Collagen/PCL Nanofibers Electrospun in Green Solvent by DOE Assisted Process. An Insight into Collagen Contribution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Protein and Polymer Solutions
2.2.2. Electrospinning Process
2.2.3. Rheological Characterization of Polymer and Protein Solutions
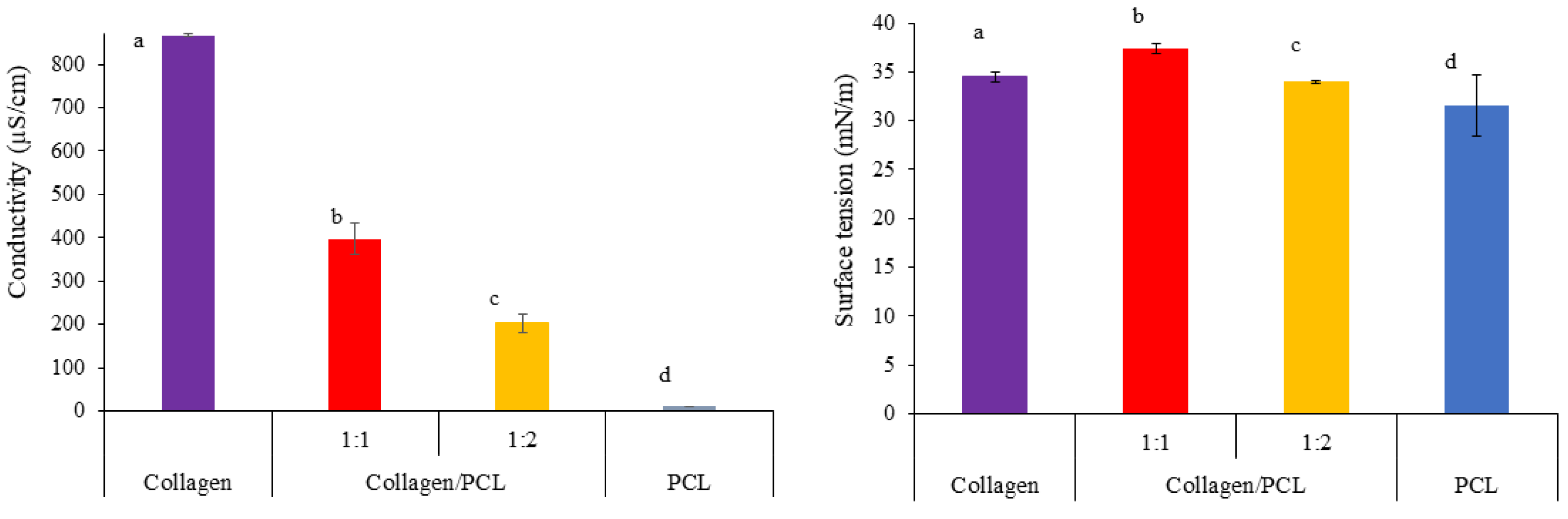
2.2.4. Conductivity and Surface Tension Measurements
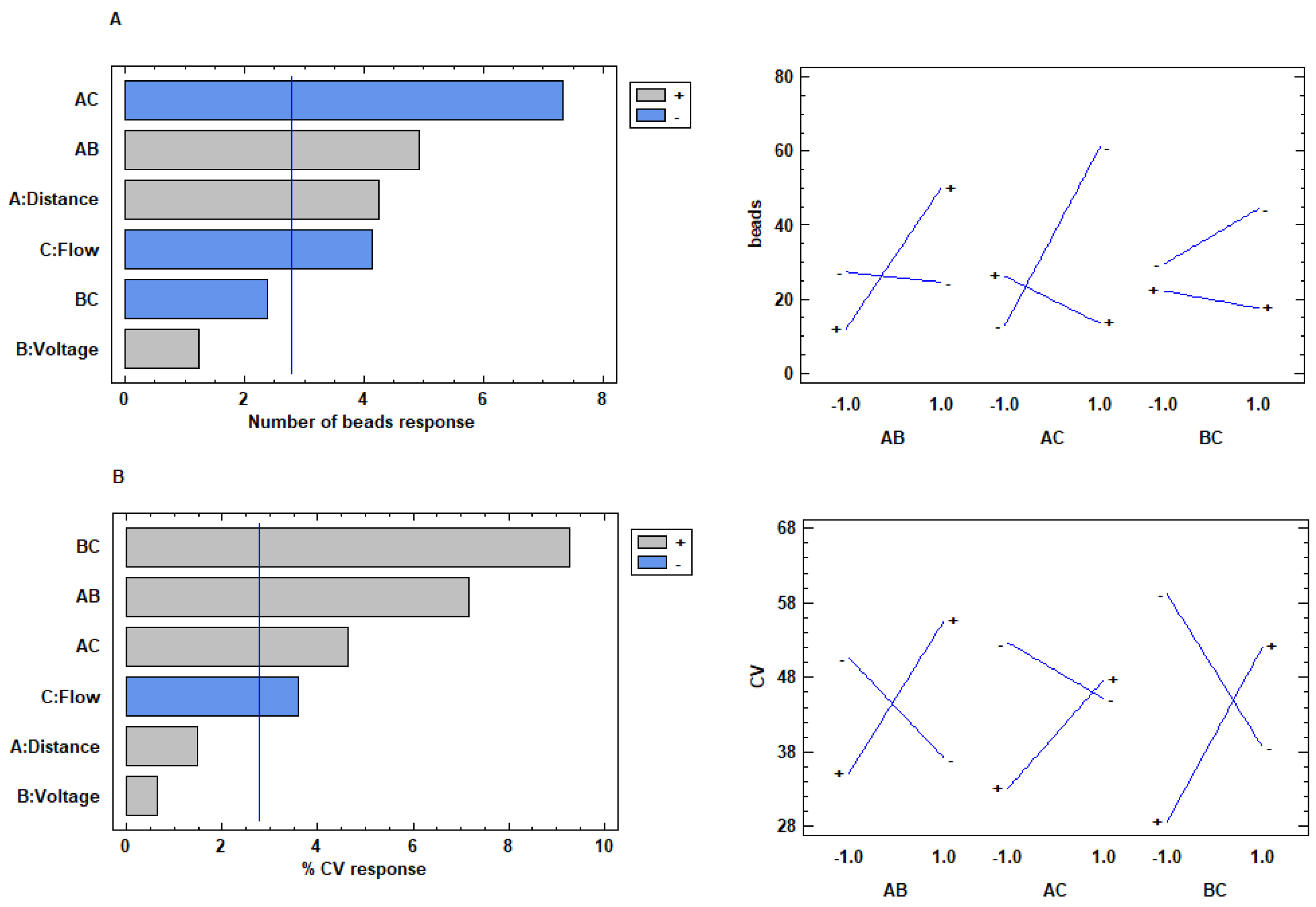
2.2.5. Experimental Design
2.2.6. Characterization of the Nanofibers
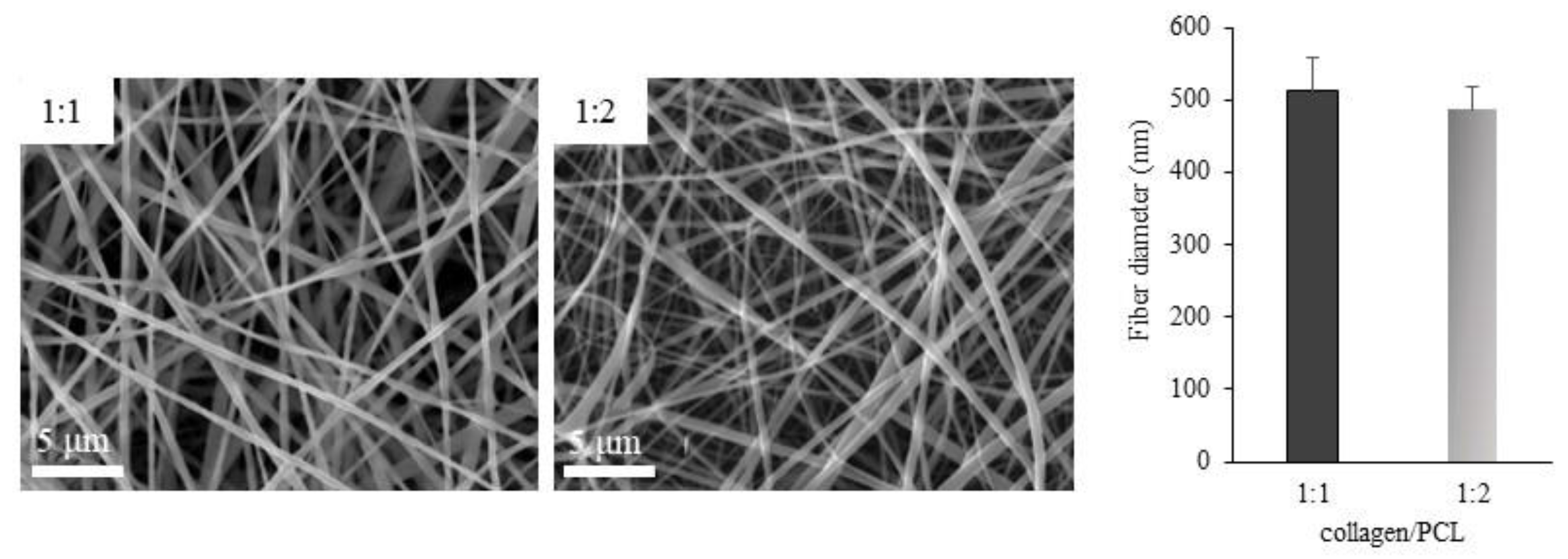
SEM Analysis
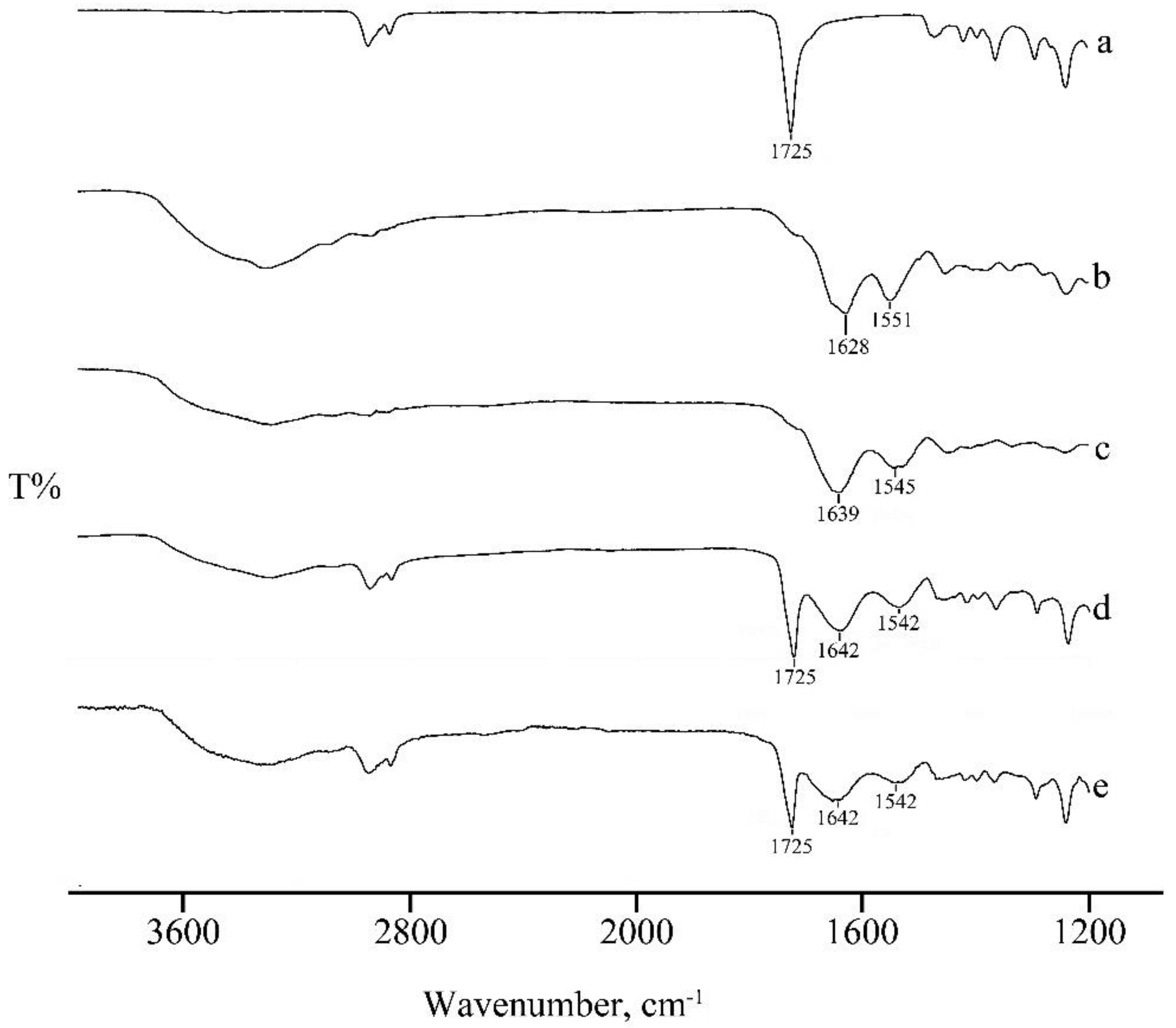
FTIR Analysis
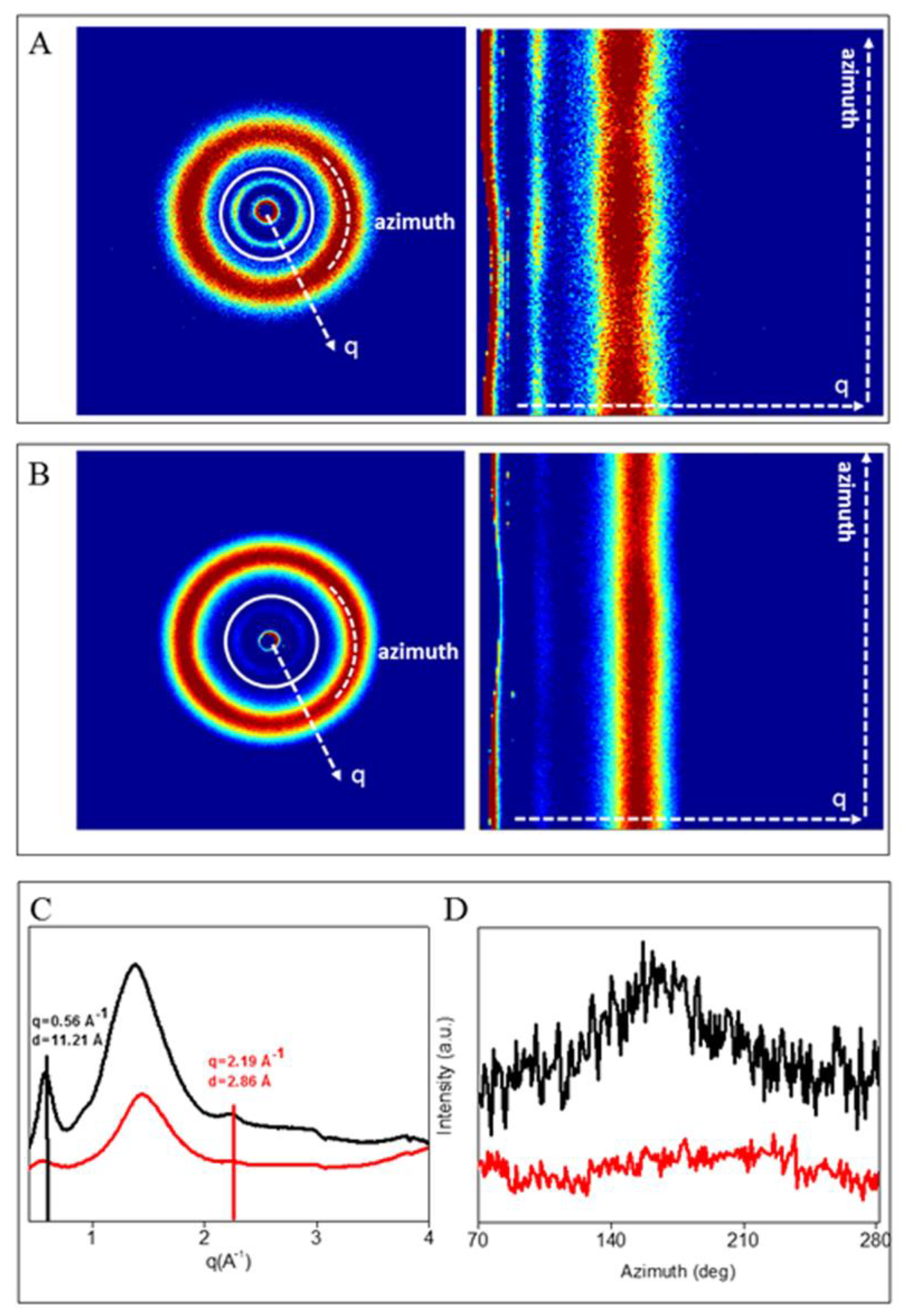
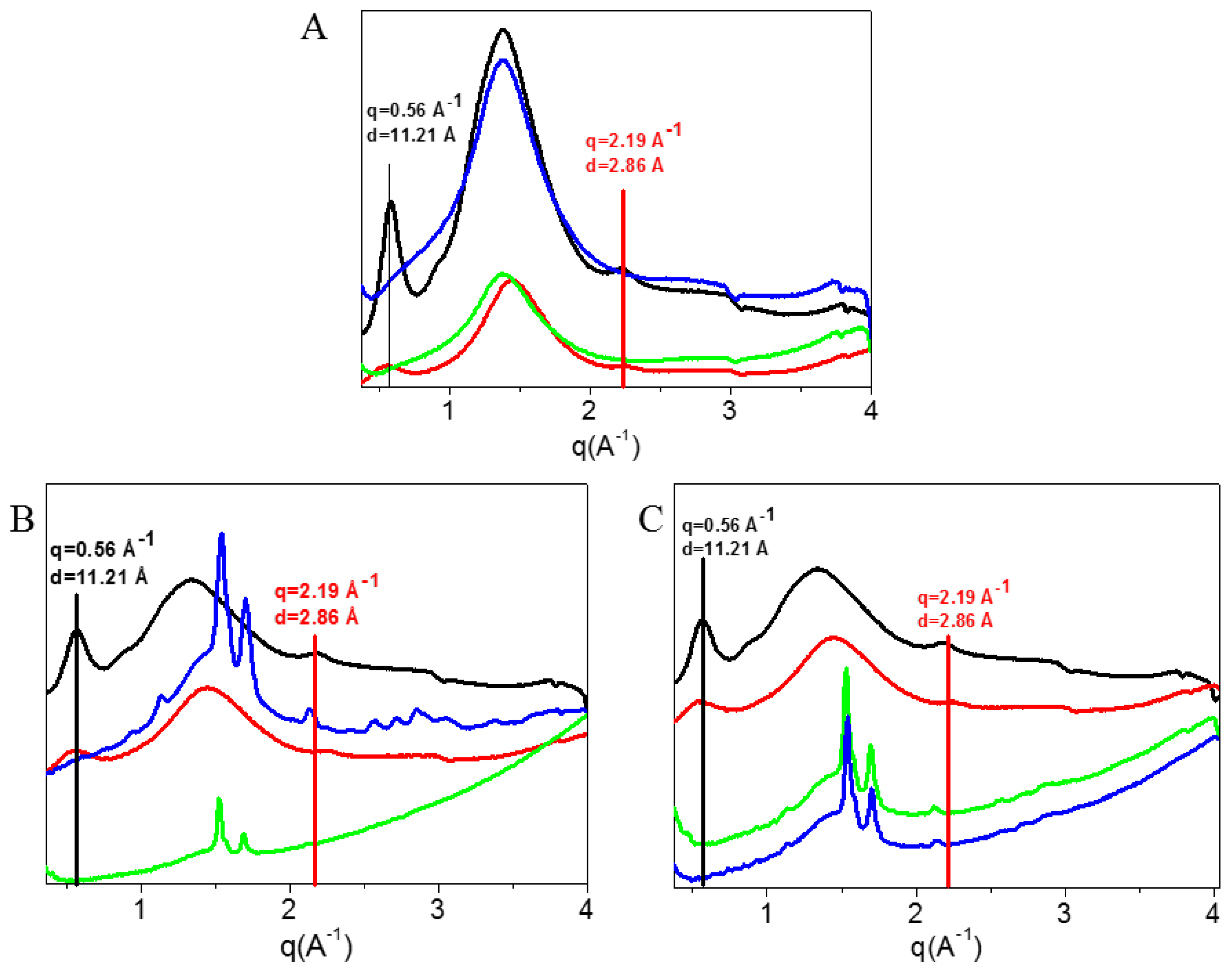
WAXS Analysis
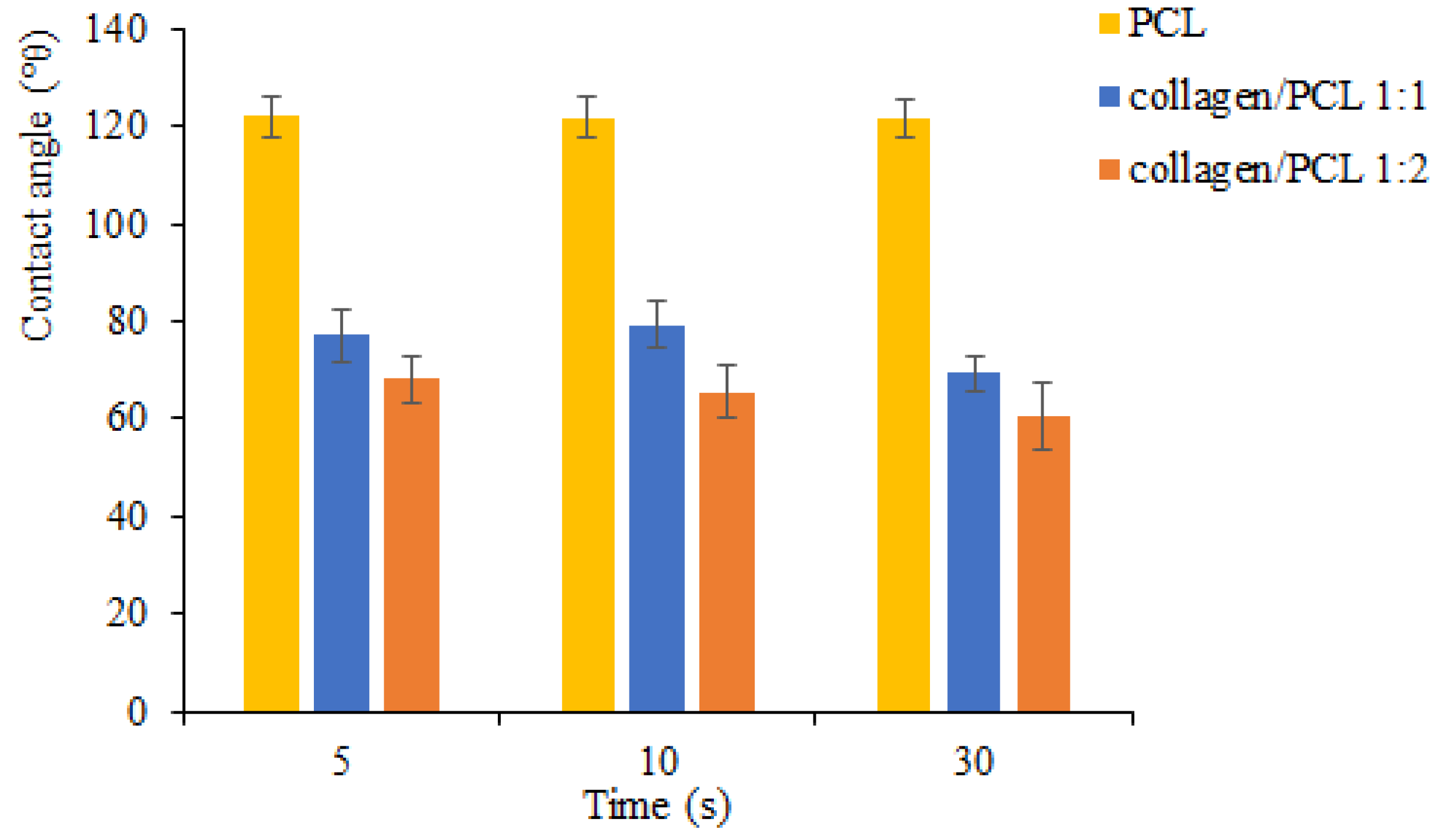
Wettability Measurements
Mechanical Properties Evaluation
2.2.7. Collagen Release Studies
Eosin Staining
Hydroxyproline Quantification
2.2.8. Cell Adhesion and Morphology Study
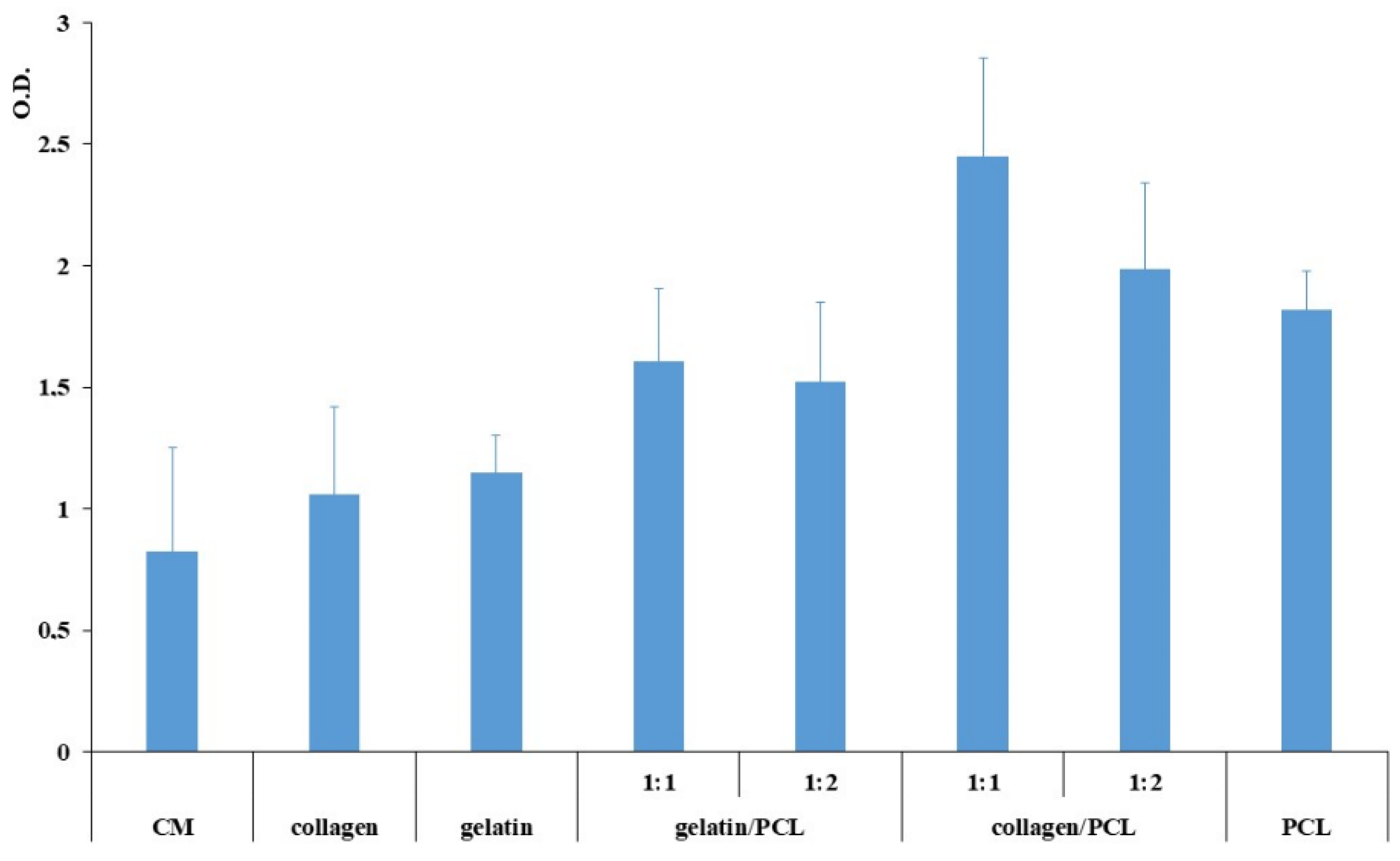
2.2.9. Biocompatibility Evaluation
3. Results and Discussion
3.1. Preparation of the Electrospun Nanofibers
3.1.1. Rheological Studies
3.1.2. Physical Properties of Collagen, PCL and Collagen/PCL Solutions
3.1.3. Design of Experiment (DOE) Based Evaluation of the Process Parameters
3.2. Characterization of Nanofibrous Membranes Based On Collagen/PCL Mixtures
3.2.1. FTIR Analysis
3.2.2. WAXS Analysis
3.2.3. Wettability
3.2.4. Mechanical Properties
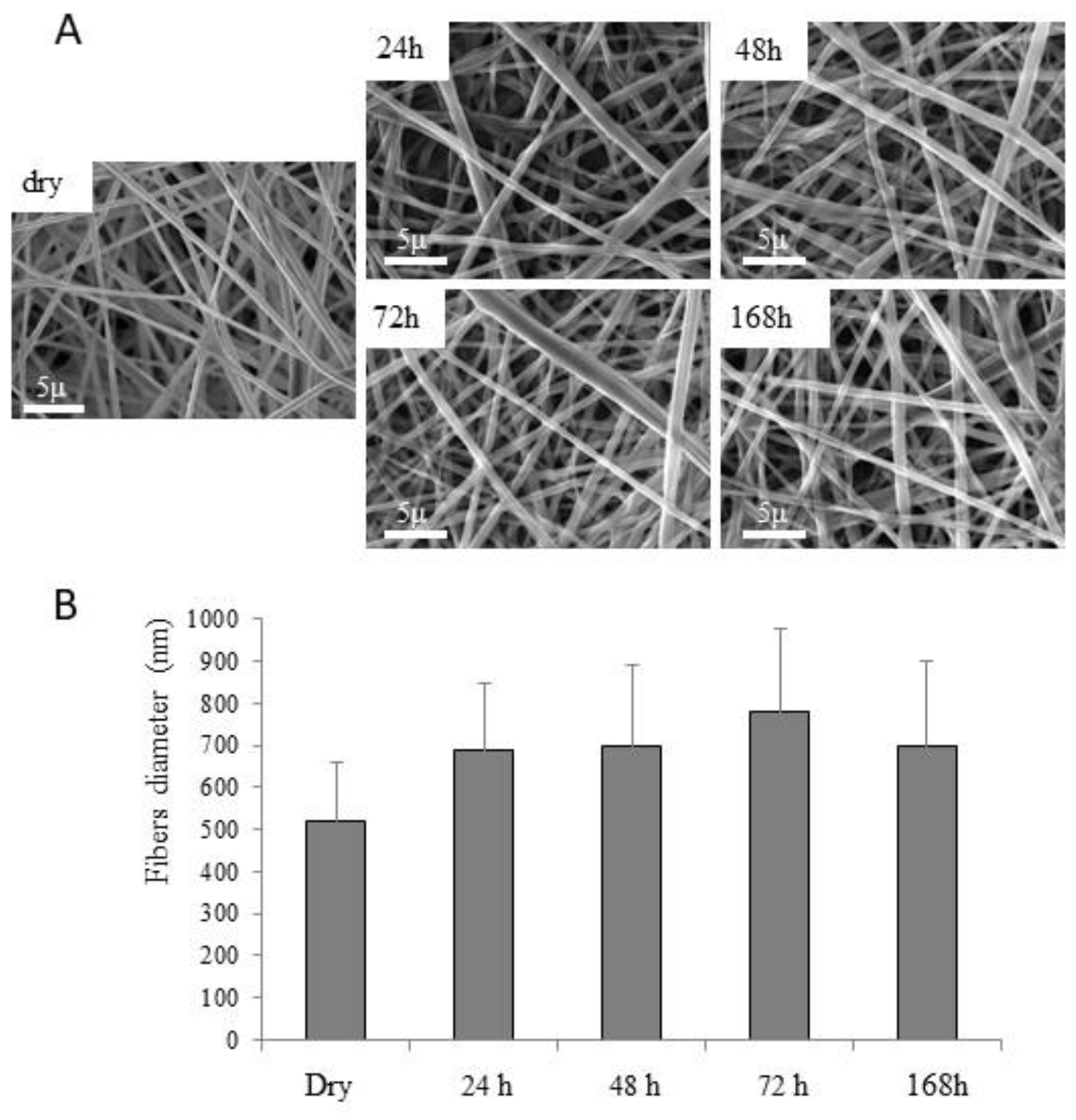
3.2.5. Morphological Stability of Nanofiber Structure in the Hydrated State
3.3. Collagen Release
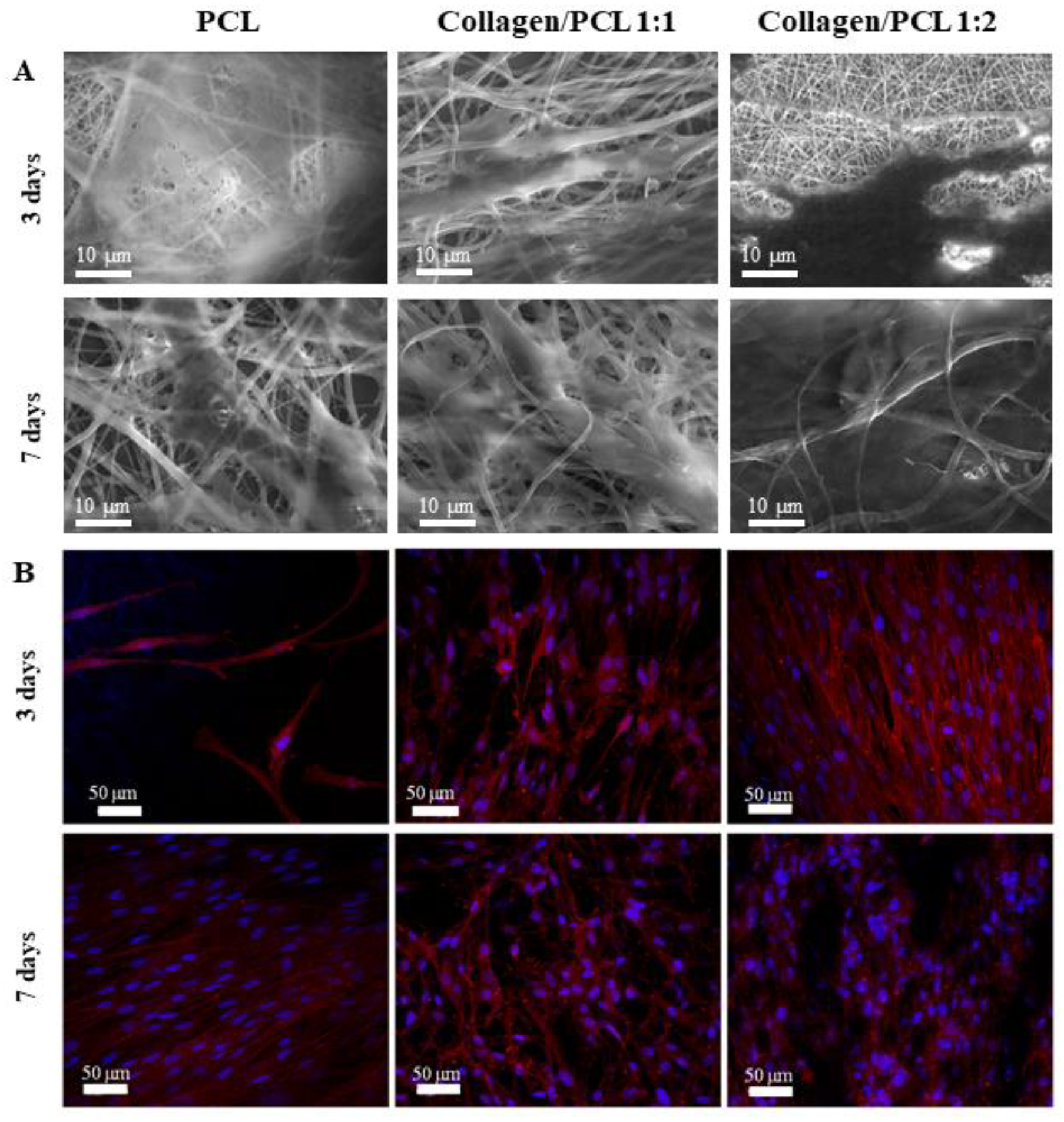
3.4. Fibroblast Growth and Adhesion
