Effect of H2O Activity on Zeolite Formation
Abstract
1. Introduction
2. Experimental Section
2.1. Samples Preparation
2.2. Sample Characterization
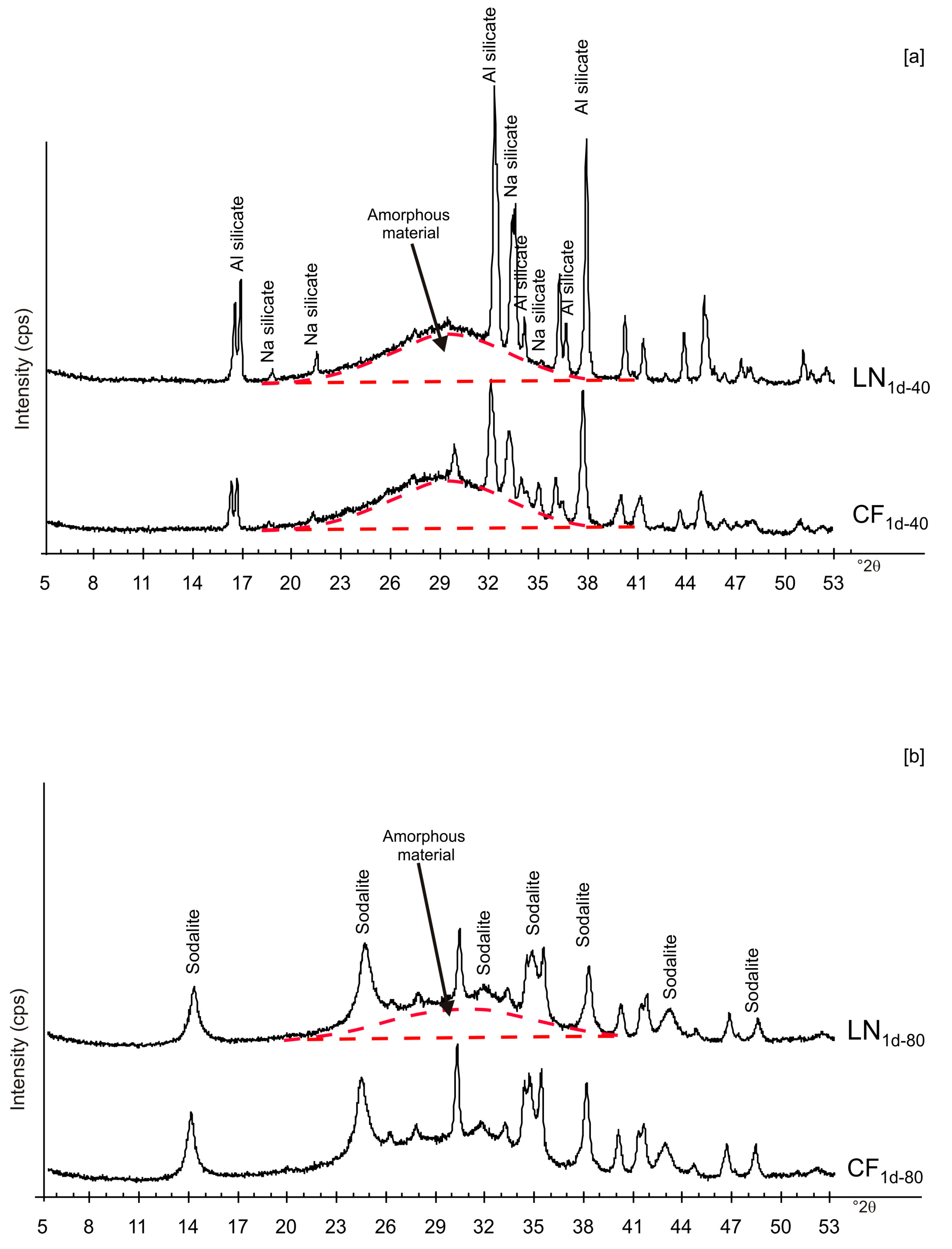
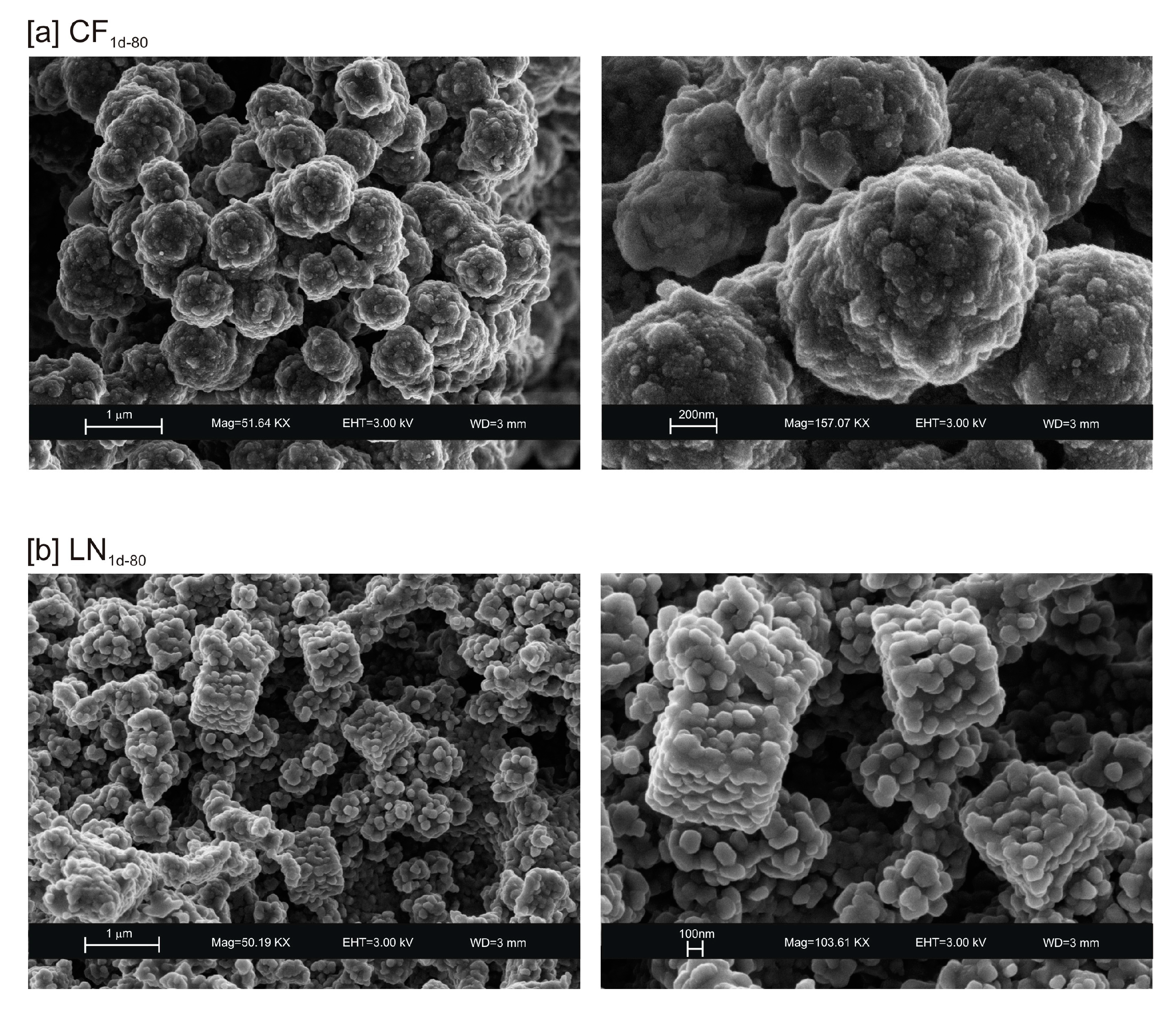
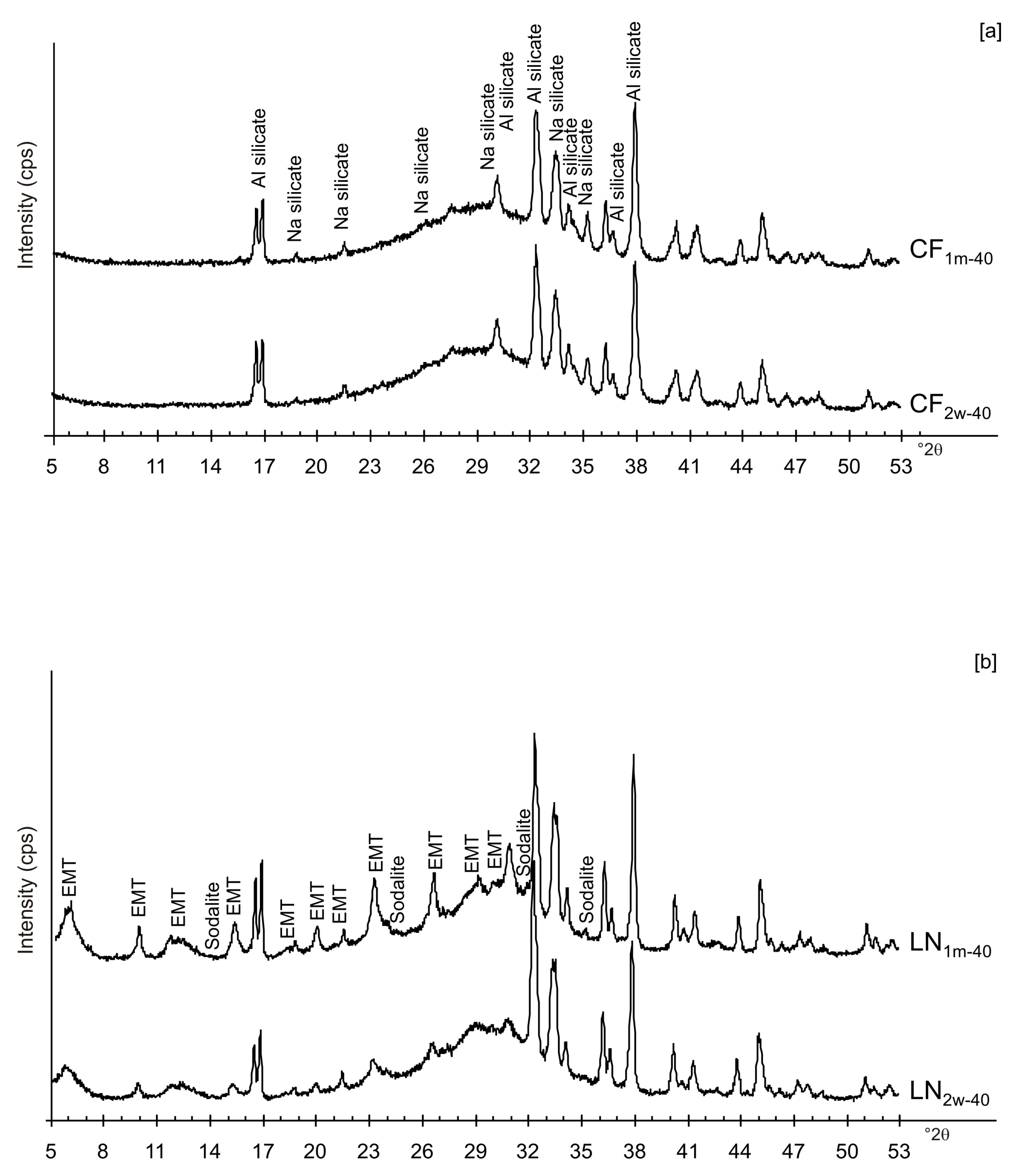
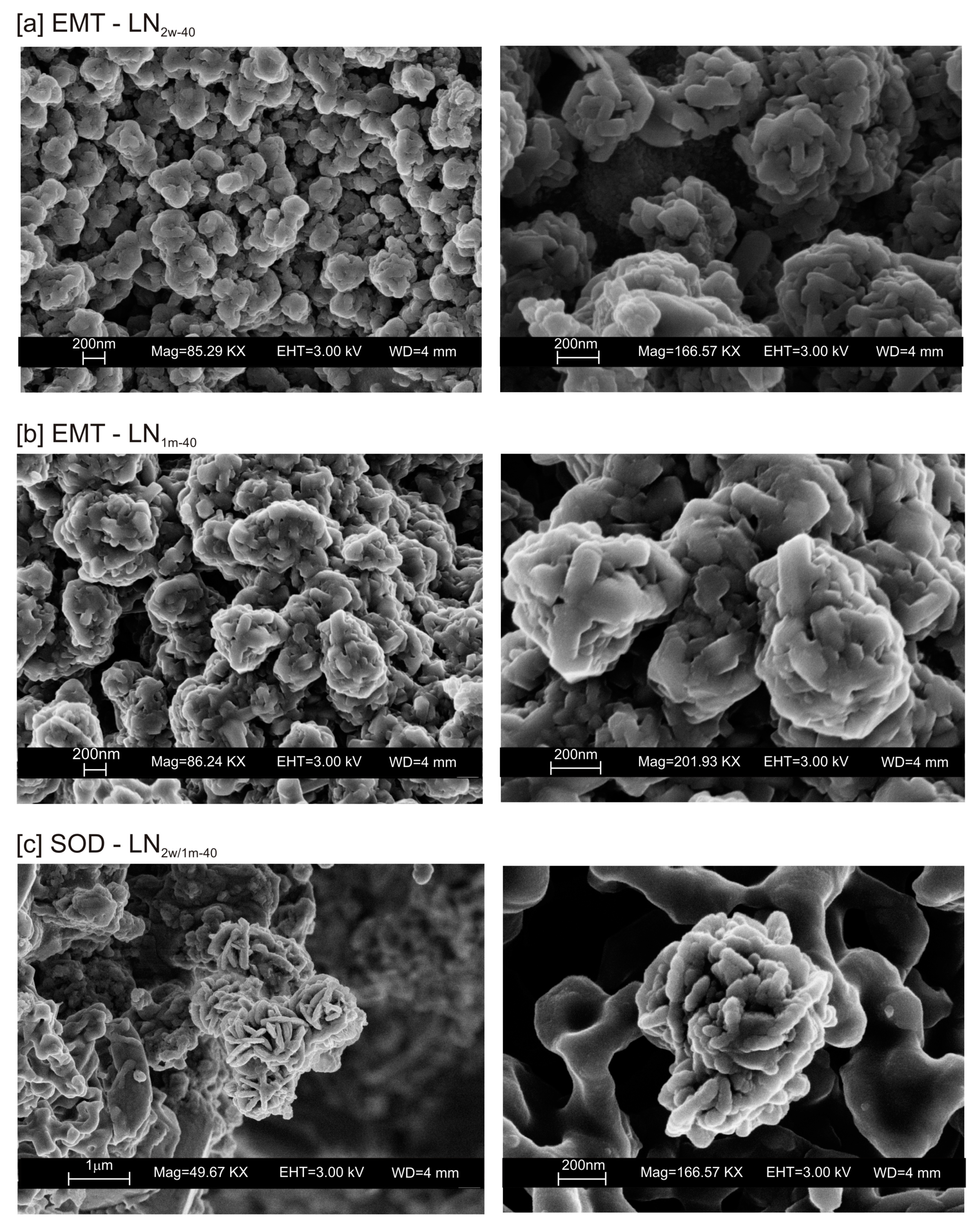
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/database/2008 (accessed on 3 October 2020).
- Belviso, C.; Cavalcante, F.; Lettino, A.; Fiore, S. A and X Type Zeolite Synthesized from Kaolinite at Low Temperature. Appl. Clay Sci. 2013, 80–81, 20162–20168. [Google Scholar]
- Rios, C.; Williams, C.; Fullen, M. Nucleation and Growth History of Zeolite LTA Synthesized from Kaolinite by Two Different Methods. Appl. Clay Sci. 2009, 42, 446–454. [Google Scholar] [CrossRef]
- Singh, P.; Aswal, V.K.; Chaudhri, S.G.; Schwieger, W. Structural Evolution During Nucleation of Si-Rich LTA Nanocrystals from Colloidal Solution. Microporous Mesoporous Mater. 2018, 259, 99–110. [Google Scholar] [CrossRef]
- Oleksiak, M.D.; Soltis, J.A.; Conato, M.T.; Penn, R.L.; Rimer, J.D. Nucleation of FAU and LTA Zeolites from Heterogeneous Aluminosilicate Precursors. Chem. Mater. 2016, 28, 4906–4916. [Google Scholar] [CrossRef]
- Belviso, C.; Agostinelli, E.; Belviso, S.; Cavalcante, F.; Pascucci, S.; Peddis, D.; Varvaro, G.; Fiore, S. Synthesis of Magnetic Zeolite at Low Temperature Using a Waste Material Mixture: Fly Ash and Red Mud. Microporous Mesoporous Mater. 2015, 202, 208–216. [Google Scholar] [CrossRef]
- Belviso, C.; Giannossa, L.C.; Huertas, F.J.; Lettino, A.; Mangone, A.; Fiore, S. Synthesis of Zeolites at Low Temperatures in Fly Ash-Kaolinite Mixtures. Microporous Mesoporous Mater. 2015, 212, 35–47. [Google Scholar] [CrossRef]
- Li, Y.; Peng, T.; Man, W.; Ju, L.; Zheng, F.; Zhang, M.; Guo, M. Hydrothermal Synthesis of Mixtures of Na-A Zeolite and Sodalite from Ti-Bearing Electric Arc Furnace Slag. RSC Adv. 2016, 6, 8358–8366. [Google Scholar] [CrossRef]
