Production of Soda Lime Glass Having Antibacterial Property for Industrial Applications
Abstract
1. Introduction
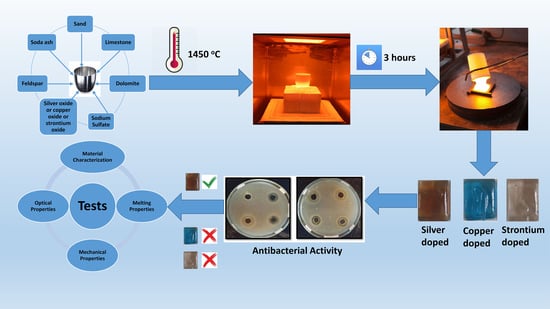
2. Experimental Procedure
2.1. Glass Composition and Melting
2.2. Characterization of Antibacterial Glasses
2.3. High Temperature Melting Observation
3. Results and Discussions
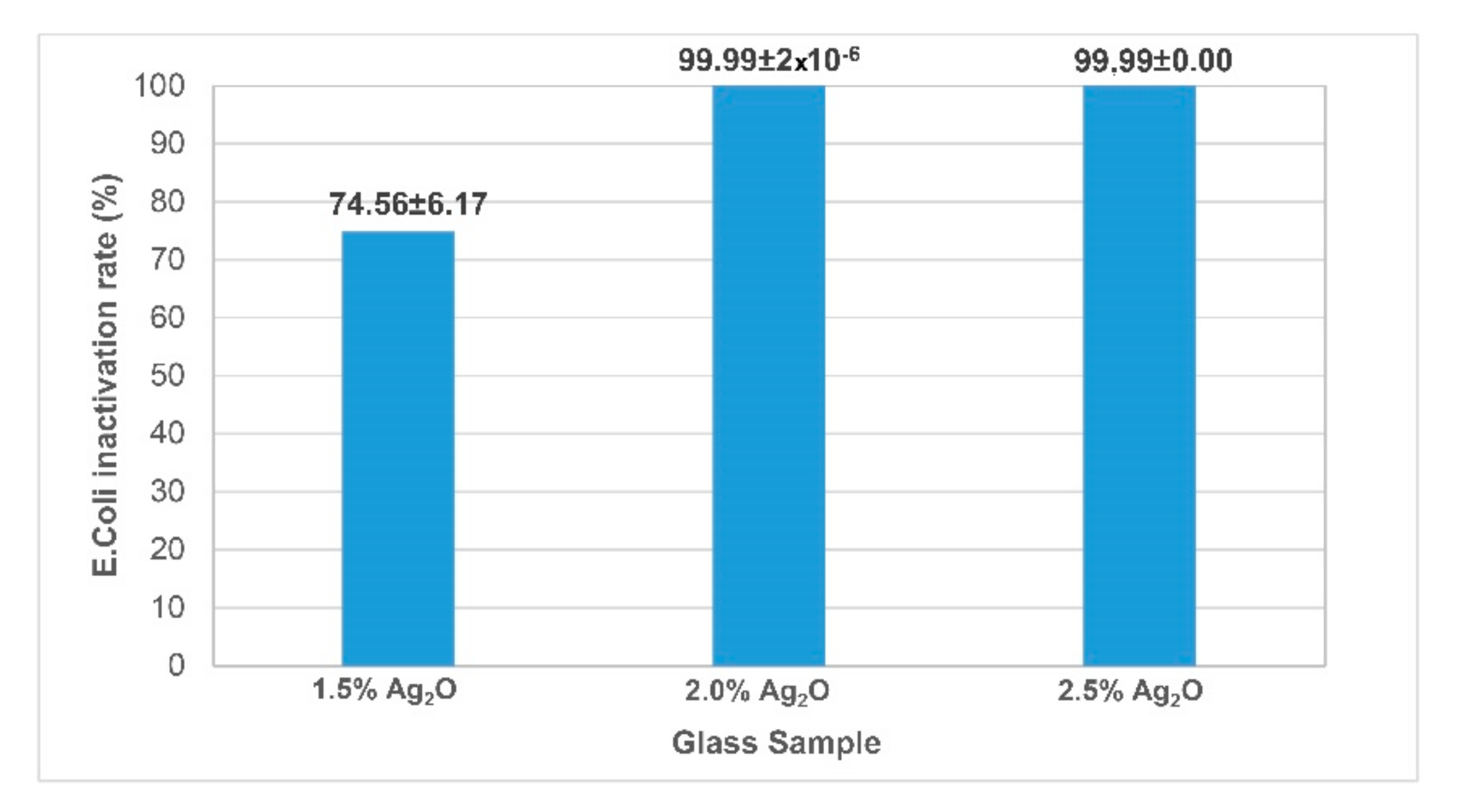
3.1. Antibacterial and Ion Release Tests of the Obtained Glass Samples
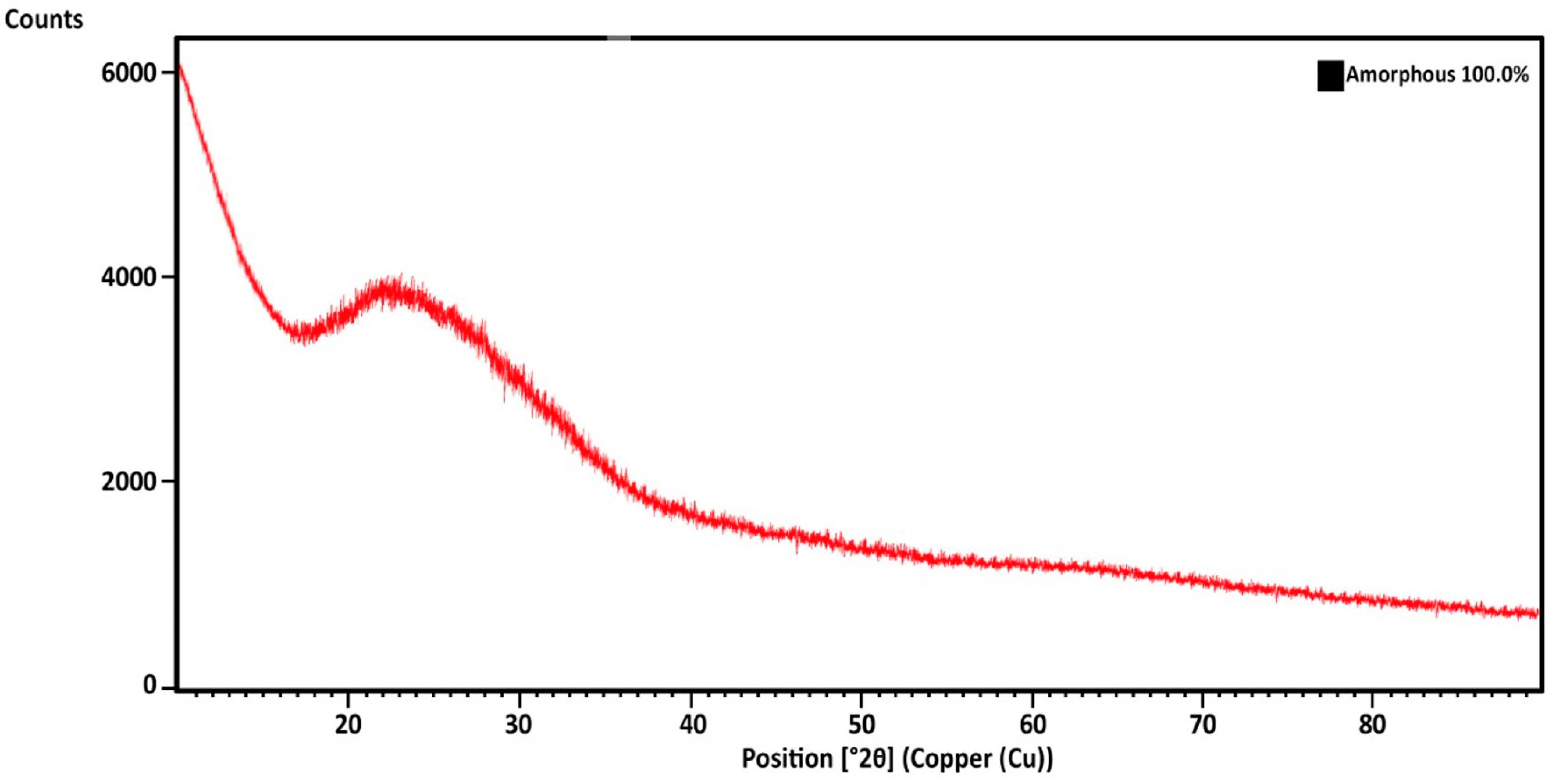
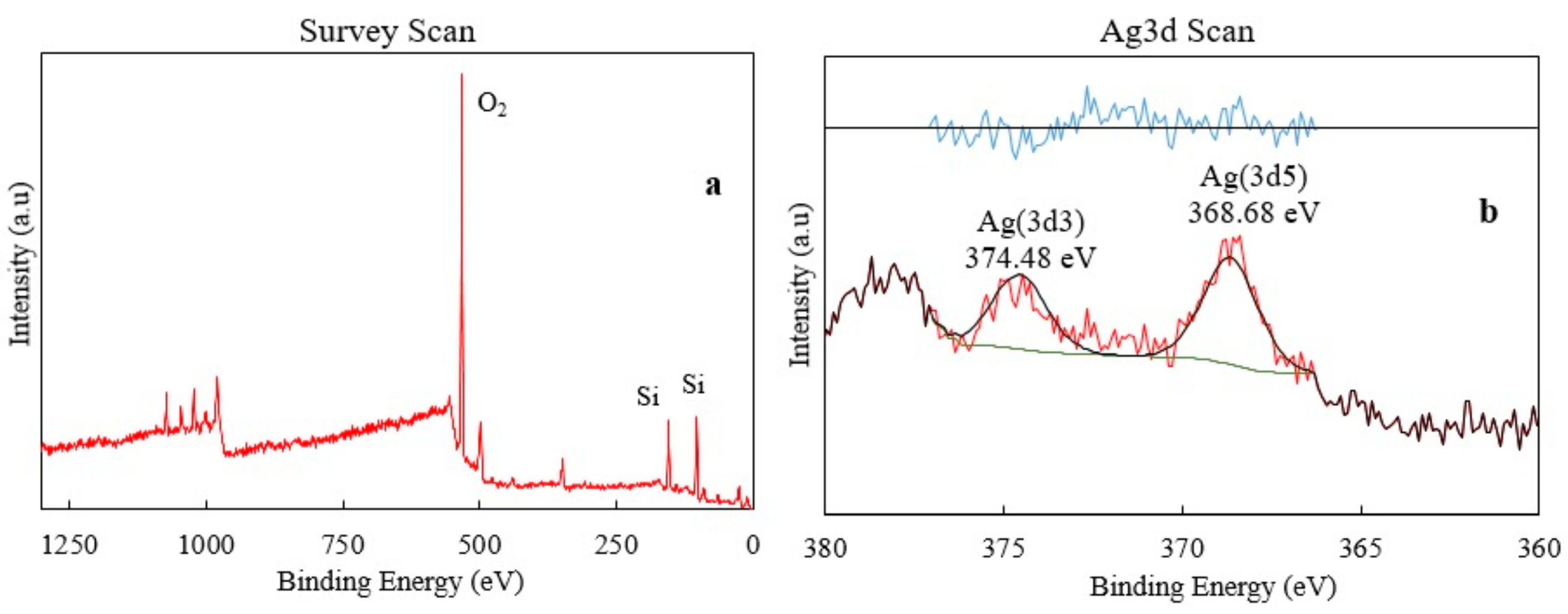
3.2. Material Characterization
3.3. High Temperature Melting Observation System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mauro, J.C.; Zanotto, E.D. Two Centuries of Glass Research: Historical Trends, Current Status, and Grand Challenges for the Future. Int. J. Appl. Glass Sci. 2014, 5, 313–327. [Google Scholar] [CrossRef]
- Lindahl, J.F.; Grace, D. The consequences of human actions on risks for infectious diseases: A review. Infect. Ecol. Epidemiol. 2015, 5, 30048. [Google Scholar] [CrossRef] [PubMed]
- Hammond, B.; Ali, Y.; Fendler, E.; Dolan, M.; Donovan, S. Effect of hand sanitizer use on elementary school absenteeism. Am. J. Infect. Control 2000, 28, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef]
- Xiu, Z.-M.; Zhang, Q.-B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles againstCampylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef]
- Guida, A.; Towler, M.R.; Wall, G.J.; Hill, R.G.; Eramo, S. Preliminary work on the antibacterial effect of strontium in glass ionomer cements. J. Mater. Sci. Lett. 2003, 22, 1401–1403. [Google Scholar] [CrossRef]
