The Effect of Zeolite Features on the Dehydration Reaction of Methanol to Dimethyl Ether: Catalytic Behaviour and Kinetics
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of the Investigated Samples
2.2. Characterisation of the Investigated Samples
2.3. Catalytic Tests and Kinetic Analysis
3. Results and Discussion
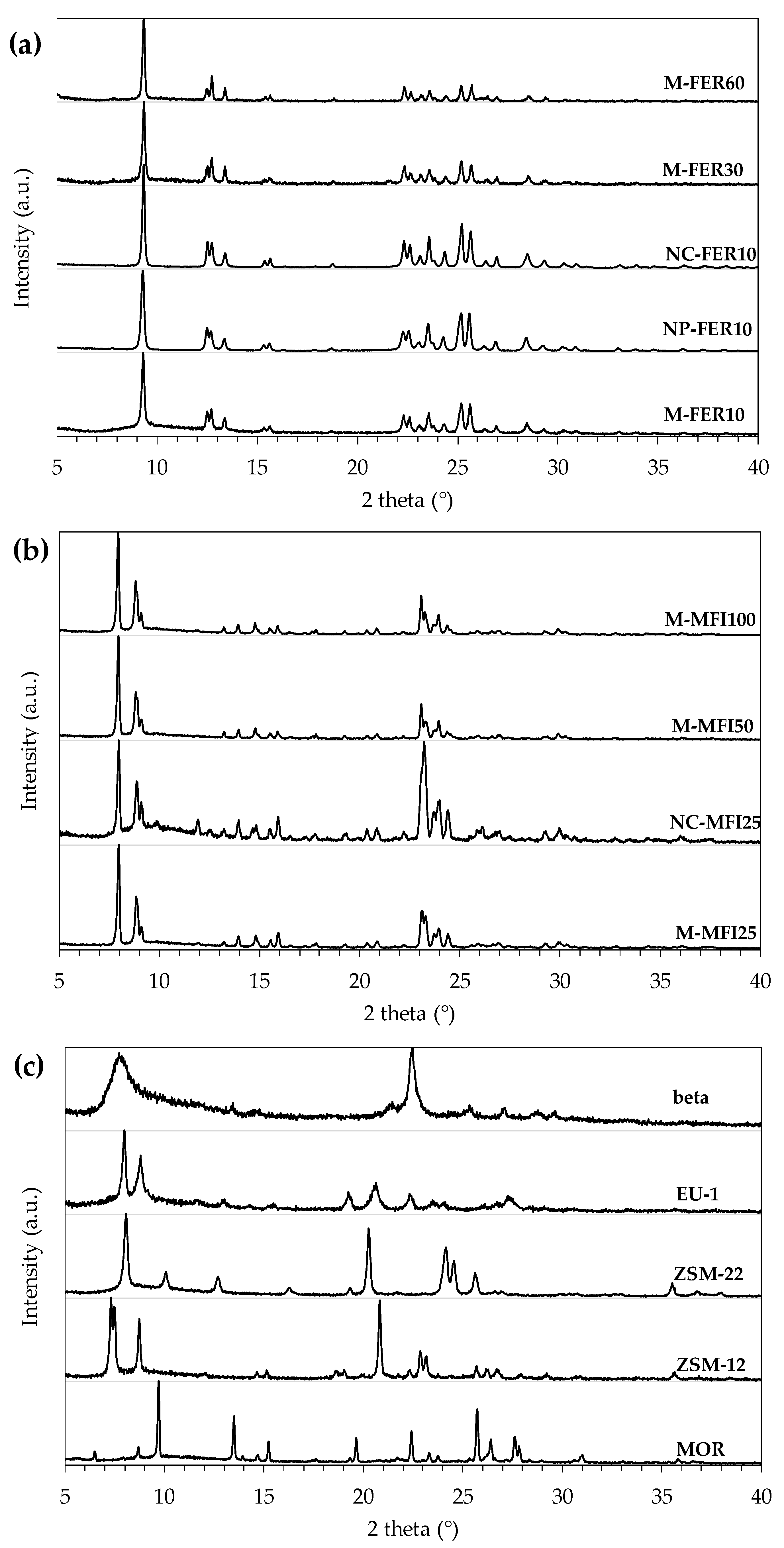
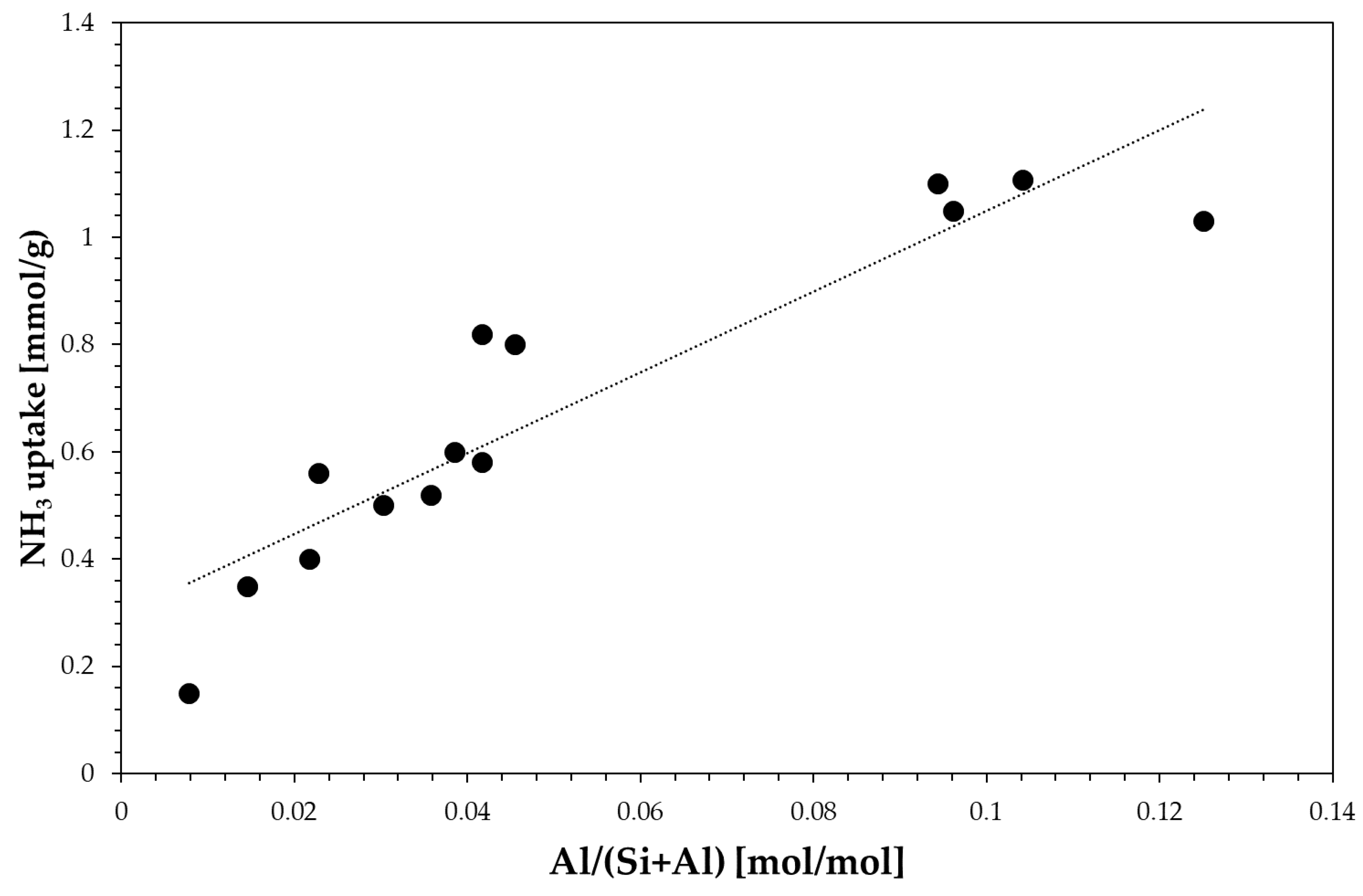
3.1. Physic-Chemical Properties of the Investigated Samples
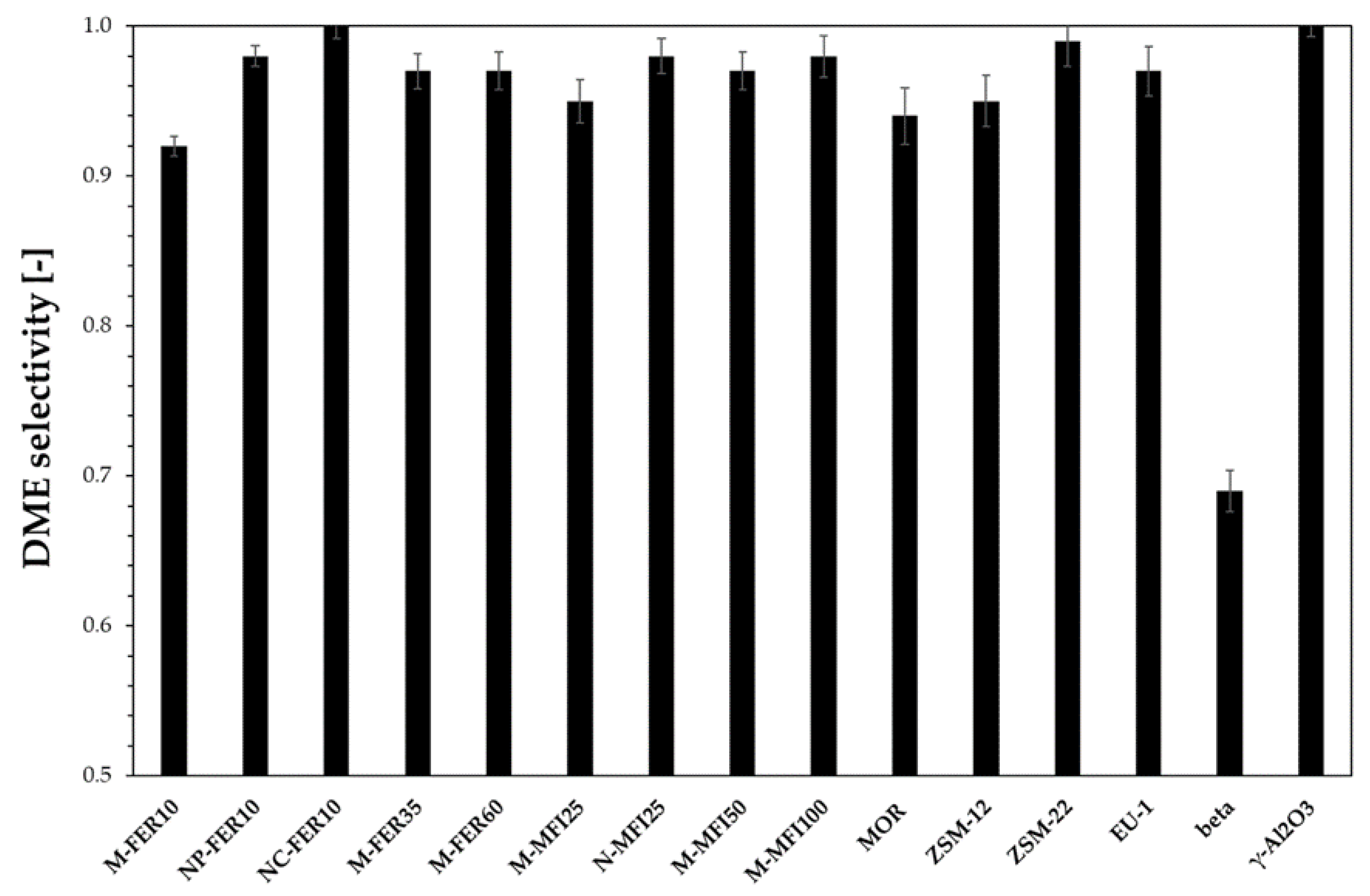
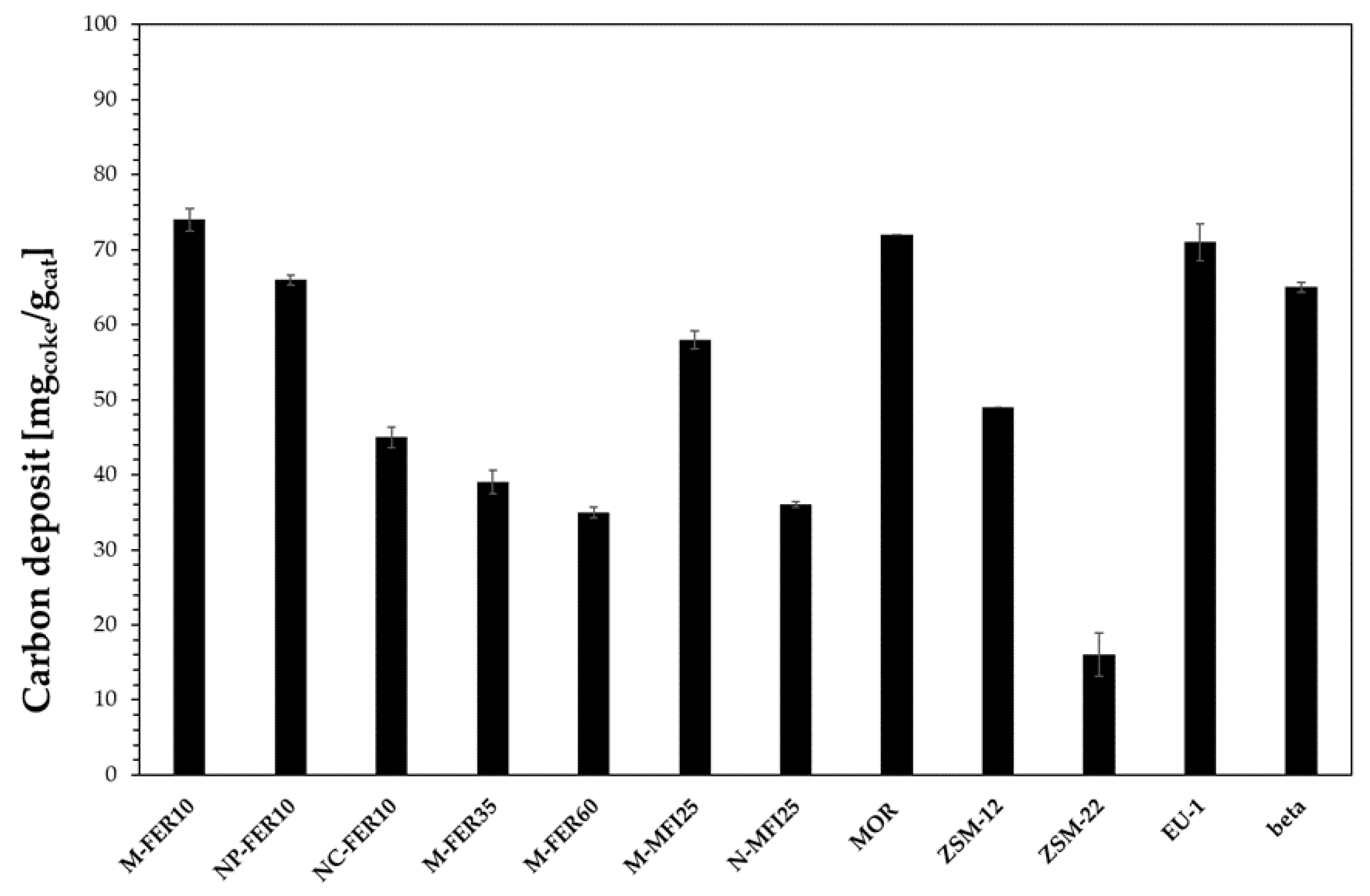
3.2. Catalytic Tests
3.3. Kinetic Analysis on MFI- and FER-Type Zeolites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kalck, P.; Le Berre, C.; Serp, P. Recent advances in the methanol carbonylation reaction into acetic acid. Coord. Chem. Rev. 2020, 402, 213078. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Lanzafame, P.; Centi, G.; Perathoner, S. Catalysis for biomass and CO2 use through solar energy: Opening new scenarios for a sustainable and low-carbon chemical production. Chem. Soc. Rev. 2014, 43, 7562–7580. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, A.; Khalilpour, K.R.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- Giuliano, A.; Catizzone, E.; Freda, C.; Cornacchia, G. Valorization of OFMSW Digestate-Derived Syngas toward Methanol, Hydrogen, or Electricity: Process Simulation and Carbon Footprint Calculation. Processes 2020, 8, 526. [Google Scholar] [CrossRef]
- Giuliano, A.; Freda, C.; Catizzone, E. Techno-Economic Assessment of Bio-Syngas Production for Methanol Synthesis: A Focus on the Water–Gas Shift and Carbon Capture Sections. Bioengineering 2020, 7, 70. [Google Scholar] [CrossRef]
- Arcoumanis, C.; Bae, C.; Crookes, R.; Kinoshita, E. The potential of di-methyl ether (DME) as an alternative fuel for compression-ignition engines: A review. Fuel 2008, 87, 1014–1030. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C. Applicability of dimethyl ether (DME) in a compression ignition engine as an alternative fuel. Energy Conv. Manage. 2014, 86, 848–863. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Source 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Haro, P.; Trippe, F.; Stahl, R.; Henrich, E. Bio-syngas to gasoline and olefins via DME—A comprehensive techno-economic assessment. Appl. Energy 2013, 108, 54–65. [Google Scholar] [CrossRef]
