J. Mech. Mater. Eng. 2015, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Vilčáková, J.; Kutějová, L.; Jurča, M.; Moučka, R. Enhanced Charpy impact strength of epoxy resin modified with vinyl-terminated polydimethylsiloxane: Research Article. J. Appl. Polym. Sci. 2017, 135, 45720. [Google Scholar] [CrossRef]
- Wyszkowska, E.; Leśniak, M.; Kurpaska, L.; Prokopowicz, R.; Jozwik, I.; Sitarz, M.; Jagielski, J. Functional properties of poly(tetrafluoroethylene) (PTFE) gasket working in nuclear reactor conditions. J. Mol. Struct. 2018, 1157, 306–311. [Google Scholar] [CrossRef]
- Mizobe, K. Effect of PTFE Retainer on Friction Coefficient in Polymer Thrust Bearings under Dry Contact. Adv. Mater. Res. 2013, 683, 90–93. [Google Scholar] [CrossRef]
- Rae, P.; Dattelbaum, D.J.P. The properties of poly (tetrafluoroethylene) (PTFE) in compression. Polymer 2004, 45, 7615–7625. [Google Scholar] [CrossRef]
- Nunes, L.C.S.; Dias, F.W.R.; Mattos, H.S.D. Mechanical behavior of polytetrafluoroethylene in tensile loading under different strain rates. Polym. Test. 2011, 30, 791–796. [Google Scholar] [CrossRef] [Green Version]
- Charitidis, C.A. Influence of accelerated aging on nanomechanical properties, creep behaviour and adhesive forces of PDMS. Plast. Rubber Compos. 2012, 41, 94–99. [Google Scholar] [CrossRef]
- Yadhuraj, S.R.; Gandla, S. Preparation and Study of PDMS Material. Mater. Today Proc. 2018, 5, 21406–21412. [Google Scholar] [CrossRef]
- Saha, B. A study on frictional behavior of PMMA against FDTS coated silicon as a function of load, velocity and temperature. Tribol. Int. 2016, 102, 44–51. [Google Scholar] [CrossRef]
- Ali, U.; Karim, K.J.B.A.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Chandrasena, L. Investigating the compatibility of PEEK polymer for the fabrication of sample cells for use in muon spin spectroscopy. J. Phys. Conf. Ser. 2014, 551, 012038. [Google Scholar] [CrossRef] [Green Version]