- Lucacel, R.C.; Radu, T.; Tătar, A.S.; Lupan, I.; Ponta, O.; Simon, V. The influence of local structure and surface morphology on the antibacterial activity of silver-containing calcium borosilicate glasses. J. Non-Cryst. Solids 2014, 404, 98–103. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Ciprioti, S.V. Investigation on bioactivity, biocompatibility, thermal behavior and antibacterial properties of calcium silicate glass coatings containing Ag. J. Non-Cryst. Solids 2015, 422, 16–22. [Google Scholar] [CrossRef]
- Schierholz, J.M.; Lucas, L.J.; Rump, A.; Pulverer, G. Efficacy of silver-coated medical devices. J. Hosp. Infect. 1998, 40, 257–262. [Google Scholar] [CrossRef]
- Clement, J.L.; Jarrett, P.S. Antibacterial Silver. Met. Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I. The increasing use of silver-based products as antimicrobial agents: A useful development or a cause for concern? J. Antimicrob. Chemother. 2007, 59, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, I.; Anghel, E.M.; Predoana, L.; Mocioiu, O.C.; Jecu, L.; Raut, I.; Munteanu, C.; Culita, D.; Zaharescu, M. Influence of ZnO addition on the structural, in vitro behavior and antimicrobial activity of sol–gel derived CaO–P2O5–SiO2 bioactive glasses. Ceram. Int. 2016, 42, 3033–3045. [Google Scholar] [CrossRef]
- Fernandes, J.S.; Gentile, P.; Pires, R.A.; Reis, R.L.; Hatton, P.V. Multifunctional bioactive glass and glass-ceramic biomaterials with antibacterial properties for repair and regeneration of bone tissue. Acta Biomater. 2017, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Sergi, R.; Bellucci, D.; Salvatori, R.; Maisetta, G.; Batoni, G.; Cannillo, V. Zinc containing bioactive glasses with ultra-high crystallization temperature, good biological performance and antibacterial effects. Mater. Sci. Eng. C 2019, 104, 1–9. [Google Scholar] [CrossRef]