- Olah, G.A. Beyond oil and gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to Olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Perathoner, S.; Centi, G. CO2 recycling: A key strategy to introduce green energy in the chemical production chain. ChemSusChem 2014, 7, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Macrì, D.; Catizzone, E.; Molino, A.; Migliori, M. Supercritical waster gasification of biomass and agro-food residues: Energy assessment from modelling approach. Renew. Energy 2020, 150, 624–636. [Google Scholar] [CrossRef]
- Giuliano, A.; Catizzone, E.; Barisano, D.; Nanna, F.; Villone, A.; De Bari, I.; Cornacchia, G.; Braccio, G. Towards Methanol Economy: A Techno-environmental Assessment for a Bio-methanol OFMSW/Biomass/Carbon Capture-based Integrated Plant. Int. J. Heat Technol. 2019, 37, 665–674. [Google Scholar] [CrossRef]
- Molino, A.; Migliori, M.; Blasi, A.; Davoli, M.; Marino, T.; Chianese, S.; Catizzone, E.; Giordano, G. Municipal waste leachate conversion via catalytic supercritical water gasification process. Fuel 2017, 206, 155–161. [Google Scholar] [CrossRef]
- Centi, G.; Genovese, C.; Giordano, G.; Katovic, A.; Perathoner, S. Performance of Fe-BEA catalysts for the selective hydroxylation of benzene with N2O. Catal. Today 2004, 91–92, 17–26. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Pino, F.; Arrigo, R.; Giordano, G.; Katovic, A.; Pedullà, V. Performance of Fe-[Al, B] catalyst in benzene hydroxylation with N2O: The role of zeolite defects as host sites for highly active iron species. Catal. Today 2005, 110, 211–220. [Google Scholar] [CrossRef]
- Katovic, A.; Giordano, G.; Bonelli, B.; Onida, B.; Garrone, E.; Lentz, P.; Nagy, J.B. Preparation and characterization of mesoporous molecular sieves containing Al, Fe or Zn. Microporous Mesoporous Mater. 2001, 44–45, 275–281. [Google Scholar] [CrossRef]