- Taillon, G. Cavitation erosion mechanisms in stainless steels and in composite metal–ceramic HVOF coatings. Wear 2016, 364–365, 201–210. [Google Scholar] [CrossRef]
- García, M.A.; Marina, M.L.; Ros, A.; Valcrcel, M. Separation modes in capillary electrophoresis. In Comprehensive Analytical Chemistry; Elsevier Science: Madrid, Spain, 2005; Volume 45, pp. 31–134. [Google Scholar] [CrossRef]
- Lee, P.T.-W.; Yang, Z. Multi-Criteria Decision Making in Maritime Studies and Logistics: Applications and Cases; Palgrave Macmillan: Cham, Switzerland, 2018; pp. 19–21. [Google Scholar] [CrossRef]
- Kittur, J. Using the PROMETHEE and TOPSIS Multi-Criteria Decision Making Methods to Evaluate Optimal Generation. In Proceedings of the 2015 International Conference on Power and Advanced Control Engineering (ICPACE), Bangalore, India, 12–14 August 2015; pp. 80–85. [Google Scholar] [CrossRef]
- Shi, L.; Han, Q. Molecular dynamics study of deformation mechanisms of poly (vinyl alcohol) hydrogel. Mol. Simul. 2018, 44, 1363–1370. [Google Scholar] [CrossRef]
- Epa, V.; Winkler, D.; Tran, L. Chapter 5—Computational Approaches. In Adverse Effects of Engineered Nanomaterials; Fadeel, B., Pietroiusti, A., Shvedova, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 85–96. [Google Scholar]
- Allison, T.C.; Tong, Y.J. Application of the condensed Fukui function to predict reactivity in core–shell transition metal nanoparticles. Electrochim. Acta 2013, 101, 334–340. [Google Scholar] [CrossRef]
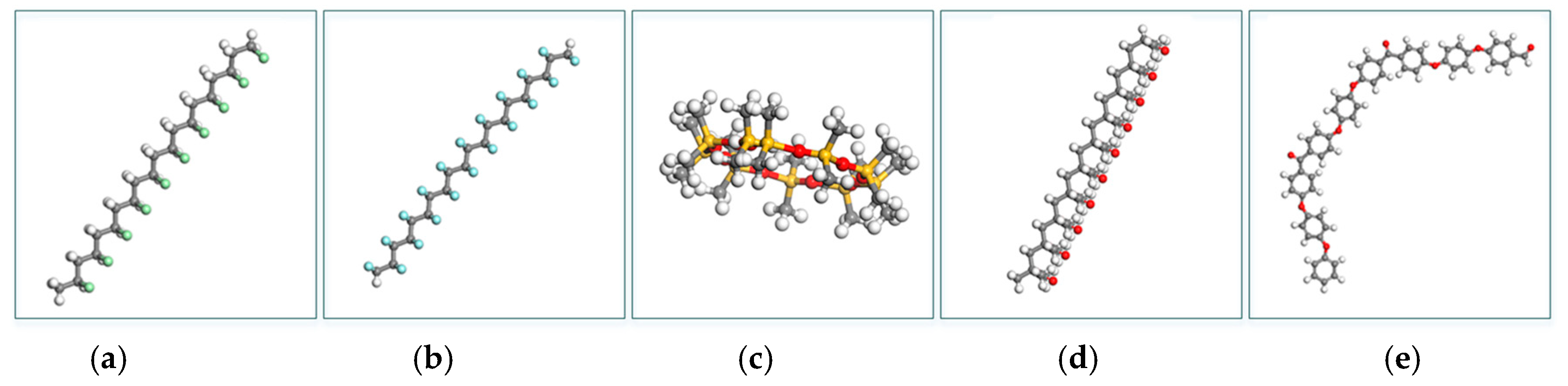


| Attribute or Criteria | Units | Polymer | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | ||||
| Non-beneficial | A1 | % | 0.4 | 0 | 0.17 | 0.3 | 0.5 | [28,29] |
| A2 | (MPa0.5) | 19.1 | 12.7 | 14.9 | 21.3 | 22.8 | [28] | |
| A3 | - | 0.8 | 0.06 | 0.8 | 0.539 | 0.4 | [28,30,31] | |
| Beneficial | A4 | (degree) | 91.9 | 122 | 110 | 74.7 | 90 | [28] |
| A5 | MPa | 51.7 | 35 | 9.7 | 72.4 | 103 | [32,33,34] | |
| A6 | D | 25 | 50 | 70 | 78 | 88 | [28,35,36] | |
| A7 | J/m | 200 | 188 | 22 | 20 | 80 | [17,28,37] | |