- Shahrbabak, M.S.N.; Sharifianjazi, F.; Rahban, D.; Salimi, A. A Comparative Investigation on Bioactivity and Antibacterial Properties of Sol-Gel Derived 58S Bioactive Glass Substituted by Ag and Zn. Silicon 2019, 11, 2741–2751. [Google Scholar] [CrossRef]
- Ranga, N.; Gahlyan, S.; Duhan, S. Antibacterial Efficiency of Zn, Mg and Sr Doped Bioactive Glass for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2020, 20, 2465–2472. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Romero, A.M.; Ragel, C.V.; LeGeros, R.Z. XRD, SEM-EDS, and FTIR studies of in vitro growth of an apatite-like layer on sol-gel glasses. J. Biomed. Mater. 1999, 44, 416–421. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Salinas, A.J.; Román, J.; Gil, M. Effect of magnesium content on the in vitro bioactivity of CaO-MgO-SiO2-P2O5 sol-gel glasses. J. Mater. Chem. 1999, 9, 515–518. [Google Scholar] [CrossRef]
- Jie, Q.; Lin, K.; Zhong, J.; Shi, Y.; Li, Q.; Chang, J.; Wang, R. Preparation of Macroporous Sol-Gel Bioglass Using PVA Particles as Pore Former. J. Sol-Gel Sci. Technol. 2004, 30, 49–61. [Google Scholar] [CrossRef]
- Gil-Albarova, J.; Garrido-Lahiguera, R.; Salinas, A.J.; Román, J.; Bueno-Lozano, A.L.; Gil-Albarova, R.; Vallet-Regi, M. The in vivo performance of a sol–gel glass and a glass-ceramic in the treatment of limited bone defects. Biomaterials 2004, 25, 4639–4645. [Google Scholar] [CrossRef]