- Raoof, F.; Taghizadeh, M.; Eliassi, A.; Yaripour, F. Effects of temperature and feed composition on catalytic dehydration of methanol to dimethyl ether over γ-alumina. Fuel 2008, 87, 2967–2971. [Google Scholar] [CrossRef]
- Azizi, Z.; Rezaeimanesh, M.; Tohidian, T.; Rahimpour, M.R. Dimethyl ether: A review of technologies and production challenges. Chem. Eng. Process. 2014, 82, 150–172. [Google Scholar] [CrossRef]
- Xu, M.; Lunsford, J.H.; Goodman, D.W.; Bhattacharyya, A. Synthesis of dimethyl ether (DME) from methanol over solid-acid catalysts. Appl. Catal. A 1997, 149, 289–301. [Google Scholar] [CrossRef]
- Catizzone, E.; Bonura, G.; Migliori, M.; Frusteri, F.; Giordano, G. CO2 recycling to dimethyl ether: State-of-the-art and perspectives. Molecules 2018, 23, 31. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Migliori, M.; Giordano, G. From 1-D to 3-D zeolite structures: Performance assessment in catalysis of vapour-phase methanol dehydration to DME. Microporous Mesoporous Mater. 2017, 243, 102–111. [Google Scholar] [CrossRef]
- Catizzone, E.; Migliori, M.; Purita, A.; Giordano, G. Ferrierite vs. γ-Al2O3: The superiority of zeolites in terms of water-resistance in vapour phase dehydration of methanol to dimethyl ether. J. Energy Chem. 2019, 30, 162–169. [Google Scholar] [CrossRef]
- Catizzone, E.; Cirelli, Z.; Aloise, A.; Lanzafame, P.; Migliori, M.; Giordano, G. Methanol conversion over ZSM-12, ZSM-22 and EU-1 zeolites: From DME to hydrocarbons production. Catal. Today 2018, 304, 39–50. [Google Scholar] [CrossRef]
- Palčić, A.; Catizzone, E. Application of nanosized zeolites in methanol conversion processes: A short review. Curr. Opin. Green Sustain. Chem. 2020, 27, 100393. [Google Scholar] [CrossRef]
- Prasad, P.S.S.; Bae, J.W.; Kang, S.-H.; Lee, Y.-J.; Jun, K.-W. Single-step synthesis of DME from syngas on Cu-ZnO-Al2O3/zeolite bifunctional catalysts: The superiority of ferrierite over the other zeolites. Fuel Process. Technol. 2008, 89, 1281–1286. [Google Scholar] [CrossRef]
- Montesano, R.; Narvaez, A.; Chadwick, D. Shape-selectivity effects in syngas-to-dimethyl ether conversion over Cu/ZnO/Al2O3 and zeolite mixtures: Carbon deposition and by-product formation. Appl. Catal. A 2014, 482, 69–77. [Google Scholar] [CrossRef]