| A8 | - | Satisfactory | Excellent | Good | Poor | Very Good | [28] | |
| Polymer | Attributes | |||||||
|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | |
| P1 | 0.4 | 19.1 | 0.4 | 91.9 | 51.7 | 25 | 200 | 2 |
| P2 | 0 | 12.7 | 0.06 | 122 | 35 | 50 | 188 | 5 |
| P3 | 0.17 | 14.9 | 0.8 | 110 | 9.7 | 70 | 22 | 3 |
| P4 | 0.3 | 21.3 | 0.539 | 74.7 | 72.4 | 78 | 20 | 1 |
| P5 | 0.5 | 22.8 | 0.4 | 90 | 103 | 88 | 80 | 4 |
| Polymer | Attributes | |||||||
|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | |
| P1 | 0.2 | 0.46835 | 0 | 0.36364 | 0.45016 | 0 | 1 | 0.25 |
| P2 | 1 | 1.2848 | 1 | 1 | 0.37513 | 0.39683 | 0.93333 | 1 |
| P3 | 0.66 | 1 | 0 | 0.7463 | 0 | 0.71429 | 0.01111 | 0.5 |
| P4 | 0.4 | 0.18987 | 0.3527 | 0 | 0.67202 | 0.84127 | 0 | 0 |
| P5 | 0 | 0 | 0.54054 | 0.32347 | 1 | 1 | 0.33333 | 0.75 |
| Max (Xij) | 0.5 | 22.8 | 0.8 | 122 | 103 | 88 | 200 | 5 |
| Min (Xij) | 0 | 14.9 | 0.06 | 74.7 | 9.7 | 25 | 20 | 1 |
| dj (a,b) | Attributes | |||||||
|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | |
| d(P1-P2) | −0.80 | −0.81645 | −1 | −0.63636 | 0.07503 | −0.39683 | 0.06667 | −0.75 |
| d(P1-P3) | −0.46 | −0.53165 | 0 | −0.38266 | 0.45016 | −0.71429 | 0.98889 | −0.25 |
| d(P1-P4) | −0.2 | 0.27848 | −0.3527 | 0.36364 | −0.22186 | −0.84127 | 1 | 0.25 |
| d(P1-P5) | 0.2 | 0.46835 | −0.54054 | 0.04017 | −0.54984 | -1 | 0.66667 | −0.5 |
| d(P2-P1) | 0.8 | 0.81645 | 1 | 0.63636 | −0.07503 | 0.39683 | −0.06667 | 0.75 |
| d(P2-P3) | 0.34 | 0.2848 | 1 | 0.2537 | 0.37513 | −0.31746 | 0.92222 | 0.5 |
| d(P2-P4) | 0.6 | 1.09493 | 0.6473 | 1 | −0.29689 | −0.44444 | 0.93333 | 1 |
| d(P2-P5) | 1 | 1.2848 | 0.45946 | 0.67653 | −0.62487 | −0.60317 | 0.6 | 0.25 |
| d(P3-P1) | 0.46 | 0.53165 | 0 | 0.38266 | −0.45016 | 0.71429 | −0.98889 | 0.25 |
| d(P3-P2) | −0.34 | −0.2848 | −1 | −0.2537 | −0.37513 | 0.31746 | −0.92222 | −0.5 |
| d(P3-P4) | 0.26 | 0.81013 | −0.3527 | 0.7463 | −0.67202 | −0.12698 | 0.01111 | 0.5 |
