Fabrication of All-Solid Organic Electrochromic Devices on Absorptive Paper Substrates Utilizing a Simplified Lateral Architecture
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Preparation of PEDOT:PSS Coatings
2.3. Absorption Evaluation of Substrates
2.4. Assessment of Solid Electrolyte and Preparation of ECDs
3. Results and Discussion
3.1. Optimal PEDOT:PSS Formulation
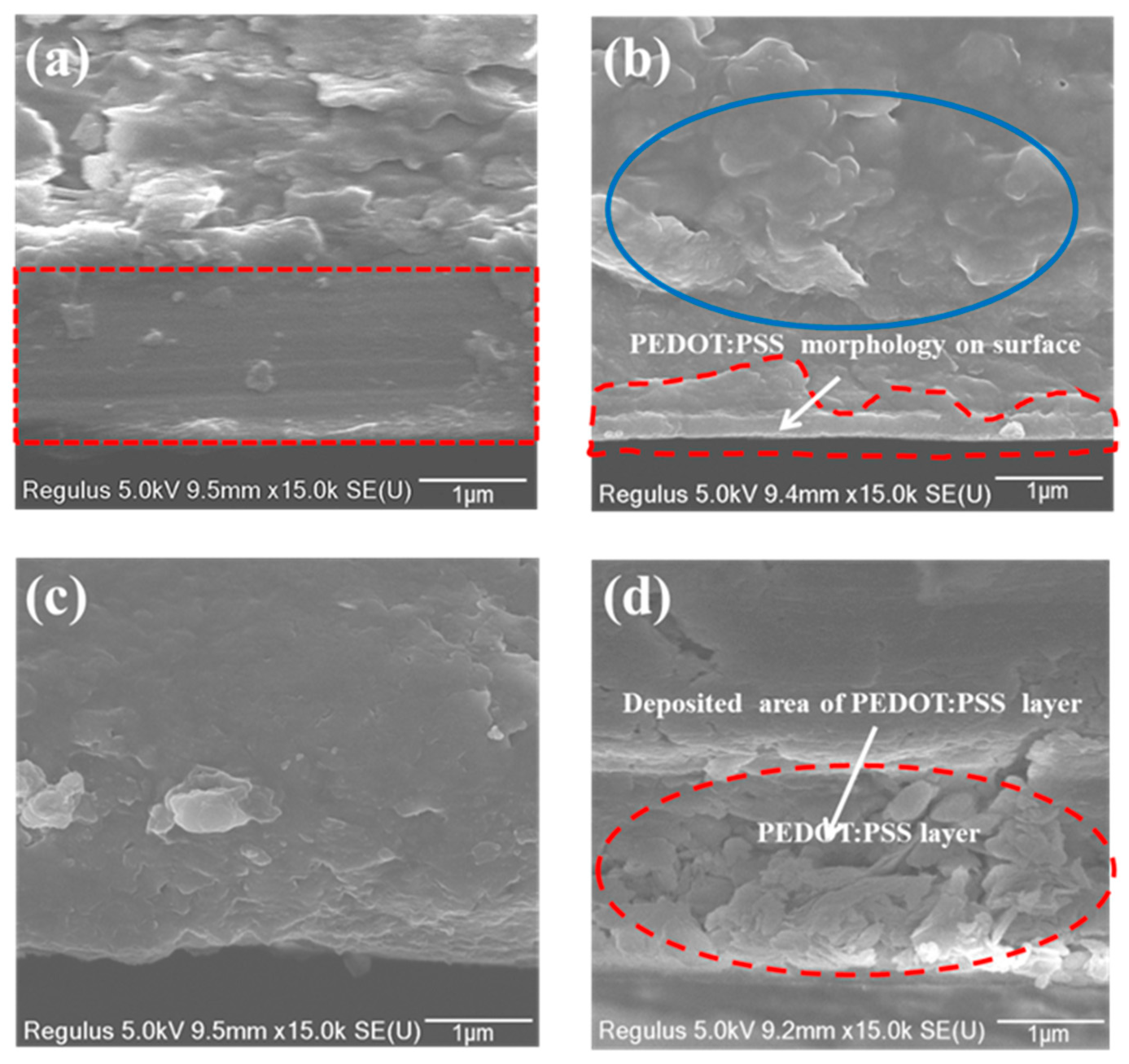
3.2. Interaction of PEDOT:PSS Formulations with the Substrates
3.3. Performance Evaluation of the Electrolytes
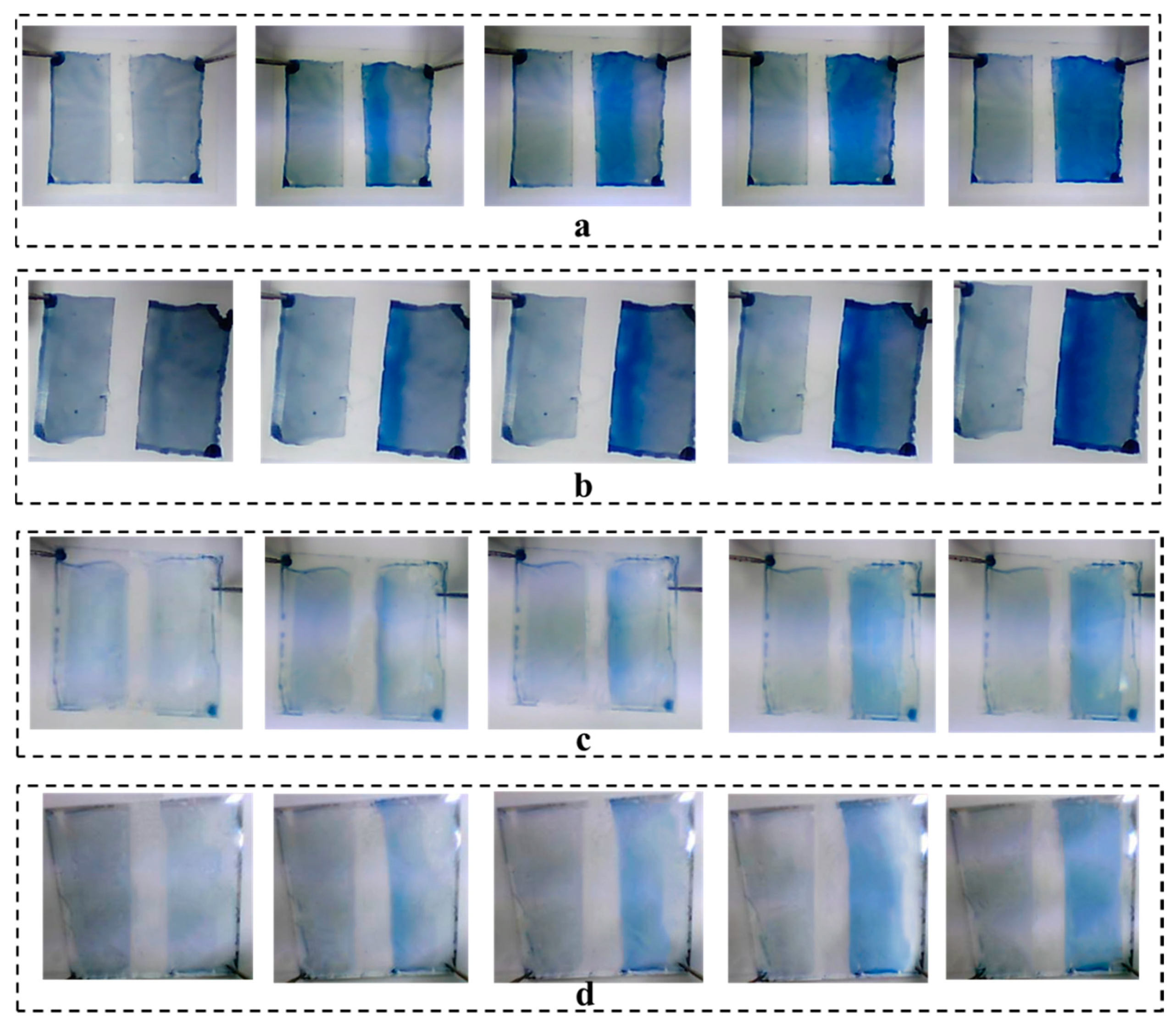
3.4. Performance of Prepared ECDs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Singh, R.; Tharion, J.; Murugan, S.; Kumar, A. ITO-Free Solution-Processed Flexible Electrochromic Devices Based on PEDOT: PSS as Transparent Conducting Electrode. ACS Appl. Mater. Interfaces 2016, 9, 19427–19435. [Google Scholar] [CrossRef]
- Chandrasekhar, P.; Zay, B.J.; Cai, C.; Chai, Y.; Lawrence, D. Matched-dual-polymer electrochromic lenses, using new cathodically coloring conducting polymers, with exceptional performance and incorporated into automated sunglasses. J. Appl. Polym. Sci. 2014, 131, 41043–41064. [Google Scholar] [CrossRef]
- Kim, B.; Koh, J.K.; Park, J.; Ahn, C.; Ahn, J.; Kim, J.H.; Jeon, S. Patternable PEDOT nanofilms with grid electrodes for transparent electrochromic devices targeting thermal camouflage. Nano Converg. 2015, 2, 19. [Google Scholar] [CrossRef]
- Chandrasekhar, P.; Zay, B.J.; Lawrence, D.; Caldwell, E.; Sheth, R.; Stephan, R.; Cornwell, J. Variable-emittance infrared electrochromic skins combining unique conducting polymers, ionic liquid electrolytes, microporous polymer membranes, and semiconductor/polymer coatings, for spacecraft thermal control. J. Appl. Polym. Sci. 2014, 131, 40850–40865. [Google Scholar] [CrossRef]