- Garci-Trenco, A.; Martinez, A. Direct synthesis of DME from syngas on hybrid CuZnAl/ZSM-5 catalysts: New insights into the role of zeolite acidity. Appl. Catal. A 2012, 411–412, 170–179. [Google Scholar] [CrossRef]
- Cai, M.; Palcic, A.; Subramanian, V.; Moldovan, S.; Ersen, O.; Valtchev, V.; Ordomsky, V.V.; Khodakov, A.Y. Direct dimethyl ether synthesis from syngas on copper-zeolite hybrid catalysts with a wide range of zeolite particle size. J. Catal. 2016, 338, 227–238. [Google Scholar] [CrossRef]
- Frusteri, F.; Migliori, M.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Aloise, A.; Giordano, G.; Bonura, G. Direct CO2-to-DME hydrogenation reaction: New evidences of a superior behavior of FER-based hyb rid systems to obtain high DME yield. J. CO2 Util. 2017, 18, 353–361. [Google Scholar] [CrossRef]
- Bonura, G.; Cordaro, M.; Spadaro, L.; Cannilla, C.; Arena, F.; Frusteri, F. Hbrid Cu-ZnO-ZrO2/H-ZSM5 system for the direct synthesis of DME by CO2 hydrogenation. Appl. Catal. B Environ. 2013, 140, 16–24. [Google Scholar] [CrossRef]
- Ge, Q.; Huang, Y.; Qiu, F.; Li, S. Bifunctional catalysts for conversion of synthesis gas to dimethyl ether. Appl. Catal. A Gen 1998, 167, 23–30. [Google Scholar] [CrossRef]
- Frusteri, F.; Bonura, G.; Cannilla, C.; Drago Ferrante, G.; Aloise, A.; Catizzone, E.; Migliori, M.; Giordano, G. Stepwise tuning of metal-oxide and acid sites of CuZnZr-MFI hybrid catalysts for the direct DME synthesis by CO2 hydrogenation. Appl. Catal. B Environ. 2015, 176–177, 522–531. [Google Scholar] [CrossRef]
- Bonura, G.; Frusteri, F.; Cannilla, C.; Drago Ferrante, G.; Aloise, A.; Catizzone, E.; Migliori, M.; Giordano, G. Catalytic features of CuZnZr-zeolite hybrid systems for the direct CO2-to-DME hydrogenation reaction. Catal. Today 2016, 277, 48–54. [Google Scholar] [CrossRef]
- Bonura, G.; Migliori, M.; Frusteri, L.; Cannilla, C.; Catizzone, E.; Giordano, G.; Frusteri, F. Acidity control of zeolite functionality on activity and stability of hybrid catalysts during DME production via CO2 hydrogenation. J. CO2 Util. 2018, 24, 398–406. [Google Scholar] [CrossRef]