| d(P3-P5) | 0.66 | 1 | −0.54054 | 0.42283 | −1 | −0.28571 | −0.32222 | −0.25 |
| d(P4-P1) | 0.2 | −0.27848 | 0.3527 | −0.36364 | 0.22186 | 0.84127 | −1 | −0.25 |
| d(P4-P2) | −0.6 | −1.09493 | −0.6473 | −1 | 0.29689 | 0.44444 | −0.93333 | −1 |
| d(P4-P3) | −0.26 | −0.81013 | 0.3527 | −0.7463 | 0.67202 | 0.12698 | −0.01111 | −0.5 |
| d(P4-P5) | 0.4 | 0.18987 | −0.18784 | −0.32347 | −0.32798 | −0.15873 | −0.33333 | −0.75 |
| d(P5-P1) | −0.2 | −0.46835 | 0.54054 | −0.04017 | 0.54984 | 1 | −0.66667 | 0.5 |
| d(P5-P2) | −1 | −1.2848 | −0.45946 | −0.67653 | 0.62487 | 0.60317 | −0.6 | −0.25 |
| d(P5-P3) | −0.66 | −1 | 0.54054 | −0.42283 | 1 | 0.28571 | 0.32222 | 0.25 |
| d(P5-P4) | −0.4 | −0.18987 | 0.18784 | 0.32347 | 0.32798 | 0.15873 | 0.33333 | 0.75 |
| Pj(a,b) | Attributes | |||||||
|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | |
| d(P1-P2) | 0 | 0 | 0 | 0 | 0.0750 | 0 | 0.0667 | 0 |
| d(P1-P3) | 0 | 0 | 0 | 0 | 0.4502 | 0 | 0.9889 | 0 |
| d(P1-P4) | 0 | 0.2785 | 0 | 0.3636 | 0 | 0 | 1 | 0.25 |
| d(P1-P5) | 0.2 | 0.4684 | 0 | 0.0402 | 0 | 0 | 0.6667 | 0 |
| d(P2-P1) | 0.8 | 0.8165 | 1 | 0.6364 | 0 | 0.3968 | 0 | 0.75 |
| d(P2-P3) | 0.34 | 0.2848 | 1 | 0.2537 | 0.3751 | 0 | 0.9222 | 0.5 |
| d(P2-P4) | 0.6 | 1.0949 | 0.6473 | 1 | 0 | 0 | 0.9333 | 1 |
| d(P2-P5) | 1 | 1.2848 | 0.4595 | 0.6765 | 0 | 0 | 0.6 | 0.25 |
| d(P3-P1) | 0.46 | 0.5317 | 0 | 0.3827 | 0 | 0.7143 | 0 | 0.25 |
| d(P3-P2) | 0 | 0 | 0 | 0 | 0 | 0.3175 | 0 | 0 |
| d(P3-P4) | 0.26 | 0.8101 | 0 | 0.7463 | 0 | 0 | 0.0111 | 0.5 |
| d(P3-P5) | 0.66 | 1 | 0 | 0.4228 | 0 | 0 | 0 | 0 |
| d(P4-P1) | 0.2 | 0 | 0.3527 | 0 | 0.2219 | 0.8413 | 0 | 0 |
| d(P4-P2) | 0 | 0 | 0 | 0 | 0.2969 | 0.4444 | 0 | 0 |
| d(P4-P3) | 0 | 0 | 0.3527 | 0 | 0.6720 | 0.1270 | 0 | 0 |
| d(P4-P5) | 0.4 | 0.1899 | 0 | 0 | 0 | 0 | 0 | 0 |
| d(P5-P1) | 0 | 0 | 0.5405 | 0 | 0.5498 | 1 | 0 | 0.5 |
| d(P5-P2) | 0 | 0 | 0 | 0 | 0.6249 | 0.6032 | 0 | 0 |
| d(P5-P3) | 0 | 0 | 0.5405 | 0 | 1 | 0.2857 | 0.3222 | 0.25 |
| d(P5-P4) | 0 | 0 | 0.1878 | 0.3235 | 0.3280 | 0.1587 | 0.3333 | 0.75 |