- Qian, T.; Li, J. Synthesis of Na-A Zeolite from Coal Gangue With the in-Situ Crystallization Technique. Adv. Powder Technol. 2015, 26, 98–104. [Google Scholar] [CrossRef]
- Fernandes-Machado, N.R.C.; Miotto, D.M.M. Synthesis of Na–A and –X Zeolites from Oil Shale Ash. Fuel 2005, 84, 2289–2294. [Google Scholar] [CrossRef]
- Belviso, S.; Cavalcante, F.; Lettino, A.; Ragone, P.; Belviso, C. Fly Ash as Raw Material for the Synthesis of Zeolite-Encapsulated Porphyrazine and Metallo Porphyrazine Tetrapyrrolic Macrocycles. Microporous Mesoporous Mater. 2016, 236, 228–234. [Google Scholar] [CrossRef]
- Collins, F.; Rozhkovskaya, A.; Outram, J.G.; Millar, G.J. A Critical Review of Waste Resources, Synthesis, and Applications for Zeolite LTA. Microporous Mesoporous Mater. 2020, 291, 109667. [Google Scholar] [CrossRef]
- Selim, M.M.; El-Mekkawi, D.M.; Aboelenin, R.M.M.; Sayed Ahmed, S.A.; Mohamed, G.M. Preparation and Characterization of Na-A Zeolite from Aluminium Scrub and Commercial Sodium Silicate for the Removal of Cd2+ from Water. J. Assoc. Arab Univ. Basic Appl. Sci. 2017, 24, 19–25. [Google Scholar]
- Mintova, S.; Olson, N.H.; Valtchev, V.; Bein, T. Nanocrystal Growth from Colloids at Room Temperature. Science 1999, 283, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhang, B.; Zhao, B.; Li, H.; Wang, N. High-efficient synthesis of zeolite LTA via a wet-gel crystallization route. Chem. Res. Chin. Univ. 2017, 33, 520–524. [Google Scholar] [CrossRef]
- Smaïhi, M.; Barida, O.; Valtchev, V. Investigation of the Crystallization Stages of LTA-Type Zeolite by Complementary Characterization Techniques. Eur. J. Inorg. Chem. 2003, 2003, 4370–4377. [Google Scholar] [CrossRef]
- Valtchev, V.P.; Tosheva, L.; Bozhilov, K.N. Synthesis of Zeolite Nanocrystals at Room Temperature. Langmuir 2005, 21, 10724–10729. [Google Scholar] [CrossRef]