3.5. Collagen vs. Gelatin Fibers Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Bonassar, J.L.; Vacanti, C.A. Tissue Engineering: The first decade and beyond. J. Cell. Biochem. 1998, 31 (Suppl. 30), 297–303. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Pezzoli, D.; Di Paolo, J.; Kumra, H.; Fois, G.; Candiani, G.; Reinhardt, D.P.; Mantovani, D. Fibronectin promotes elastin deposition, elasticity and mechanical strength in cellularised collagen-based scaffolds. Biomaterials 2018, 180, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Miele, D.; Faccendini, A.; Del Favero, E.; Di Cola, E.; Cornaglia, A.I.; Boselli, C.; Luxbacher, T.; et al. Chitosan/glycosaminoglycan scaffolds for skin reparation. Carbohydr. Polym. 2019, 220, 219–227. [Google Scholar] [CrossRef]
- Kolácná, L.; Bakesová, J.; Varga, F.; Kostáková, E.; Plánka, L.; Nečas, A.; Lukás, D.; Amler, E.; Pelouch, V. Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix. Physiol. Res. 2007, 56, 51. [Google Scholar]
- Dreesmann, L.; Ahlers, M.; Schlosshauer, B. The pro-angiogenic characteristics of a cross-linked gelatin matrix. Biomaterials 2007, 28, 5536–5543. [Google Scholar] [CrossRef]
- Neve, A.; Cantatore, F.P.; Maruotti, N.; Corrado, A.; Ribatti, D. Extracellular Matrix Modulates Angiogenesis in Physiological and Pathological Conditions. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Georgiana, M.; Violeta, M.; Titorencu, I. Collagen-based drug delivery systems for tissue engineering. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; IntechOpen: London, UK, 2011; ISBN 978-953-307-661-4. [Google Scholar] [CrossRef]
- Dong, B.; Arnoult, O.; Smith, M.E.; Wnek, G.E. Electrospinning of Collagen Nanofiber Scaffolds from Benign Solvents. Macromol. Rapid Commun. 2009, 30, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Fullana, M.J.; Wnek, G.E. Electrospun collagen and its applications in regenerative medicine. Drug Deliv. Transl. Res. 2012, 2, 313–322. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.