Surface Physical and Chemical Modification of Pure Iron by Using Atmospheric Pressure Plasma Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Application of the Atmospheric Pressure Plasma Treatment (APPT) Method
2.3. Characterization of Material Surface Properties
2.4. Simulation Method
3. Results and Discussion
3.1. Surface Wetting Behavior of Pure Iron
3.2. Macromechanical Behavior of Pure Iron
3.3. Surface Micromechanical Behavior of Pure Iron
3.4. Molecular Dynamics Simulation Results
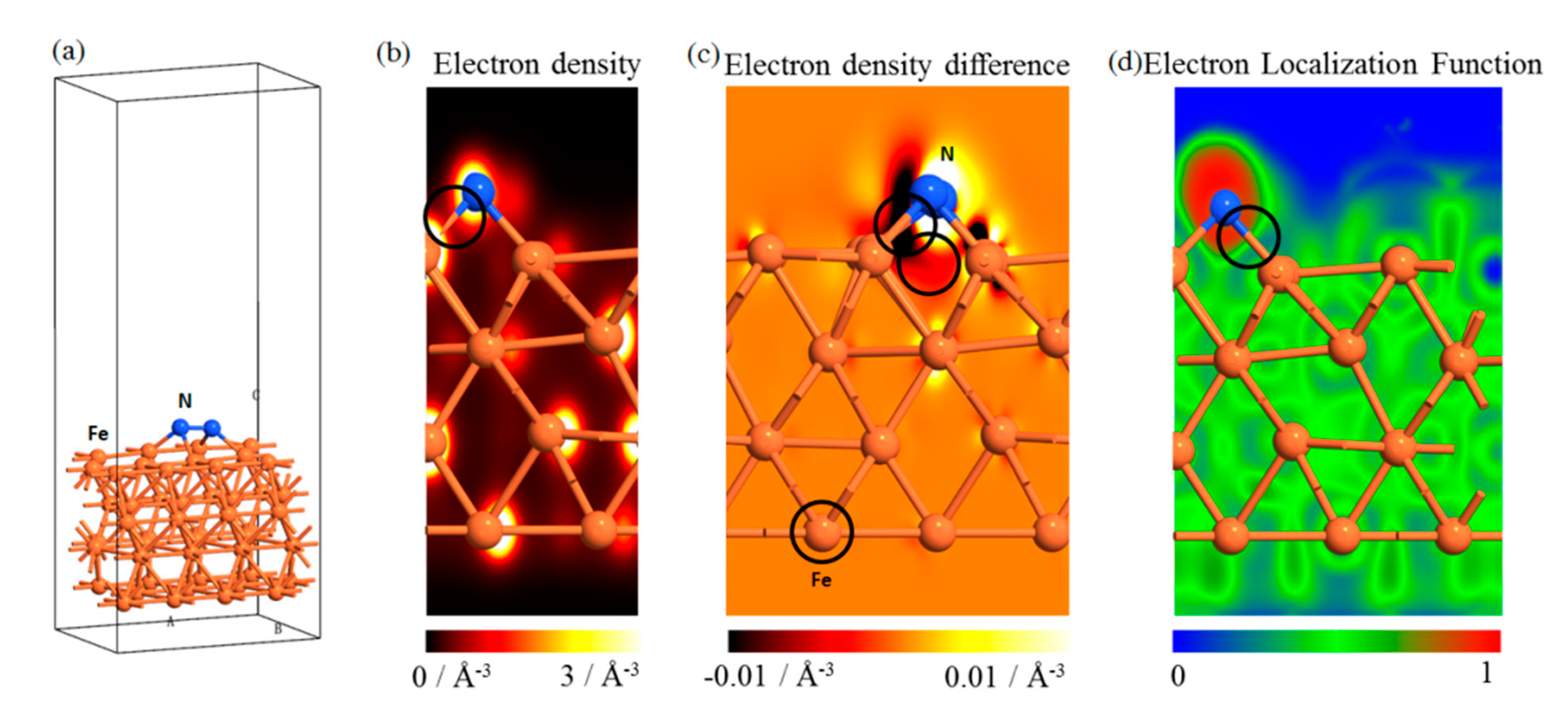
3.4.1. Physical and Chemical Process of Pure Iron Treated by APPT
3.4.2. Micromechanical Properties of Pure Iron Treated by APPT
3.4.3. Wetting Behavior of Pure Iron Treated by APPT
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bagherifard, S.; Molla, M.F.; Kajanek, D.; Donnini, R.; Donnini, R.; Hadzima, B.; Guagliano, M. Accelerated biodegradation and improved mechanical performance of pure iron through surface grain refinement. Acta Biomater. 2019, 98, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, E.; Olivetti, E.; Fiorillo, F.; Forton, E.; Martino, L.; Rocchino, L. Microstructure and magnetic properties of pure iron for cyclotron electromagnets. J. Alloys Compd. 2014, 615, S291–S295. [Google Scholar] [CrossRef]
- Shinko, T.; Hénaff, G.; Halm, D. Hydrogen-affected fatigue crack propagation at various loading frequencies and gaseous hydrogen pressures in commercially pure iron. Int. J. Fatigue 2019, 121, 197–207. [Google Scholar] [CrossRef]
- Fan, K.; Jin, Z.; Guo, J.; Wang, Z.; Jiang, G. Investigation on the surface layer formed during electrochemical modification of pure iron. Appl. Surf. Sci. 2019, 466, 466–471. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.G.; Pan, Y.A.; Kang, R.; Namba, Y.; Shore, P.; Yue, X.; Wang, B.; Guo, D. A critical review on the chemical wear and wear suppression of diamond tools in diamond cutting of ferrous metals. Int. J. Extrem. Manuf. 2020, 2, 12001. [Google Scholar]
- Nagashima, F.; Yoshino, M.; Terano, M. Microstructure control of pure iron by utilizing metal cutting method. Procedia Manuf. 2018, 15, 1541–1548. [Google Scholar] [CrossRef]
- Yildiz, Y.; Nalbant, M. A review of cryogenic cooling in machining processes. Int. J. Mach. Tools Manuf. 2008, 48, 947–964. [Google Scholar] [CrossRef]