- Catizzone, E.; Freda, C.; Braccio, G.; Frusteri, F.; Bonura, G. Dimethyl ether as circular hydrogen carrier: Catalytic aspects of hydrogenation/dehydrogenation steps. J. Energy Chem. 2020, 58, 55–77. [Google Scholar] [CrossRef]
- Ying, J.Y. Design and synthesis of nanostructured catalysts. Chem. Eng. Sci. 2006, 61, 1540–1548. [Google Scholar] [CrossRef]
- Chng, L.L.; Erathodiyil, N.; Ying, J.Y. Nanostructured catalysts for organic transformations. Acc. Chem. Res. 2013, 46, 1825–1837. [Google Scholar] [CrossRef]
- Zeng, M.; Yuan, S.; Huang, D.; Cheng, Z. Accelerated Design of Catalytic Water-Cleaning Nanomotors via Machine Learning. ACS Appl. Mater. Interfaces 2019, 11, 40099–40106. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, A.; Migliori, M.; Cozza, D.; Catizzone, E.; Giordano, G.; Barbieri, G. Methanol conversion to dimethyl ether in catalytic zeolite membrane reactors. ACS Sustain. Chem. Eng. 2020, 8, 10471–10479. [Google Scholar] [CrossRef]
- Comboni, D.; Pagliaro, F.; Lotti, P.; Gatta, G.D.; Merlini, M.; Milani, S.; Migliori, M.; Giordano, G.; Catizzone, E.; Collimgs, I.E.; et al. The elastic behavior of zeolitic frameworks: The case of MFI type under high-pressure methanol intrusion. Catal. Today 2020, 345, 88–96. [Google Scholar] [CrossRef]
- Catizzone, E.; van Daele, S.; Bianco, M.; Di Michele, A.; Aloise, A.; Migliori, M.; Valtchev, V. Catalytic application of ferrierite nanocrystals in vapour-phase dehydration of methanol to dimethyl ether. Appl. Catal. B Environ. 2019, 243, 273–282. [Google Scholar] [CrossRef]
- Catizzone, E.; Aloise, A.; Giglio, E.; Ferrarelli, G.; Bianco, M.; Migliori, M.; Giordano, G. MFI vs. FER zeolite during methanol dehydration to dimethyl ether: The crystal size plays a key role. Catal. Commun. 2021, 149, 106214. [Google Scholar] [CrossRef]
- Bonura, G.; Cannilla, C.; Frusteri, L.; Catizzone, E.; Todaro, S.; Migliori, M.; Giordano, G.; Frusteri, F. Interaction effects between CuO-ZnO-ZrO2 methanol phase and zeolite surface affecting stability of hybrid systems during one-step CO2 hydrogenation to DME. Catal. Today 2020, 345, 175–182. [Google Scholar] [CrossRef]