-Y.L.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres – Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Kokol, V.; Collagen, V.S. Gelatine-Based biomaterials and their biocompatibility: Review and perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; IntechOpen: London, UK, 2011; ISBN 978-953-307-661-4. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; Wang, W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomed. 2014, 9, 2335–2344. [Google Scholar] [CrossRef]
- Aguirre-Chagala, Y.E.; Altuzar, V.; León-Sarabia, E.; Tinoco-Magaña, J.C.; Yañez-Limón, J.M.; Mendoza-Barrera, C. Physicochemical properties of polycaprolactone/collagen/elastin nanofibers fabricated by electrospinning. Mater. Sci. Eng. C 2017, 76, 897–907. [Google Scholar] [CrossRef]
- Bertram, U.; Steiner, D.; Poppitz, B.; Dippold, D.; Köhn, K.; Beier, J.P.; Detsch, R.; Boccaccini, A.R.; Schubert, D.W.; Horch, R.E.; et al. Vascular Tissue Engineering: Effects of Integrating Collagen into a PCL Based Nanofiber Material. BioMed Res. Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Dippold, D.; Cai, A.; Hardt, M.; Boccaccini, A.R.; Horch, R.; Beier, J.P.; Schubert, D.W. Novel approach towards aligned PCL-Collagen nanofibrous constructs from a benign solvent system. Mater. Sci. Eng. C 2017, 72, 278–283. [Google Scholar] [CrossRef]
- Barrientos, I.J.H.; Paladino, E.; Szabó, P.; Brozio, S.; Hall, P.J.; Oseghale, C.I.; Passarelli, M.K.; Moug, S.J.; Black, R.A.; Wilson, C.G.; et al. Electrospun collagen-based nanofibres: A sustainable material for improved antibiotic utilisation in tissue engineering applications. Int. J. Pharm. 2017, 531, 67–79. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.-M.; Lim, C.T.; Ramakrishna, S. Characterization of the Surface Biocompatibility of the Electrospun PCL-Collagen Nanofibers Using Fibroblasts. Biomacromolecules 2005, 6, 2583–2589. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.; Ontoria-Oviedo, I.; Castellano, D.; Sepúlveda, P.; Ribes-Greus, A. Polycaprolactone/gelatin-based scaffolds with tailored performance: In vitro and in vivo validation. Mater. Sci. Eng. C 2020, 107, 110296. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, V.Y.; Gnanamani, A.; Rengaswami, G.D.V.; Madhusoothanan, M.; Sekaran, G. Electrospinning of type I collagen and PCL nanofibers using acetic acid. J. Appl. Polym. Sci. 2012, 125, 3221–3227. [Google Scholar] [CrossRef]
- Dulnik, J.; Denis, P.; Sajkiewicz, P.; Kołbuk, D.; Choińska, E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Dulnik, J.; Kołbuk, D.; Denis, P.; Sajkiewicz, P. The effect of a solvent on cellular response to PCL/gelatin and PCL/collagen electrospun nanofibres. Eur. Polym. J. 2018, 104, 147–156. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Sels, H.; De Smet, H.; Geuens, J. SUSSOL—Using Artificial Intelligence for Greener Solvent Selection and Substitution. Molecules 2020, 25, 3037. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Baldwin, R.L. Mechanism of Helix Induction by Trifluoroethanol: A Framework for Extrapolating the Helix-Forming Properties of Peptides from Trifluoroethanol/Water Mixtures Back to Water. Biochemistry 1997, 36, 8413–8421. [Google Scholar] [CrossRef]
- Bürck, J.; Heissler, S.; Geckle, U.; Ardakani, M.F.; Schneider, R.; Ulrich, A.S.; Kazanci, M. Resemblance of Electrospun Collagen Nanofibers to Their Native Structure. Langmuir 2013, 29, 1562–1572. [Google Scholar] [CrossRef]
- Caramella, C.; Ferrari, F.; Bonferoni, M.C.; Ronchi, M.; Colombo, P. Rheological properties and diffusion dissolution behaviour of hydrophilic polymers. Boll. Chim. Farm. 1989, 128, 298–302. [Google Scholar] [PubMed]