- Umbrello, D.; Pu, Z.; Caruso, S.; Outeiro, J.C.; Jayal, A.D.; Dillon, O.W., Jr.; Jawahir, I.S. The effects of cryogenic cooling on surface integrity in hard machining. Procedia Eng. 2011, 19, 371–376. [Google Scholar] [CrossRef]
- Rotella, G.; Dillon, O.W.; Umbrello, D.; Settineri, L.; Jawahir, I.S. The effects of cooling conditions on surface integrity in machining of Ti6Al4V alloy. Int. J. Adv. Manuf. Technol. 2014, 71, 47–55. [Google Scholar] [CrossRef]
- Kong, J.X.; Li, L.; Xu, D.M.; He, N. Study on tool life and tool wear mechanisms in dry cutting pure iron. Mater. Sci. Forum 2013, 770, 74–77. [Google Scholar] [CrossRef]
- Kong, J.X.; Xia, Z.H.; Xu, D.M.; He, N. Investigation on notch wear mechanism in finish turning pure iron material with uncoated carbide tools under different cooling/lubrication conditions. Int. J. Adv. Manuf. Technol. 2016, 86, 97–105. [Google Scholar] [CrossRef]
- Kong, J.X.; Hu, K.; He, N.; Zhao, W. Effect of cooling/lubrication modes on tool wear mechanisms in turning pure iron material. Mocaxue Xuebao Tribol. 2015, 35, 378–385. [Google Scholar]
- Katahira, K.; Ohmori, H.; Takesue, S.; Komotori, J.; Yamazaki, K. Effect of atmospheric-pressure plasma jet on polycrystalline diamond micro-milling of silicon carbide. Cirp Ann. 2015, 64, 129–132. [Google Scholar] [CrossRef]
- Bastawros, A.F.; Chandra, A.; Poosarla, P.A. Atmospheric pressure plasma enabled polishing of single crystal sapphire. Cirp Ann. 2015, 64, 515–518. [Google Scholar] [CrossRef]
- Chen, F.; Liu, S.; Liu, J.; Huang, S.; Xia, G.; Song, J.; Xu, W.; Sun, J.; Liu, X. Surface modification of tube inner wall by transferred atmospheric pressure plasma. Appl. Surf. Sci. 2016, 389, 967–976. [Google Scholar] [CrossRef]
- Zou, R.; Yu, Z.; Zhang, C.; Yan, C.; Liu, X. High-speed micro electrical discharge machining with fine surface quality in atmospheric pressure nitrogen plasma jet. J. Mater. Process. Technol. 2019, 273, 116270. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, F.; Liu, J.; Zhang, J.; Chen, Y.; Zhang, Z.; Shen, H.; Kong, J.; Sun, J. Atmospheric pressure plasma-assisted precision turning of pure iron material. Int. J. Adv. Manuf. Technol. 2020, 106, 5187–5197. [Google Scholar] [CrossRef]
- GB/T 228.1-2010. Metallic Materials Tensile Testing Part 1: Room Temperature Test Method(S); Standardization Administration: Beijing, China, 2010.