- Agger, J.R.; Pervaiz, N.; Cheetham, A.K.; Anderson, M.W. Crystallization in Zeolite A Studied by Atomic Force Microscopy. J. Am. Chem. Soc. 1998, 120, 10754–10759. [Google Scholar] [CrossRef]
- Yoon, K.B. Mono and Multilayer Assembly of Zeolite Microcrystals on Substrates. Bull. Korean Chem. Soc. 2006, 27, 17–26. [Google Scholar]
- Yoon, K.B. Organization of Zeolite Microcrystals for Production of Functional Materials. Acc. Chem. Res. 2007, 40, 29–40. [Google Scholar] [CrossRef]
- Leiggener, C.; Calzaferri, G. Monolayers of Zeolite A Containing Luminescent Silver Sulfide Clusters. Chem. Phys. Chem. 2004, 5, 1593–1596. [Google Scholar] [CrossRef]
- Walton, R.I.; Millange, F.; O’Hare, D.; Davies, A.T.; Sankar, G.; Catlow, C.R.A. An In Situ Energy-Dispersive X-ray Diffraction Study of the Hydrothermal Crystallization of Zeolite A. 1. Influence of Reaction Conditions and Transformation into Sodalite. J. Phys. Chem. B 2001, 105, 83–90. [Google Scholar] [CrossRef]
- Subotic, B.; Sekovanic, L. Transformation of Zeolite A into Hydroxysodalite. J. Cryst. Growth 1986, 75, 561–572. [Google Scholar] [CrossRef]
- Greer, H.; Wheatley, P.S.; Ashbrook, S.E.; Morris, R.E.; Zhou, W. Early Stage Reversed Crystal Growth of Zeolite A and Its Phase Transformation to Sodalite. J. Am. Chem. Soc. 2009, 131, 17986–17992. [Google Scholar] [CrossRef] [PubMed]
- Belviso, C. Ultrasonic vs Hydrothermal Method: Different Approaches to Convert Fly Ash into Zeolite. How They Affect the Stability of Synthetic Products Over Time? Ultrason. Sonochemistry 2018, 43, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Belviso, C.; Lettino, A.; Cavalcante, F. Influence of Synthesis Method on LTA Time-Dependent Stability. Molecules 2018, 23, 2122. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The Hydrothermal Synthesis of Zeolites: History and Development from the Earliest Days to the Present Time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef] [PubMed]