- Oki, A.; Parveen, B.; Hossain, S.; Adeniji, S.; Donahue, H. Preparation andin vitro bioactivity of zinc containing sol-gel-derived bioglass materials. J. Biomed. Mater. Res. 2004, 69, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.; Nedelec, J.-M.; Moretto, P.; Jallot, E. Micro-PIXE characterization of interactions between a sol–gel derived bioactive glass and biological fluids. Nucl. Instrum. Meth. B 2006, 245, 511–518. [Google Scholar] [CrossRef][Green Version]
- Zhang, K.; Washburn, N.R.; Simon, C.G., Jr. Cytotoxicity of three-dimensionally ordered macroporous sol–gel bioactive glass (3DOM-BG). Biomaterials 2005, 26, 4532–4539. [Google Scholar] [CrossRef]
- Meseguer-Olmo, L.; Ros-Nicolás, M.J.; Vicente-Ortega, V.; Alcaraz-Baños, M.; Clavel-Sainz, M.; Arcos, D.; Ragel, C.V.; Vallet-Regí, M.; Meseguer-Ortiz, C. A bioactive sol-gel glass implant for in vivo gentamicin release. Experimental model in Rabbit. J. Orthop. Res. 2006, 24, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Jaroch, D.B.; Clupper, D.C. Modulation of zinc release from bioactive sol–gel derived SiO2-CaO-ZnO glasses and ceramics. J. Biomed. Mater. Res. 2007, 82, 575–588. [Google Scholar] [CrossRef]
- Balamurugan, A.; Balossier, G.; Michel, J.; Kannan, S.; Benhayoune, H.; Rebelo, A.H.S.; Ferreira, J.M.F. Sol gel derived SiO2-CaO-MgO-P2O5 bioglass system—Preparation andin vitro characterization. J. Biomed. Mater. Res. 2007, 83, 546–553. [Google Scholar] [CrossRef]
- Balamurugan, A.; Balossier, G.; Kannan, S.; Michel, J.; Rebelo, A.H.; Ferreira, J.M. Development and in vitro characterization of sol–gel derived CaO–P2O5–SiO2–ZnO bioglass. Acta Biomater. 2007, 3, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Barton, S.D.; Click, C.A.; Howse, T.K.G. Glass Container Composition. U.S. Patent 8,785,337 B2, 22 July 2014. [Google Scholar]