- Porter, R.S.; Johnson, J.F. The Entanglement Concept in Polymer Systems. Chem. Rev. 1966, 66, 1–27. [Google Scholar] [CrossRef]
- Cordenonsi, L.M.; Faccendini, A.; Rossi, S.; Bonferoni, M.C.; Malavasi, L.; Raffin, R.; Schapoval, E.E.S.; Del Fante, C.; Vigani, B.; Miele, D.; et al. Platelet lysate loaded electrospun scaffolds: Effect of nanofiber types on wound healing. Eur. J. Pharm. Biopharm. 2019, 142, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Vigani, B.; Rossi, S.; Sandri, G.; Bonferoni, M.C.; Milanesi, G.; Bruni, G.; Ferrari, F. Coated electrospun alginate-containing fibers as novel delivery systems for regenerative purposes. Int. J. Nanomed. 2018, 13, 6531–6550. [Google Scholar] [CrossRef]
- Saquing, C.D.; Tang, C.; Monian, B.; Bonino, C.A.; Manasco, J.L.; Alsberg, E.; Khan, S.A. Alginate–Polyethylene Oxide Blend Nanofibers and the Role of the Carrier Polymer in Electrospinning. Ind. Eng. Chem. Res. 2013, 52, 8692–8704. [Google Scholar] [CrossRef]
- Drosou, C.; Krokida, M.; Biliaderis, C.G. Composite pullulan-whey protein nanofibers made by electrospinning: Impact of process parameters on fiber morphology and physical properties. Food Hydrocoll. 2018, 77, 726–735. [Google Scholar] [CrossRef]
- Motamedi, A.S.; Mirzadeh, H.; Hajiesmaeilbaigi, F.; Bagheri-Khoulenjani, S.; Shokrgozar, M. Effect of electrospinning parameters on morphological properties of PVDF nanofibrous scaffolds. Prog. Biomater. 2017, 6, 113–123. [Google Scholar] [CrossRef]
- Yang, L.; Fitié, C.F.; Van Der Werf, K.O.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Mechanical properties of single electrospun collagen type I fibers. Biomaterials 2008, 29, 955–962. [Google Scholar] [CrossRef]