- Bushuev, Y.; Sastre, G.; De Julián-OrtizJ, V. The Structural Directing Role of Water and Hydroxyl Groups in the Synthesis of Beta Zeolite Polymorphs. J. Phys. Chem. C 2009, 114, 345–356. [Google Scholar] [CrossRef]
- Demontis, P.; Gulín-González, J.; Jobic, H.; Masia, M.; Sale, R.; Suffritti, G.B. Dynamical Properties of Confined Water Nanoclusters: Simulation Study of Hydrated Zeolite Na-A: Structural and Vibrational Properties. ACS Nano 2008, 2, 1603–1614. [Google Scholar] [CrossRef]
- Angell, C.A. Insights into Phases of Liquid Water from Study of Its Unusual Glass-Forming Properties. Science 2008, 319, 582–587. [Google Scholar] [CrossRef]
- Marcolli, C. Deposition Nucleation Viewed as Homogeneous or Immersion Freezing in Pores and Cavities. Atmos. Chem. Phys. Discuss. 2014, 14, 2071–2104. [Google Scholar] [CrossRef]
- Jelassi, J.; Castricum, H.; Bellissent-Funel, M.-C.; Dore, J.; Webber, J.B.W.; Sridi-Dorbez, R. Studies of Water and Ice in Hydrophilic and Hydrophobic Mesoporous Silicas: Pore Characterisation and Phase Transformations. Phys. Chem. Chem. Phys. 2010, 12, 2838. [Google Scholar] [CrossRef]
- Seyed-Yazdi, J.; Farman, H.; Dore, J.C.; Webber, J.B.W.; Findenegg, G.H. Structural Characterization of Water/Ice Formation in Sba-15 Silicas: III. the Triplet Profile for 86 Å Pore Diameter. J. Phys. Condens. Matter 2008, 20, 205108. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Dore, J.C.; Webber, J.B.W.; Khushalani, D.; Jähnert, S.; Findenegg, G.H.; Hansen, T. Neutron Diffraction and NMR Relaxation Studies of Structural Variation and Phase Transformations for Water/Ice in SBA-15 Silica: I. the Over-Filled Case. J. Phys. Condens. Matter 2006, 18, 10009–10028. [Google Scholar] [CrossRef]
- Jażdżewska, M.; Sliwinska-Bartkowiak, M.; Domin, K.; Chudoba, D.M.; Beskrovnyi, A.I.; Neov, D.S.; Gubbins, K.E. Structure of Ice Confined in Carbon and Silica Nanopores. Bull. Mater. Sci. 2019, 42, 184. [Google Scholar] [CrossRef]
- Bordonskii, G.S.; Orlov, A.O. Phase Transitions of Water in Zeolite Pores. Tech. Phys. Lett. 2019, 45, 205–207. [Google Scholar] [CrossRef]