- Brooke, R.; Fabretto, M.; Pering, S.; Switalska, E.; Reeks, L.; Evans, D.; Murphy, P. Diffuse color patterning using blended electrochromic polymers for proof-of-concept adaptive camouflage plaques. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Zhang, M.; Yang, Z.; Gane, P. Comparing build architecture and electrolyte of ITO-free flexible patterned organic screen-printed ECDs based on a novel PEDOT: PSS compound film post-treated with strong and weak organic acids. Org. Electron. 2020, 81, 105674. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.K.; Singh, R.; Gopinathan, S.; Murugan, S.; Gawali, S.; Saha, B.; Biswas, J.; Lodha, S.; Kumar, A. Solution-Processed Poly(3,4-ethylenedioxythiophene) Thin Films as Transparent Conductors: Effect of p-Toluenesulfonic Acid in Dimethyl Sulfoxide. ACS Appl. Mater. Interfaces 2014, 6, 17792–17803. [Google Scholar] [CrossRef]
- Ye, S.; Rathmell, A.R.; Chen, Z.; Wiley, B.J.; Wiley, B.J. Metal Nanowire Networks: The Next Generation of Transparent Conductors. Adv. Mater. 2014, 26, 6670–6687. [Google Scholar] [CrossRef]
- Hsu, P.C.; Wang, S.; Wu, H.; Narasimgan, V.K.; Kong, D.; Lee, H.Y.; Cui, Y. Performance enhancement of metal nanowire transparent conducting electrodes by mesoscale metal wires. Nat. Commun. 2013, 4, 2522–2529. [Google Scholar] [CrossRef] [Green Version]
- Tu, K.H.; Li, S.S.; Li, W.C.; Wang, D.Y.; Yang, J.R.; Chen, C.W. Solution processable nanocarbon platform for polymer solar cells. Energy Environ. Sci. 2011, 4, 3521–3526. [Google Scholar] [CrossRef]
- Gomez De Arco, L.; Zhang, Y.; Schlenker, C.W.; Ryu, K.; Thompson, M.E.; Zhou, C. Continuous, highly flexible, and transparent graphene films by chemical vapor deposition for organic photovoltaics. ACS Nano 2010, 4, 2865–2873. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Highly conductive poly (3, 4-ethylenedioxythiophene): Poly (styrene sulfonate) films treated with an amphiphilic fluoro compound as the transparent electrode of polymer solar cells. Energy Environ. Sci. 2012, 5, 5325–5332. [Google Scholar] [CrossRef]