| Attributes | π(a,b) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Weight → | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | |
| 0.2 | 0.18 | 0.08 | 0.16 | 0.06 | 0.06 | 0.14 | 0.12 | ||
| wj × d(P1-P2) | 0 | 0 | 0 | 0 | 0.0045 | 0 | 0.0093 | 0 | 0.0138356 |
| wj × d(P1-P3) | 0 | 0 | 0 | 0 | 0.0270 | 0 | 0.1384 | 0 | 0.1654542 |
| wj × d(P1-P4) | 0 | 0.0501 | 0 | 0.0582 | 0 | 0 | 0.14 | 0.03 | 0.2783088 |
| wj × d(P1-P5) | 0.04 | 0.0843 | 0 | 0.0064 | 0 | 0 | 0.0933 | 0 | 0.224064 |
| wj × d(P2-P1) | 0.16 | 0.1470 | 0.08 | 0.1018 | 0 | 0.0238 | 0 | 0.09 | 0.6025884 |
| wj × d(P2-P3) | 0.068 | 0.0512 | 0.08 | 0.0406 | 0.0225 | 0 | 0.1291 | 0.06 | 0.4514746 |
| wj × d(P2-P4) | 0.12 | 0.1971 | 0.0518 | 0.16 | 0 | 0 | 0.1307 | 0.12 | 0.7795376 |
| wj × d(P2-P5) | 0.2 | 0.2313 | 0.0368 | 0.1082 | 0 | 0 | 0.084 | 0.03 | 0.6902656 |
| wj × d(P3-P1) | 0.092 | 0.0957 | 0 | 0.0612 | 0 | 0.0429 | 0 | 0.03 | 0.32178 |
| wj × d(P3-P2) | 0 | 0 | 0 | 0 | 0 | 0.0191 | 0 | 0 | 0.0190476 |
| wj × d(P3-P4) | 0.052 | 0.1458 | 0 | 0.1194 | 0 | 0 | 0.0016 | 0.06 | 0.3787868 |
| wj × d(P3-P5) | 0.132 | 0.18 | 0 | 0.0677 | 0 | 0 | 0 | 0 | 0.3796528 |
| wj × d(P4-P1) | 0.04 | 0 | 0.0282 | 0 | 0.0133 | 0.0505 | 0 | 0 | 0.1320038 |
| wj × d(P4-P2) | 0 | 0 | 0 | 0 | 0.0178 | 0.0267 | 0 | 0 | 0.0444798 |
| wj × d(P4-P3) | 0 | 0 | 0.0282 | 0 | 0.0403 | 0.0076 | 0 | 0 | 0.076156 |
| wj × d(P4-P5) | 0.08 | 0.0342 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1141766 |
| wj × d(P5-P1) | 0 | 0 | 0.0432 | 0 | 0.0330 | 0.06 | 0 | 0.06 | 0.1962336 |
| wj × d(P5-P2) | 0 | 0 | 0 | 0 | 0.0375 | 0.0362 | 0 | 0 | 0.0736824 |
| wj × d(P5-P3) | 0 | 0 | 0.0432 | 0 | 0.06 | 0.0171 | 0.0451 | 0.03 | 0.1954966 |
| wj × d(P5-P4) | 0 | 0 | 0.0150 | 0.0518 | 0.0197 | 0.0095 | 0.0467 | 0.09 | 0.2326512 |
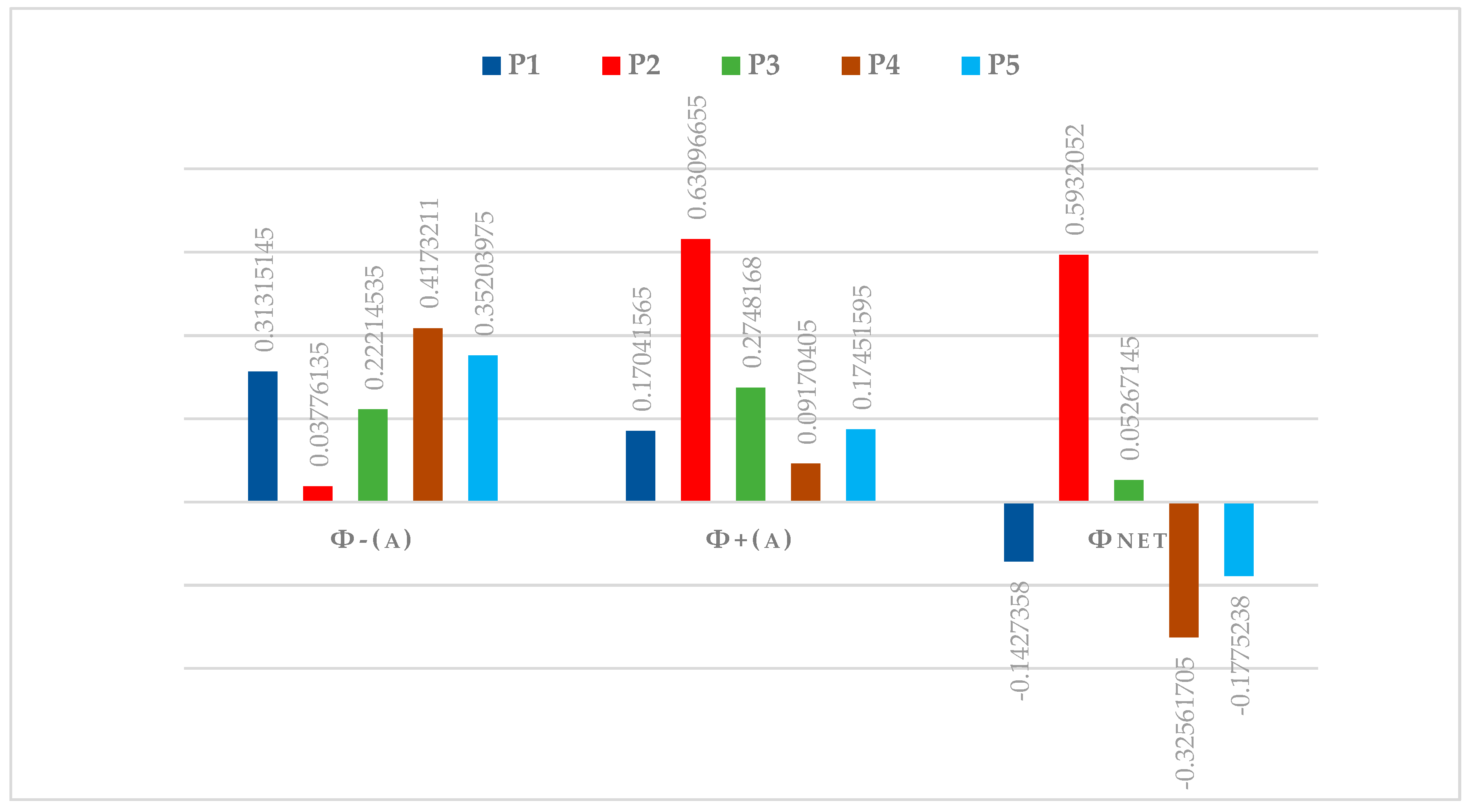
| Aggregate Preference Function | P1 | P2 | P3 | P4 | P5 | Leaving Flow ϕ+(a) |
|---|---|---|---|---|---|---|