- Limmer, D.T.; Chandler, D. Phase Diagram of Supercooled Water Confined to Hydrophilic Nanopores. J. Chem. Phys. 2012, 137, 44509. [Google Scholar] [CrossRef]
- Menshikov, L.I.; Menshikov, P.L.; Fedichev, P.O. Phenomenological Model of Hydrophobic and Hydrophilic Interactions. J. Exp. Theor. Phys. 2017, 125, 1173–1188. [Google Scholar] [CrossRef]
- Janssen, A.H.; Talsma, H.; Van Steenbergen, M.J.; De Jong, K.P. Homogeneous Nucleation of Water in Mesoporous Zeolite Cavities. Langmuir 2004, 20, 41–45. [Google Scholar] [CrossRef][Green Version]
- Kyakuno, H.; Matsuda, K.; Nakai, Y.; Fukuoka, T.; Maniwa, Y.; Nishihara, H.; Kyotani, T. Amorphous Water in Three-Dimensional Confinement of Zeolite-Templated Carbon. Chem. Phys. Lett. 2013, 571, 54–60. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, G.; Hossain, S.M.C.; Gao, D. Coupled Experimental-Modeling Analyses of Heat Transfer in Ex-Vivo VS55-Perfused Porcine Hepatic Tissue Being Plunged in Liquid Nitrogen for Vitreous Cryopreservation. Int. J. Heat Mass Transf. 2017, 106, 970–979. [Google Scholar] [CrossRef]
- Fahy, G.; Macfarlane, D.; Angell, C.; Meryman, H. Vitrification as an Approach to Cryopreservation. Cryobiology 1984, 21, 407–426. [Google Scholar] [CrossRef]
- Faizullin, M.Z.; Koverda, V.P. Vitrification and Crystallization of Low-Temperature Amorphous Condensates of Water and Methane—Water Mixture. Russ. J. Phys. Chem. A 2012, 86, 229–234. [Google Scholar] [CrossRef]
- Poudyal, R.L.; Kobayashi, R.; Suzuki, T.; Watanabe, M. Effect of Different Freezing and Storage Condition on the Physical Properties of Protein Coagulum (Firm Tofu). Int. J. Refrig. 2019, 107, 11–19. [Google Scholar] [CrossRef]
- Olmo, A.; Baena, R.; Risco, R. Use of a Droplet Nucleation Analyzer in the Study of Water Freezing Kinetics Under the Influence of Ultrasound Waves. Int. J. Refrig. 2008, 31, 262–269. [Google Scholar] [CrossRef]