- Guldiren, D.; Aydin, S. Characterization and antimicrobial properties of soda lime glass prepared by silver/sodium ion exchange. Mater. Sci. Eng. C 2016, 67, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Tejeda, L.; Malpartida, F.; Esteban-Cubillo, A.; Pecharromán, C.; Moya, J.S. The antibacterial and antifungal activity of a soda-lime glass containing silver nanoparticles. Nanotechnology 2009, 20, 085103. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Tejeda, L.; Malpartida, F.; Esteban-Cubillo, A.; Pecharroman, C.; Moya, J.S. Antibacterial and antifungal activity of a soda-lime glass containing copper nanoparticles. Nanotechnology 2009, 20, 505701. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US); National Research Council (US); Committee on the Review of the Use of Scientific Criteria and Performance Standards for Safe Food. International Microbiological Criteria for Dairy Products; National Academies Press (US): Washington, DC, USA, 2003. [Google Scholar]
- Guldiren, D.; Aydın, S. Antimicrobial property of silver, silver-zinc and silver-copper incorporated soda lime glass prepared by ion exchange. Mater. Sci. Eng. C 2017, 78, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Lee, B.S.; Byun, T.G.; Song, K.C. Preparation and antibacterial activity of silver-doped organic–inorganic hybrid coatings on glass substrates. Colloids Surf. A Physicochem. Eng. Asp. 2010, 355, 167–171. [Google Scholar] [CrossRef]
- 37. Agency for Toxic Substances and Disease Registry U.S. Public Health Service. Toxicological Profile for Silver. 1990. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp146.pdf (accessed on 10 April 2020).