- Liu, T.; Teng, W.K.; Chan, B.P.; Chew, S.Y. Photochemical crosslinked electrospun collagen nanofibers: Synthesis, characterization and neural stem cell interactions. J. Biomed. Mater. Res. Part A 2010, 95, 276–282. [Google Scholar] [CrossRef]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; De Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-Ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis, and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, 2000017. [Google Scholar] [CrossRef]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Campa, L.; Natali, M.L.; Salvatore, L.; Madaghiele, M.; De Caro, L.; et al. Investigations of Processing–Induced Structural Changes in Horse Type-I Collagen at Sub and Supramolecular Levels. Front. Bioeng. Biotechnol. 2019, 7, 203. [Google Scholar] [CrossRef] [PubMed]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of processing on structural, mechanical and biological properties of collagen-based substrates for regenerative medicine. Sci. Rep. 2018, 8, 1429. [Google Scholar] [CrossRef]
- Rich, A.; Crick, F. The molecular structure of collagen. J. Mol. Biol. 1961, 3, 483–484. [Google Scholar] [CrossRef]
- Okuyama, K.; Xu, X.; Iguchi, M.; Noguchi, K. Revision of collagen molecular structure. Biopolymers 2006, 84, 181–191. [Google Scholar] [CrossRef]
- Sun, L.; Hou, H.; Li, B.-F.; Zhang, Y. Characterization of acid- and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef]
- Powell, H.M.; Boyce, S.T. Engineered Human Skin Fabricated Using Electrospun Collagen–PCL Blends: Morphogenesis and Mechanical Properties. Tissue Eng. Part A 2009, 15, 2177–2187. [Google Scholar] [CrossRef]
- Chakrapani, V.Y.; Kumar, T.S.; Raj, D.; Kumary, T.V. Electrospun Cytocompatible Polycaprolactone Blend Composite with Enhanced Wettability for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2017, 17, 2320–2328. [Google Scholar] [CrossRef]