- Kiani, H.; Zhang, Z.; Sun, D.-W. Effect of Ultrasound Irradiation on Ice Crystal Size Distribution in Frozen Agar Gel Samples. Innov. Food Sci. Emerg. Technol. 2013, 18, 126–131. [Google Scholar] [CrossRef]
- Dalvi-Isfahan, M.; Hamdami, N.; Xanthakis, E.; Le-Bail, A. Review on the Control of Ice Nucleation by Ultrasound Waves, Electric and Magnetic Fields. J. Food Eng. 2017, 195, 222–234. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, M.; Xu, B.; Adhikari, B.; Sun, J. The Principles of Ultrasound and Its Application in Freezing Related Processes of Food Materials: A Review. Ultrason. Sonochemistry 2015, 27, 576–585. [Google Scholar] [CrossRef]
- Inaba, H.; Takeya, K.; Nozu, S. Fundamental Study on Continuous Ice Making Using Flowing Supercooled Water. JSME Int. J. Ser. B 1994, 37, 385–393. [Google Scholar] [CrossRef]
- Simonetti, F.; Fox, M. Experimental Methods for Ultrasonic Testing of Complex-Shaped Parts Encased in Ice. NDT E Int. 2019, 103, 1–11. [Google Scholar] [CrossRef]
- Mohd Khairi, M.T.; Ibrahim, S.; Md Yunus, M.A.; Faramarzi, M.; Abid, A. Experimental Methods for Ultrasonic Testing of Complex-Shaped Parts Encased in Ice. Measurement 2019, 146, 490–523. [Google Scholar]
- Saclier, M.; Peczalski, R.; Andrieu, J. Effect of Ultrasonically Induced Nucleation on Ice Crystals’ Size and Shape During Freezing in Vials. Chem. Eng. Sci. 2010, 65, 3064–3071. [Google Scholar] [CrossRef]
- Inada, T.; Zhang, X.; Yabe, A.; Kozawa, Y. Active Control of Phase Change from Supercooled Water to Ice by Ultrasonic Vibration 1. Control of Freezing Temperature. Int. J. Heat Mass Transf. 2001, 44, 4523–4531. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, X.; Cheng, B.; Zhang, D.; Zhou, G. Study on Heat and Mass Transfer of Droplet Cooling in Ultrasound Wave. Int. J. Heat Mass Transf. 2017, 107, 916–924. [Google Scholar] [CrossRef]