- Zhi, Y.; Shi, H.; Mu, L.; Liu, Y.; Mei, D.; Camaioni, D.M.; Lercher, J.A. Dehydration Pathways of 1-Propanol on HZSM-5 in the Presence and Absence of Water. J. Am. Chem. Soc. 2015, 137, 15781–15794. [Google Scholar] [CrossRef]
- Liang, J.; Mi, Y.; Song, G.; Peng, H.; Li, Y.; Yan, R.; Liu, W.; Wang, Z.; Wu, P.; Liu, F. Environmental benign synthesis of Nano-SSZ-13 via FAU trans-crystallization: Enhanced NH3-SCR performance on Cu-SSZ-13 with nano-size effect. J. Hazard. Mater. 2020, 398, 122986. [Google Scholar] [CrossRef]
- Kortunov, P.; Chmelik, C.; Kärger, J.; Rakoczy, R.A.; Ruthven, D.M.; Traa, Y.; Vasenkov, S.; Weitkamp, J. Sorption kinetics and intracrystalline diffusion of methanol in ferrierite: An example of disguised kinetics. Adsorption 2005, 11, 235–244. [Google Scholar] [CrossRef]
- Bowen, T.C.; Wyss, J.C.; Noble, R.D.; Falconer, J.L. Measurements of diffusion through a zeolite membrane using isotopic-transient pervaporation. Microporous Mesoporous Mater. 2004, 71, 199–210. [Google Scholar] [CrossRef]


| Sample | Topology | Channel Orientation | Membered Rings | Channel Openings (Å) |
|---|---|---|---|---|
| ZSM-22 | TON | 1D | 10 | 4.6 × 5.7 |
| EU-1 | EUO | 1D | 10 | 4.1 × 5.4 |
| ZSM-22 | MTW | 1D | 12 | 5.6 × 6.0 |
| MOR | MOR | 1D | 12//8 | 6.5 × 7.0 < > 2.6 × 5.7 |
| M-FER10 NP-FER10 NC-FER10 M-FER30 M-FER60 | FER | 2D | 10 × 8 | 4.2 × 5.4 < > 3.5 × 4.8 |
| M-MFI25 NC-MFI25 M-MFI50 M-MFI100 | MFI | 3D | 10 | 5.1 × 5.5 < >5.3 × 5.6 |
| beta | BEA | 3D | 12 | 6.6 × 7.7 < > 5.6 × 5.6 |
| Sample Name | Framework | Synthesis Molar Gel Composition | Crystallisation | Ref. | |
|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | ||||
| M-FER10 | FER | 0.6 C4H9N * − 0.08 Na2O − 0.05 Al2O3 − 1 SiO2 − 20 H2O | 180 | 120 | [44] |
| NP-FER10 | FER | 0.6 C4H9N * − 0.015 NaC12H25SO4 * − 0.08 Na2O − 0.05 Al2O3 − 1 SiO2 − 20 H2O | 180 | 60 | [44] |
| NC-FER10 | FER | 0.6 C4H9N * − 0.015 NaC12H25SO4 * − 0.08 Na2O − 0.05 Al2O3 − 1 SiO2 − 20 H2O + 3% wt of seeds ** | 160 | 60 | [44] |
| M-FER30 | FER | 2 C5H5N * − 0.0575 Na2O − 0.017 Al2O3 − 1 SiO2 − 25 H2O | 160 | 120 | [25] |
| M-FER60 | FER | 2 C5H5N * − 0.0575 Na2O − 0.008 Al2O3 − 1 SiO2 − 25 H2O | 160 | 120 | [25] |