- Ico, G.; Showalter, A.; Bosze, W.; Gott, S.C.; Kim, B.S.; Rao, M.P.; Myung, N.V.; Nam, J. Size-dependent piezoelectric and mechanical properties of electrospun P(VDF-TrFE) nanofibers for enhanced energy harvesting. J. Mater. Chem. A 2016, 4, 2293–2304. [Google Scholar] [CrossRef]
- Janković, B.; Pelipenko, J.; Škarabot, M.; Muševič, I.; Kristl, J. The design trend in tissue-engineering scaffolds based on nanomechanical properties of individual electrospun nanofibers. Int. J. Pharm. 2013, 455, 338–347. [Google Scholar] [CrossRef]

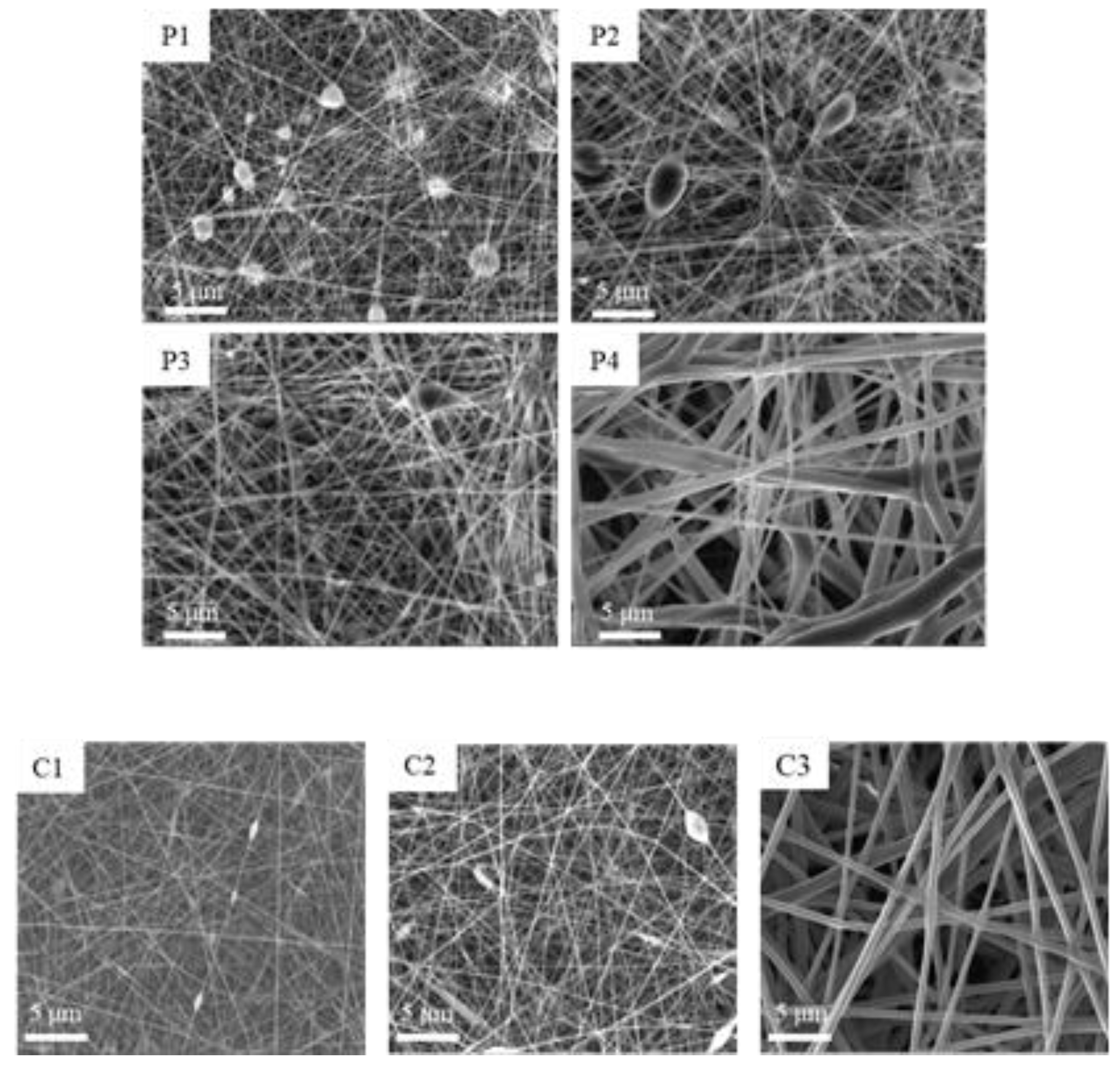

| Sample | Distance (cm) | Voltage (kV) | Flow (ml /h) |
|---|---|---|---|
| C.P. | 0 | 0 | 0 |
| 1 | −1 | −1 | −1 |
| 2 | +1 | −1 | −1 |
| 3 | −1 | +1 | −1 |
| 4 | +1 | +1 | −1 |
| C.P. | 0 | 0 | 0 |
| 5 | −1 | −1 | +1 |
| 6 | +1 | −1 | +1 |
| 7 | −1 | +1 | +1 |
| 8 | +1 | +1 | +1 |
| C.P. | 0 | 0 | 0 |
| Formulation | Concentration (% w/w) | Process Parameters | Fiber Dimensions (nm) |
|---|---|---|---|
| P1 | 10 | 20 Kv-15 cm-0.8 mL/h | 110.93 ± 29.40 |
| P2 | 15 | 197.08 ± 58.55 | |
| P3 | 20 | 211.60 ± 59.51 | |
| P4 | 30 | 494.80 ± 93.30 | |
| C1 | 10 | 25 kV-15 cm-0.4 mL/h | 175.55 ± 59.21 |
| C2 | 15 | 25 kV-15 cm-0.4 mL/h | 159.63 ± 53.01 |
| C3 | 20 | 25 kV-15 cm-0.4 mL/h | 668.24 ± 152.42 |
| Diffraction Signal | q (Å−1) | D(Å) |
|---|---|---|
| Equatorial Refl | 0.56 ± 0.02 | 11± 0.03 |
| Meridional Refl | 2.19 ± 0.02 | 2.8± 0.03 |
| Diffuse Scatter | 0.8 < q < 2.09 | 7.8 < d < 3 |
| Sample | q (Å−1) | FWHM (Å) |
|---|---|---|
| Native collagen | 0.56 ± 0.02 | 93.3± 3.6 |
| Native collagen in acetic acid 90% v/v | 0.56 ± 0.02 | 160.0± 11.04 |
| DRY | Tensile Strength (MPa) | Elongation (%) | Young’s Modulus (MPa) | HYDRATED | Tensile Strength (MPa) | Elongation (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|---|---|