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul. Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef]
- Reiter, T.; Panick, T.; Schuhladen, K.; Roether, J.A.; Hum, J.; Boccaccini, A.R. Bioactive glass based scaffolds coated with gelatin for the sustained release of icariin. Bioact. Mater. 2019, 4, 1–7. [Google Scholar] [CrossRef]
- NSG, Group. Properties of Soda-Lime Silica Float Glass. Tech. Bull. 2013, ATS-129, 1–3. [Google Scholar]
- Buckett, J.; Marsh, J.S.; Torr, A.C. Soda-Lime-Silica Glass Composition. World Intellectual Property. Organization Patent WO/02/16277 A1, 28 February 2002. [Google Scholar]
- Arslan, B.; Akdemir, M.; Sesigür, H.; Oran, M. An Experimental Study on the Batch Optimization of Tableware Glasses. In Proceedings of the XI ESG Conference, Parma, Italy, 21–24 September 2014. [Google Scholar]
- Demirok, G.; Oran, M.; Sesigür, H.; Arslan, B. Fining: Impact of sodium sulphate and anthracite in soda-lime silicate glass. In Proceedings of the 2017 ICG Annual Meeting & 32nd Şişecam Glass Symposium, Istanbul, Turkey, 22–25 October 2017. [Google Scholar]
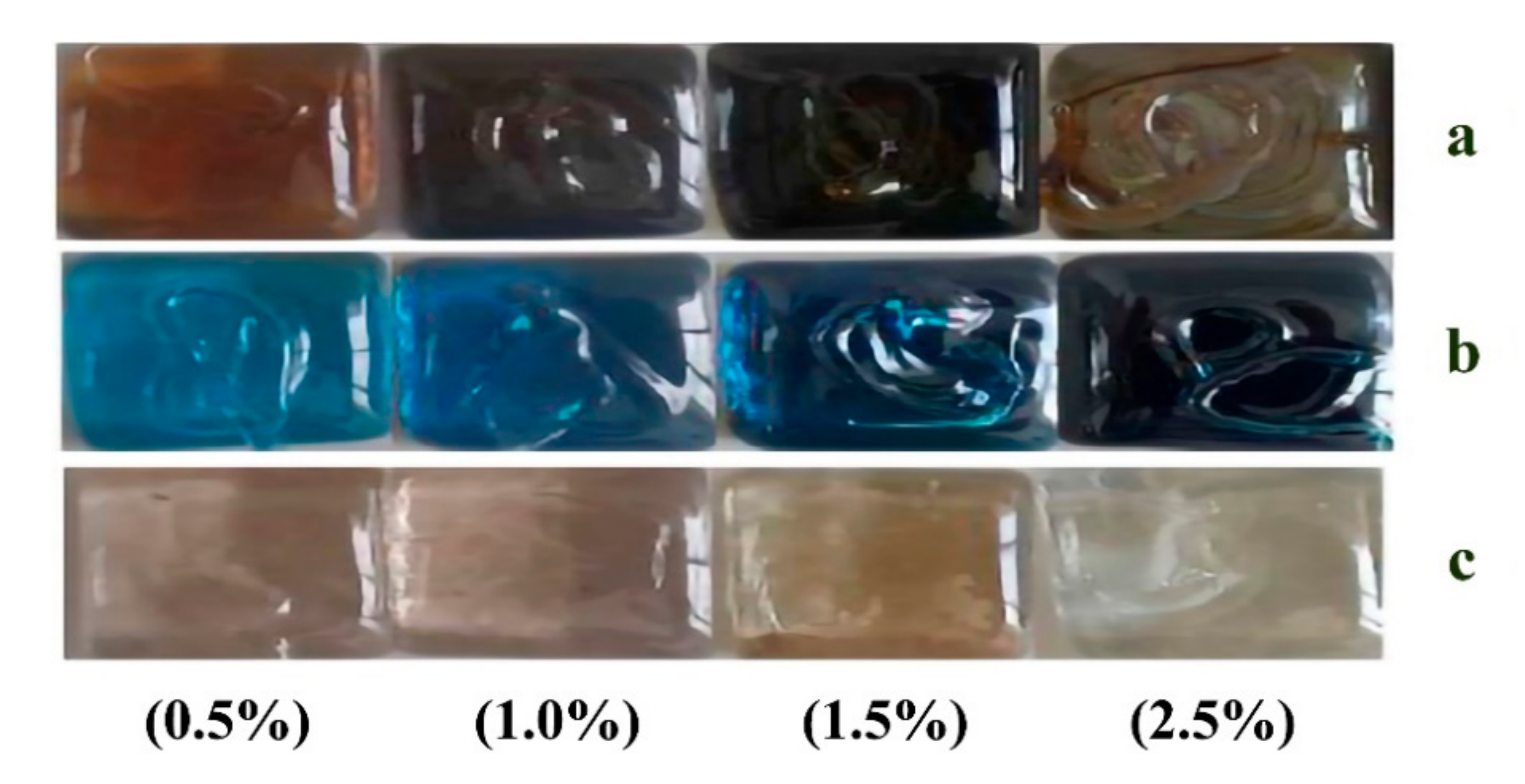


| SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | TiO2 (%) | CaO (%) | MgO (%) | Na2O (%) | K2O (%) | SO3 (%) |
|---|---|---|---|---|---|---|---|---|
| 71.40 | 1.71 | 0.06 | 0.06 | 9.79 | 3.28 | 13.17 | 0.31 | 0.24 |
| SiO2 (wt.%) | Al2O3 (wt.%) | Fe2O3 (wt.%) | TiO2 (wt.%) | CaO (wt.%) | MgO (wt.%) | Na2O (wt.%) | K2O (wt.%) | SO3 (wt.%) | Ion (wt.%) |
|---|---|---|---|---|---|---|---|---|---|
| 71.40 | 1.71 | 0.06 | 0.06 | 9.29 | 3.28 | 13.17 | 0.31 | 0.24 | 0.50 |
| 70.90 | 1.71 | 0.06 | 0.06 | 9.29 | 3.28 | 13.17 | 0.31 | 0.24 | 1.00 |
| 70.40 | 1.71 | 0.06 | 0.06 | 9.29 | 3.28 | 13.17 | 0.31 | 0.24 | 1.50 |
| 69.40 | 1.71 | 0.06 | 0.06 | 9.29 | 3.28 | 13.17 | 0.31 | 0.24 | 2.50 |
| The Released Amount of Silver Ion (Glass Doped with 2% Ag2O) | The Released Amount of Silver Ion (Glass Doped with 2.5% Ag2O) | |