| M-MFI25 | MFI | 0.10 Na2O − 0.08 C12H28NBr * − 0.02 Al2O3 − 1 SiO2 − 20 H2O | 170 | 120 | [35] |
| M-MFI50 | MFI | 0.10 Na2O − 0.08 C12H28NBr * 0.01 Al2O3 − 1 SiO2− 20 H2O | 170 | 120 | [35] |
| M-MFI100 | MFI | 0.10 Na2O − 0.08 C12H28NBr * − 0.005 Al2O3 − 1 SiO2 − 20 H2O | 170 | 120 | [35] |
| NC-MFI25 | MFI | 0.10 Na2O − 0.08 C12H28NBr * 0.02 Al2O3 − 1 SiO2 − 20 H2O | 170 | 90 | [45] |
| MOR | MOR | 0.20Na2O − 0.02Al2O3 − 1.0SiO2 − 20H2O | 170 | 120 | [46] |
| ZSM-12 | MTW | 0.1 N2O − 0.2 C7H18NBr * − 0.01 Al2O3 − 1 SiO2 − 20 H2O | 140 | 150 | [26] |
| ZSM-22 | TON | 0.140 K2O − 0.3 C8H20N2 * − 0.011 Al2O3 − 1 SiO2 − 40 H2O | 160 | 80 | [26] |
| EU-1 | EUO | 0.3 Na2O − 0.15 C12H30N2Br2 * − 0.017 Al2O3 − 1 SiO2 − 45 H2O | 160 | 340 | [26] |
| beta | BEA | 0.10 Na2O − 0.2 C8H21NO * − 0.02 Al2O3 − 1 SiO2 − 10 H2O | 150 | 120 | [46] |
| Sample | Specific Surface Area a (m2/g) | Micropore Volume b (cm3/g) | Mesopore Volume b (cm3/g) | Si/Al c (mol/mol) | Total Acidity d (mmol/g) | Strong Acid Sites Fraction e (-) | Crystal Size (µm) |
|---|---|---|---|---|---|---|---|
| M-FER10 | 332 | 0.136 | 0.086 | 9.6 | 1.10 | 0.70 | 5–10 |
| NP-FER10 | 314 | 0.125 | 0.093 | 8.6 | 1.12 | 0.72 | 0.1–0.5 |
| NC-FER10 | 304 | 0.122 | 0.071 | 9.4 | 1.10 | 0.70 | <0.1 |
| M-FER30 | 272 | 0.108 | 0.065 | 23 | 0.82 | 0.77 | 10–20 |
| M-FER60 | 275 | 0.110 | 0.054 | 45 | 0.40 | 0.78 | 10–20 |
| M-MFI25 | 386 | 0.126 | 0.073 | 27 | 0.52 | 0.58 | ~5 |
| NC-MFI25 | 371 | 0.124 | 0.074 | 23 | 0.58 | 0.52 | 0.1–0.5 |
| M-MFI50 | 316 | 0.124 | 0.070 | 68 | 0.35 | 0.55 | ~5 |
| M-MFI100 | 382 | 0.101 | 0.112 | 127 | 0.15 | 0.54 | ~5 |
| MOR | 348 | 0.152 | 0.028 | 7 | 1.03 | 0.74 | 5–10 |
| ZSM-12 | 294 | 0.115 | 0.031 | 32 | 0.50 | 0.82 | 2–3 |
| ZSM-22 | 210 | 0.074 | 0.104 | 43 | 0.56 | 0.68 | 5–10 |
| EU-1 | 384 | 0.146 | 0.061 | 21 | 0.80 | 0.72 | <1 |
| beta | 468 | 0.202 | 0.148 | 25 | 0.60 | 0.58 | <1 |
| Sample | |||
|---|---|---|---|
| M-FER10 | 60.4 | 49.7 | −175.0 |
| NP-FER10 | 58.2 | 51.2 | −165.8 |
| NC-FER10 | 61.7 | 47.6 | −177.1 |
| M-FER30 | 52.4 | 45.0 | −185.6 |
| M-FER60 | 52.3 | 47.2 | −187.9 |
| M-MFI25 | 105.5 | 70.7 | −132.6 |
| NC-MFI25 | 73.0 | 60.1 | −152.7 |