- Thompson, R.W.; Huber, M.J. Analysis of the Growth of Molecular Sieve Zeolite NaA in a Batch Precipitation System. J. Cryst. Growth 1982, 56, 711–722. [Google Scholar] [CrossRef]
- Heard, C.J.; Grajciar, L.; Rice, C.M.; Pugh, S.M.; Nachtigall, P.; Ashbrook, S.E.; Morris, R.E. Fast Room Temperature Lability of Aluminosilicate Zeolites. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gouseti, O.; Lopez-Quiroga, E.; Fryer, P.J.; Bakalis, S. Water Crystallization in Highly Concentrated Carbohydrate-Based Systems. Cryst. Growth Des. 2019, 19, 2081–2088. [Google Scholar] [CrossRef]
- Breuer, R.G.; Barsotti, L.R.; Kelly, A.C. Behaviour of Silica in Sodium Aluminate Solutions. In International Symposium on the Extract Metallurgy of Aluminium; Interscience: New York, NY, USA, 1963; pp. 133–157. [Google Scholar]
- Liu, Q.; Navrotsky, A. Synthesis of Nitrate Sodalite: An in Situ Scanning Calorimetric Study. Geochim. Cosmochim. Acta 2007, 71, 2072–2078. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Passaro, M.D.; Spinelli, N.; Apicella, B. Insights on Clusters Formation Mechanism by Time of Flight Mass Spectrometry. 1. The Case of Ethanol–Water Clusters. J. Am. Soc. Mass Spectrom. 2015, 26, 1665–1675. [Google Scholar] [CrossRef]

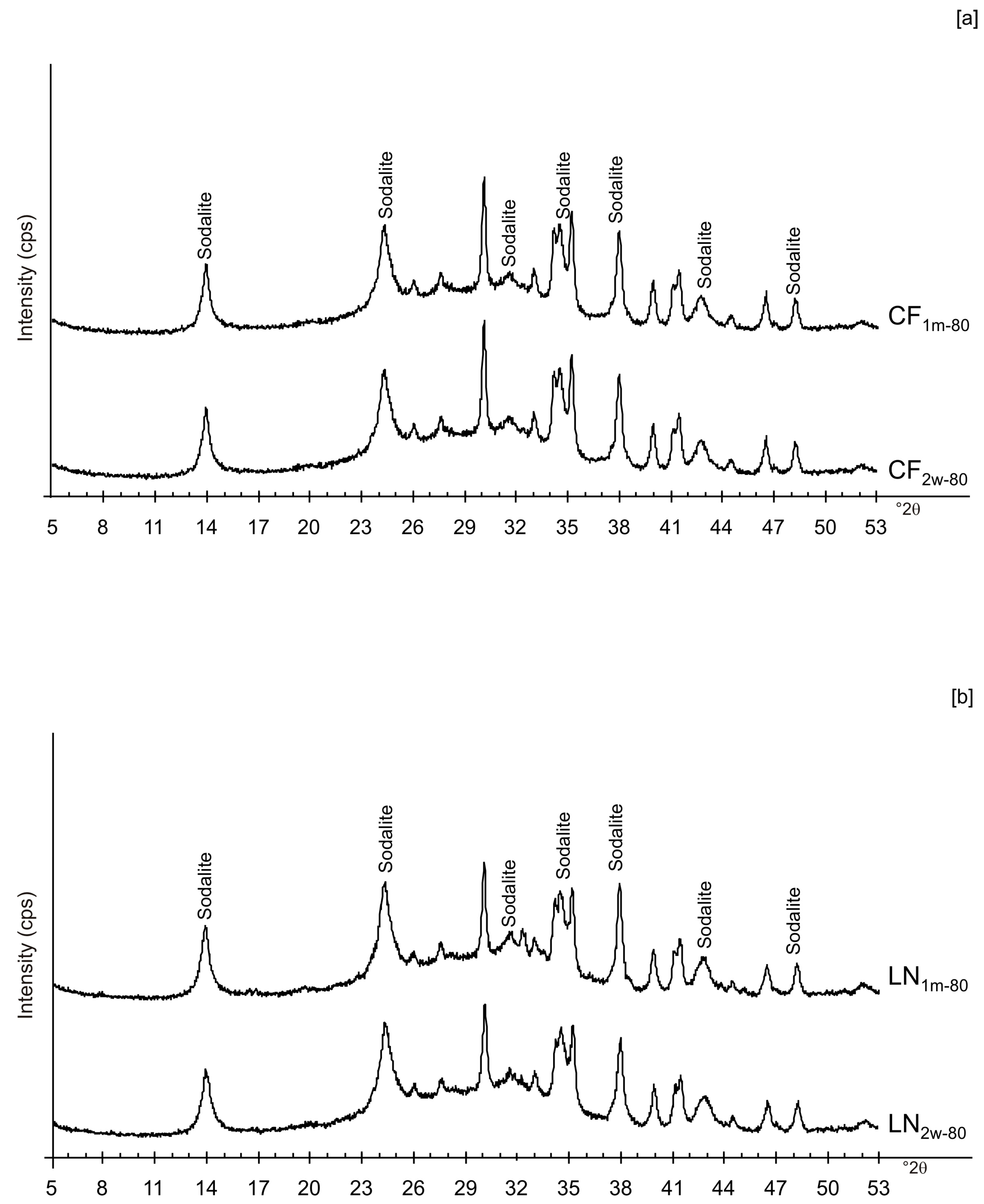
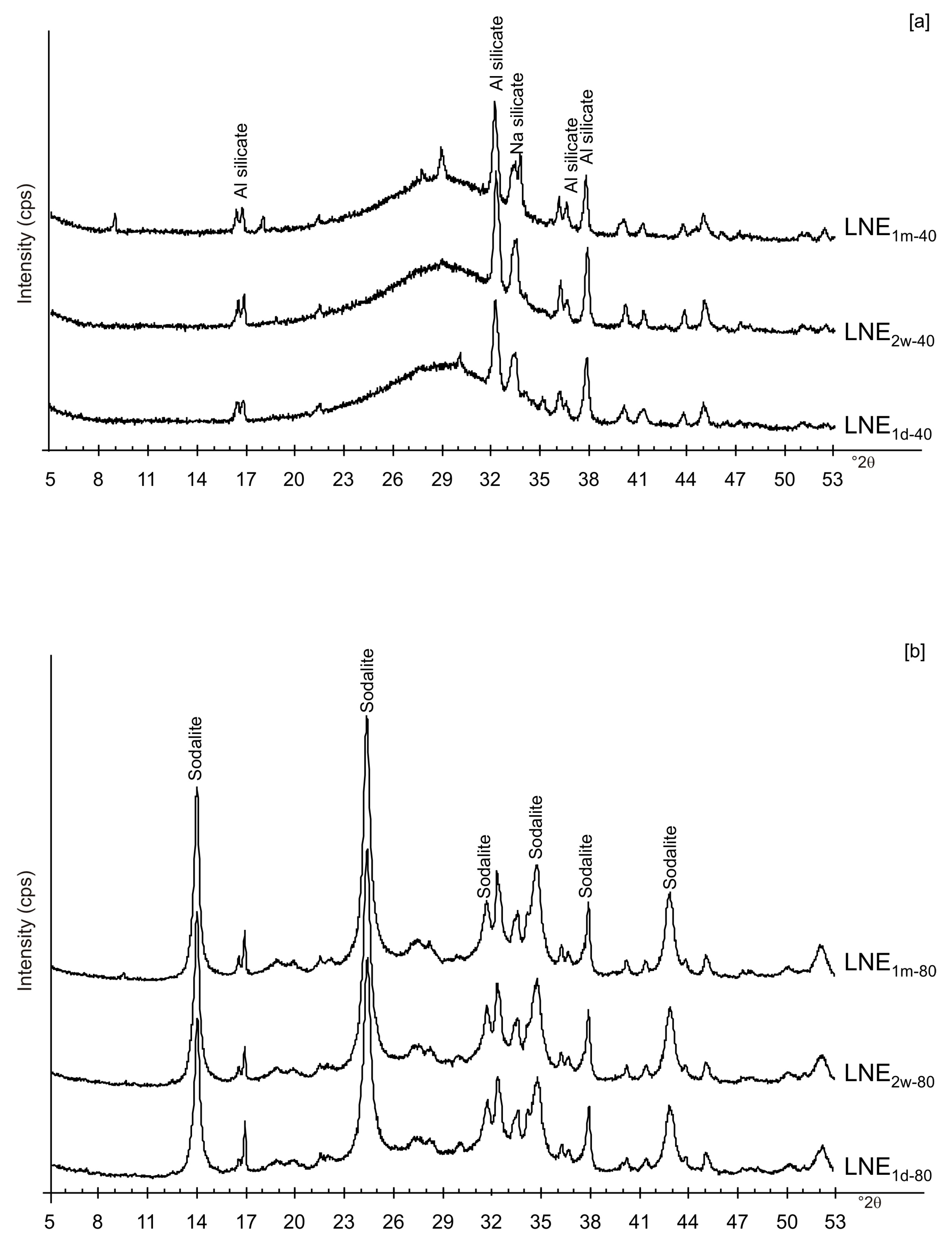

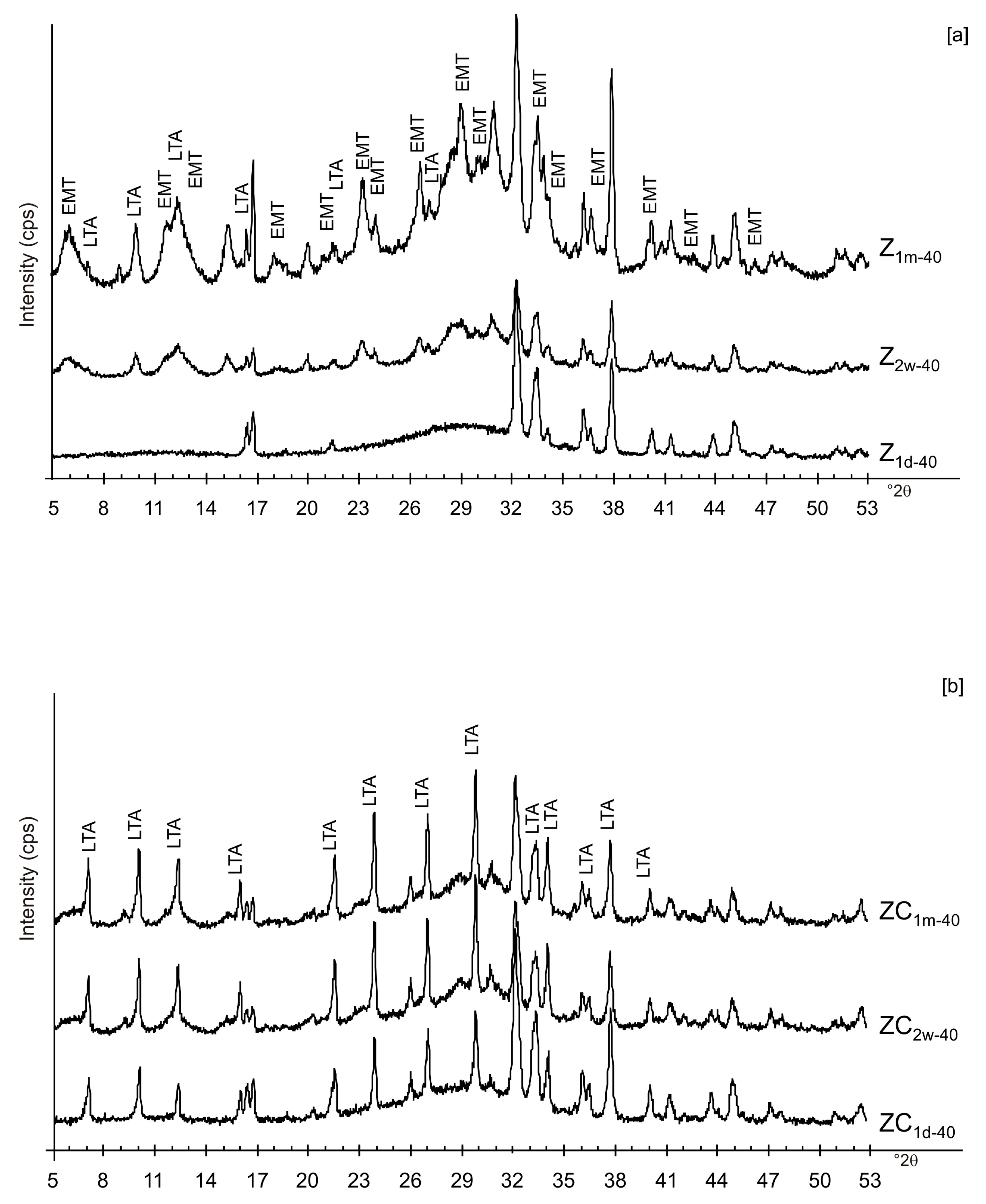
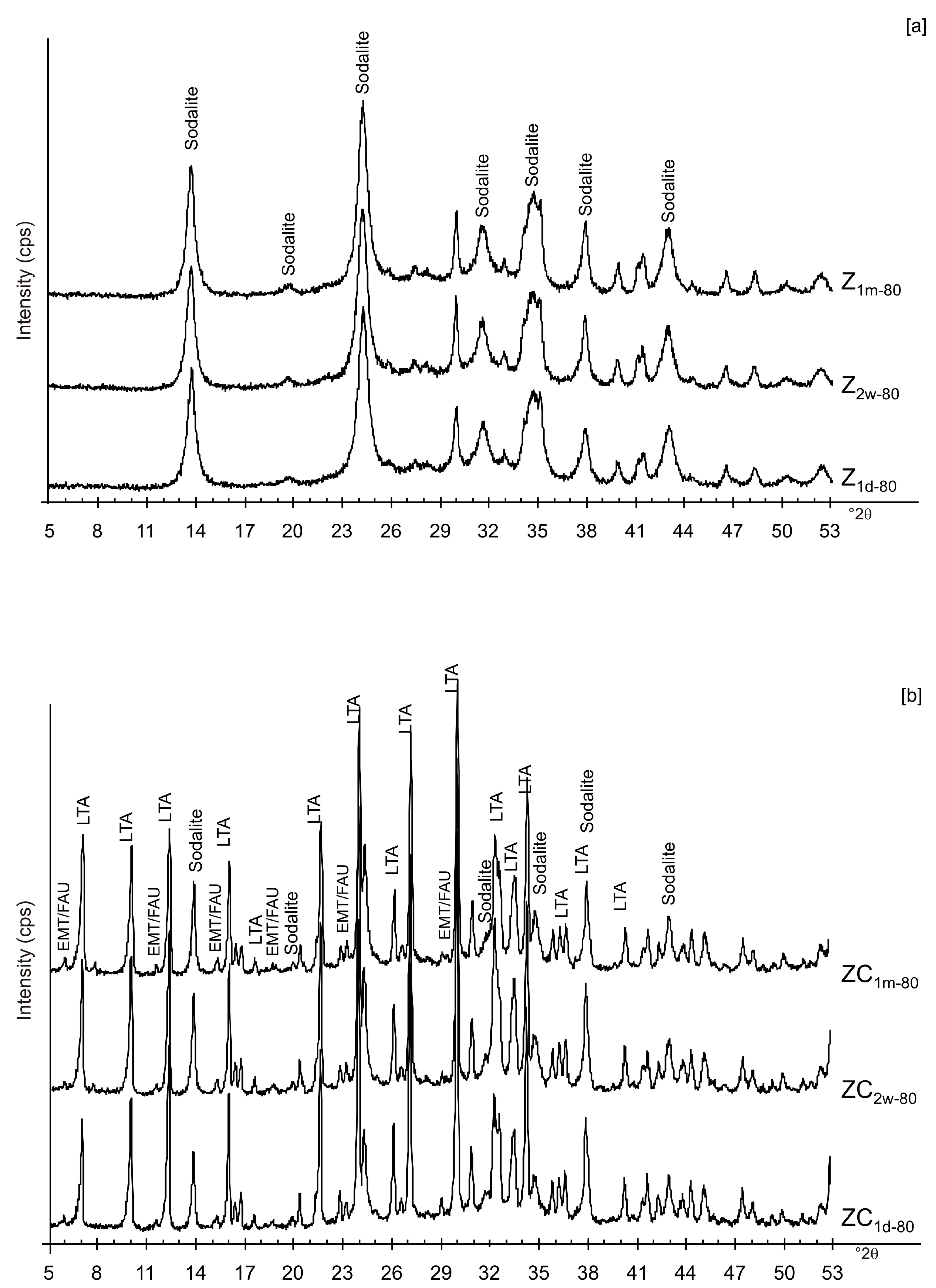
| Sample Code | Freezing Process | Additional Treatments | Drying Temperature | Aging Time at Solid State | Main Mineralogical Composition | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Liquid Nitrogen | Conventional Freezer | Ethanol Washing | Centrifuge | 40 °C | 80 °C | 1 Day | 2 Weeks | 1 Month | ||
| LN1d-40 | x | x | x | Amorphous, Al and Na silicate | ||||||
| LN1d-80 | x | x | x | Amorphous, Sodalite | ||||||
| LN2w-40 | x | x | x | Amorphous, EMT, Sodalite | ||||||
| LN2w-80 | x | x | x | Amorphous, Sodalite | ||||||
| LN1m-40 | x | x | x | Amorphous, EMT, Sodalite | ||||||
| LN1m-80 | x | x | x | Amorphous, Sodalite | ||||||