|---|---|---|
| A day in acetic acid | 0.28 | 0.59 |
| A day in water | 0.16 | 0.22 |
| A week in acetic acid | 0.72 | 0.89 |
| A week in water | 0.33 | 0.43 |
| Reference | Antibacterial | |
|---|---|---|
| Thermal expansion coefficient (10−7/°C) | 86.5 | 85.5 |
| Density (g/cm3) | 2.493 | 2.512 |
| Refractive index | 1.5200 | 1.5205 |
| Color parameters (standard 3 mm) | ||
| Brightness (%) | 72.0 | 19.7 |
| Dominant wavelength (nm) | 556.5 | 588.7 |
| Viscosity | Temperature (°C) | ||
|---|---|---|---|
| Reference | Antibacterial (First Measurement) | Antibacterial (Second Measurement) | |
| log η = 2.25 (±0.018) (melting temperature) | 1372 | 1388 | 1388 |
| log η = 2.50 (±0.014) | 1309 | 1323 | 1323 |
| log η = 2.75 (±0.009) | 1252 | 1265 | 1264 |
| log η = 3.00 (±0.011) (Gob temperature) | 1200 | 1212 | 1211 |
| log η = 3.25 (±0.012) | 1154 | 1162 | 1162 |
| log η = 3.50 (±0.015) | 1111 | 1119 | 1118 |
| log η = 4.00 (±0.016) | 1036 | 1042 | 1041 |
| log η = 7.65 (softening temperature) | 734 (±2.3) | 739 (±2.3) | – |
| Working range (WR) Tlogη=3 − Tlogη=7.65 | 466 | 473 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirel, B.; Erol Taygun, M. Production of Soda Lime Glass Having Antibacterial Property for Industrial Applications. Materials 2020, 13, 4827. https://doi.org/10.3390/ma13214827
Demirel B, Erol Taygun M. Production of Soda Lime Glass Having Antibacterial Property for Industrial Applications. Materials. 2020; 13(21):4827. https://doi.org/10.3390/ma13214827
Chicago/Turabian StyleDemirel, Barış, and Melek Erol Taygun. 2020. "Production of Soda Lime Glass Having Antibacterial Property for Industrial Applications" Materials 13, no. 21: 4827. https://doi.org/10.3390/ma13214827
APA StyleDemirel, B., & Erol Taygun, M. (2020). Production of Soda Lime Glass Having Antibacterial Property for Industrial Applications. Materials, 13(21), 4827. https://doi.org/10.3390/ma13214827