| Collagen | 4.06 ± 0.05 | 33.2 ± 13.1 | 1.26 ± 0.1 | Collagen | / | / | / |
| Collagen/PCL 1:1 (w/w) | 2.50 ± 0.03 | 12.8 ± 0.8 | 0.58 ± 0.06 | Collagen/PCL 1:1 (w/w) | 0.89 ± 0.01 | 356.2 ± 45.9 | 0.04 ± 0.01 |
| Collagen/PCL 1:2 (w/w) | 3.53 ± 0.13 | 63.3 ± 8.7 | 0.10 ± 0.01 | Collagen/PCL 1:2 (w/w) | 2.75 ± 0.18 | 169.0 ± 22.5 | 0.06 ± 0.01 |
| PCL | 2.15 ± 0.13 | 140.5 ± 55.5 | 0.05 ± 0.01 | PCL | 0.96 ± 0.07 | 230.4 ± 20.3 | 0.01 ± 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miele, D.; Catenacci, L.; Rossi, S.; Sandri, G.; Sorrenti, M.; Terzi, A.; Giannini, C.; Riva, F.; Ferrari, F.; Caramella, C.; et al. Collagen/PCL Nanofibers Electrospun in Green Solvent by DOE Assisted Process. An Insight into Collagen Contribution. Materials 2020, 13, 4698. https://doi.org/10.3390/ma13214698
Miele D, Catenacci L, Rossi S, Sandri G, Sorrenti M, Terzi A, Giannini C, Riva F, Ferrari F, Caramella C, et al. Collagen/PCL Nanofibers Electrospun in Green Solvent by DOE Assisted Process. An Insight into Collagen Contribution. Materials. 2020; 13(21):4698. https://doi.org/10.3390/ma13214698
Chicago/Turabian StyleMiele, Dalila, Laura Catenacci, Silvia Rossi, Giuseppina Sandri, Milena Sorrenti, Alberta Terzi, Cinzia Giannini, Federica Riva, Franca Ferrari, Carla Caramella, and et al. 2020. "Collagen/PCL Nanofibers Electrospun in Green Solvent by DOE Assisted Process. An Insight into Collagen Contribution" Materials 13, no. 21: 4698. https://doi.org/10.3390/ma13214698
APA StyleMiele, D., Catenacci, L., Rossi, S., Sandri, G., Sorrenti, M., Terzi, A., Giannini, C., Riva, F., Ferrari, F., Caramella, C., & Bonferoni, M. C. (2020). Collagen/PCL Nanofibers Electrospun in Green Solvent by DOE Assisted Process. An Insight into Collagen Contribution. Materials, 13(21), 4698. https://doi.org/10.3390/ma13214698