| CF1d-40 | x | x | x | Amorphous, Al and Na silicate | ||||||
| CF1d-80 | x | x | x | Amorphous, Sodalite | ||||||
| CF2w-40 | x | x | x | Amorphous, Al and Na silicate | ||||||
| CF2w-80 | x | x | x | Amorphous, Sodalite | ||||||
| CF1m-40 | x | x | x | Amorphous, Al and Na silicate | ||||||
| CF1m-80 | x | x | x | Amorphous, Sodalite | ||||||
| LNE1d-40 | x | x | x | x | Amorphous, Al and Na silicate | |||||
| LNE1d-80 | x | x | x | x | Amorphous, Sodalite | |||||
| LNE2w-40 | x | x | x | x | Amorphous, Al and Na silicate | |||||
| LNE2w-80 | x | x | x | x | Amorphous, Sodalite | |||||
| LNE1m-40 | x | x | x | x | Amorphous, Al and Na silicate | |||||
| LNE1m-80 | x | x | x | x | Amorphous, Sodalite | |||||
| ZC1d-40 | x | x | x | Amorphous, LTA | ||||||
| ZC1d-80 | x | x | x | LTA, EMT, sodalite | ||||||
| ZC2w-40 | x | x | x | Amorphous, LTA | ||||||
| ZC2w-80 | x | x | x | LTA, EMT, sodalite | ||||||
| ZC1m-40 | x | x | x | Amorphous, LTA | ||||||
| ZC1m-80 | x | x | x | LTA, EMT, sodalite | ||||||
| Z1d-40 | x | x | Amorphous, Al and Na silicate | |||||||
| Z1d-80 | x | x | Sodalite | |||||||
| Z2w-40 | x | x | Amorphous, EMT, LTA | |||||||
| Z2w-80 | x | x | Sodalite | |||||||
| Z1m-40 | x | x | Amorphous, EMT, LTA | |||||||
| Z1m-80 | x | x | Sodalite | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belviso, C.; Cavalcante, F. Effect of H2O Activity on Zeolite Formation. Materials 2020, 13, 4780. https://doi.org/10.3390/ma13214780
Belviso C, Cavalcante F. Effect of H2O Activity on Zeolite Formation. Materials. 2020; 13(21):4780. https://doi.org/10.3390/ma13214780
Chicago/Turabian StyleBelviso, Claudia, and Francesco Cavalcante. 2020. "Effect of H2O Activity on Zeolite Formation" Materials 13, no. 21: 4780. https://doi.org/10.3390/ma13214780
APA StyleBelviso, C., & Cavalcante, F. (2020). Effect of H2O Activity on Zeolite Formation. Materials, 13(21), 4780. https://doi.org/10.3390/ma13214780