- Bellido, E.P.; Seminario, J.M. Molecular dynamics simulations of ion-bombarded graphene. J. Phys. Chem. C 2012, 116, 4044–4049. [Google Scholar] [CrossRef]
- Yermolenko, O.A.; Kornich, G.V.; Betz, G. Molecular dynamics simulations of low-energy argon ion sputtering of copper clusters on polyethylene surfaces. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2011, 269, 1604–1608. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Z.; Wang, Y.; Gao, X.; Kong, R. Effect of ion implantation on material removal mechanism of 6H-SiC in nano-cutting: A molecular dynamics study. Comput. Mater. Sci. 2020, 174, 109476. [Google Scholar] [CrossRef]
- Lee, B.J.; Lee, T.H.; Kim, S.J. A modified embedded-atom method interatomic potential for the Fe-N system: A comparative study with the Fe-C system. Acta Mater. 2006, 54, 4597–4607. [Google Scholar] [CrossRef]
- Hyodo, K.; Munetoh, S.; Tsuchiyama, T.; Takaki, S. Empirical interatomic potential for Fe-N binary system based on Finnis-Sinclair potential. Comput. Mater. Sci. 2020, 174, 109500. [Google Scholar] [CrossRef]
- Muñoz, J.; Bravo, J.A.; Calzada, M.D. Aluminum metal surface cleaning and activation by atmospheric-pressure remote plasma. Appl. Surf. Sci. 2017, 407, 72–81. [Google Scholar] [CrossRef]
- Bónová, L.; Zahoranová, A.; Kováčik, D.; Zahoran, M.; Mičušík, M.; Černák, M. Atmospheric pressure plasma treatment of flat aluminum surface. Appl. Surf. Sci. 2015, 331, 79–86. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Chen, F.Z.; Zheng, H.X.; Yang, X.L.; Wu, L.B.; Song, J.L.; Xu, W.J. Diamond-cutting ferrous metals assisted by cold plasma and ultrasonic elliptical vibration. Int. J. Adv. Manuf. Technol. 2016, 85, 673–681. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Z.; Men, X.; Yang, J.; Xu, X.; Zhou, X. Plasma/thermal-driven the rapid wettability transition on a copper surface. Appl. Surf. Sci. 2011, 257, 3753–3757. [Google Scholar] [CrossRef]
- Goryaeva, A.M.; Fusco, C.; Bugnet, M.; Amodeo, J. Influence of an amorphous surface layer on the mechanical properties of metallic nanoparticles under compression. Phys. Rev. Mater. 2019, 3, 33606. [Google Scholar] [CrossRef]
- Jing, Y.; Meng, Q. Molecular dynamics simulations of the mechanical properties of crystalline/amorphous silicon core/shell nanowires. Phys. B-Condens. Matter 2010, 405, 2413–2417. [Google Scholar] [CrossRef]
- Guenole, J.; Godet, J.; Brochard, S. Plasticity in crystalline-amorphous core-shell Si nanowires controlled by native interface defects. Phys. Rev. B 2013, 87, 45201. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Qu, Z.; Sun, F.; Qie, Z.; Gao, J.; Zhao, G. The change of hydrogen bonding network during adsorption of multi-water molecules in lignite: Quantitative analysis based on AIM and DFT. Mater. Chem. Phys. 2020, 247, 122863. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, J.; Du, D.; Song, A.; Zhang, F.; Huang, W. Surface Physical and Chemical Modification of Pure Iron by Using Atmospheric Pressure Plasma Treatment. Materials 2020, 13, 4775. https://doi.org/10.3390/ma13214775
Kong J, Du D, Song A, Zhang F, Huang W. Surface Physical and Chemical Modification of Pure Iron by Using Atmospheric Pressure Plasma Treatment. Materials. 2020; 13(21):4775. https://doi.org/10.3390/ma13214775
Chicago/Turabian StyleKong, Jinxing, Dongxing Du, Aisheng Song, Fan Zhang, and Wen Huang. 2020. "Surface Physical and Chemical Modification of Pure Iron by Using Atmospheric Pressure Plasma Treatment" Materials 13, no. 21: 4775. https://doi.org/10.3390/ma13214775
APA StyleKong, J., Du, D., Song, A., Zhang, F., & Huang, W. (2020). Surface Physical and Chemical Modification of Pure Iron by Using Atmospheric Pressure Plasma Treatment. Materials, 13(21), 4775. https://doi.org/10.3390/ma13214775