| M-MFI50 | 82.8 | 72.7 | −136.7 |
| M-MFI100 | 70.7 | 57.7 | −174.7 |
| Sample | Effectiveness Factor | |||
|---|---|---|---|---|
| 140 °C | 160 °C | 180 °C | 200 °C | |
| M-FER10 | 0.2102 | 0.1017 | 0.0504 | 0.0260 |
| (1.000) | (0.4840) | (0.2396) | (0.1237) | |
| NP-FER10 | 0.9916 | 0.9817 | 0.9629 | 0.9298 |
| (1.000) | (0.9900) | (0.9711) | (0.9377) | |
| NC-FER10 | 0.9994 | 0.9987 | 0.9972 | 0.9944 |
| (1.000) | (0.9993) | (0.9978) | (0.9950) | |
| M-FER30 | 0.0483 | 0.0243 | 0.0129 | 0.0072 |
| (1.000) | (0.5025) | (0.2664) | (0.1485) | |
| M-FER60 | 0.1099 | 0.0566 | 0.0304 | 0.0170 |
| (1.000) | (0.5149) | (0.2763) | (0.1551) | |
| M-MFI25 | 0.9297 | 0.7412 | 0.3924 | 0.1508 |
| (1.000) | (0.7973) | (0.4221) | (0.1622) | |
| NC-MFI25 | 0.9990 | 0.9973 | 0.9935 | 0.9853 |
| (1.000) | (0.9983) | (0.9945) | (0.9863) | |
| M-MFI50 | 0.9490 | 0.8524 | 0.6500 | 0.3883 |
| (1.000) | (0.8983) | (0.6850) | (0.4092) | |
| M-MFI100 | 0.9609 | 0.9018 | 0.7826 | 0.5928 |
| (1.000) | (0.9385) | (0.8144) | (0.6170) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catizzone, E.; Giglio, E.; Migliori, M.; Cozzucoli, P.C.; Giordano, G. The Effect of Zeolite Features on the Dehydration Reaction of Methanol to Dimethyl Ether: Catalytic Behaviour and Kinetics. Materials 2020, 13, 5577. https://doi.org/10.3390/ma13235577
Catizzone E, Giglio E, Migliori M, Cozzucoli PC, Giordano G. The Effect of Zeolite Features on the Dehydration Reaction of Methanol to Dimethyl Ether: Catalytic Behaviour and Kinetics. Materials. 2020; 13(23):5577. https://doi.org/10.3390/ma13235577
Chicago/Turabian StyleCatizzone, Enrico, Emanuele Giglio, Massimo Migliori, Paolo C. Cozzucoli, and Girolamo Giordano. 2020. "The Effect of Zeolite Features on the Dehydration Reaction of Methanol to Dimethyl Ether: Catalytic Behaviour and Kinetics" Materials 13, no. 23: 5577. https://doi.org/10.3390/ma13235577
APA StyleCatizzone, E., Giglio, E., Migliori, M., Cozzucoli, P. C., & Giordano, G. (2020). The Effect of Zeolite Features on the Dehydration Reaction of Methanol to Dimethyl Ether: Catalytic Behaviour and Kinetics. Materials, 13(23), 5577. https://doi.org/10.3390/ma13235577