| P1 | - | 0.0138356 | 0.1654542 | 0.2783088 | 0.224064 | 0.17041565 |
| P2 | 0.6025884 | - | 0.4514746 | 0.7795376 | 0.6902656 | 0.63096655 |
| P3 | 0.32178 | 0.0190476 | - | 0.3787868 | 0.3796528 | 0.2748168 |
| P4 | 0.1320038 | 0.0444798 | 0.076156 | - | 0.1141766 | 0.09170405 |
| P5 | 0.1962336 | 0.0736824 | 0.1954966 | 0.2326512 | - | 0.17451595 |
| Entering Flow ϕ−(a) | 0.31315145 | 0.03776135 | 0.22214535 | 0.4173211 | 0.35203975 |
| Polymer | Leaving Flow | Entering Flow | Net Flow | Ranking |
|---|---|---|---|---|
| P1 | 0.17041565 | 0.31315145 | −0.1427358 | 3 |
| P2 | 0.63096655 | 0.03776135 | 0.5932052 | 1 |
| P3 | 0.2748168 | 0.22214535 | 0.05267145 | 2 |
| P4 | 0.09170405 | 0.4173211 | −0.32561705 | 5 |
| P5 | 0.17451595 | 0.35203975 | −0.1775238 | 4 |
| Alternative Polymer | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|
| ϕnet | −0.1427358 | 0.5932052 | 0.05267145 | −0.32561705 | −0.1775238 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlela, M.K.; Xu, H.; Sun, F.; Wang, H.; Madenge, G.D. Material Analysis and Molecular Dynamics Simulation for Cavitation Erosion and Corrosion Suppression in Water Hydraulic Valves. Materials 2020, 13, 453. https://doi.org/10.3390/ma13020453
Mlela MK, Xu H, Sun F, Wang H, Madenge GD. Material Analysis and Molecular Dynamics Simulation for Cavitation Erosion and Corrosion Suppression in Water Hydraulic Valves. Materials. 2020; 13(2):453. https://doi.org/10.3390/ma13020453
Chicago/Turabian StyleMlela, Masoud Kamoleka, He Xu, Feng Sun, Haihang Wang, and Gabriel Donald Madenge. 2020. "Material Analysis and Molecular Dynamics Simulation for Cavitation Erosion and Corrosion Suppression in Water Hydraulic Valves" Materials 13, no. 2: 453. https://doi.org/10.3390/ma13020453
APA StyleMlela, M. K., Xu, H., Sun, F., Wang, H., & Madenge, G. D. (2020). Material Analysis and Molecular Dynamics Simulation for Cavitation Erosion and Corrosion Suppression in Water Hydraulic Valves. Materials, 13(2), 453. https://doi.org/10.3390/ma13020453