- Wang, P.C.; Liu, L.H.; Mengistie, D.A.; Li, K.H.; Wen, B.; Liu, T.S.; Chu, C.W. Transparent electrodes based on conducting polymers for display applications. Displays 2013, 34, 301–314. [Google Scholar] [CrossRef]
- Valtakari, D.; Liu, J.; Kumar, V.; Xu, C.; Toivakka, M.; Saarinen, J.J. Conductivity of PEDOT: PSS on Spin-Coated and Drop Cast Nanofibrillar Cellulose Thin Films. Nanoscale Res. Lett. 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J. “Secondary doping” methods to significantly enhance the conductivity of PEDOT: PSS for its application as transparent electrode of optoelectronic devices. Displays 2013, 34, 423–436. [Google Scholar] [CrossRef]
- Vosgueritchian, M.; Lipomi, D.J.; Bao, Z. Highly Conductive and Transparent PEDOT: PSS Films with a Fluorosurfactant for Stretchable and Flexible Transparent Electrodes. Adv. Funct. Mater. 2011, 22, 421–428. [Google Scholar] [CrossRef]
- Liu, C.; Shi, H.; Xu, J.; Jiang, Q.; Song, H.; Zhu, Z. Improved thermoelectric properties of PEDOT:PSS nanofilms treated with oxalic acid. J. Electron. Mater. 2015, 44, 1791–1795. [Google Scholar] [CrossRef]
- Alemu, D.; Wei, H.-Y.; Ho, K.-C.; Chu, C.-W. Highly conductive PEDOT: PSS electrode by simple film treatment with methanol for ITO-free polymer solar cells. Energy Environ. Sci. 2012, 5, 9662–9671. [Google Scholar] [CrossRef]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.R.; Kim, B.J.; Lee, K. Highly conductive PEDOT: PSS nanofibrils induced by solution-processed crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, M.; Chen, X.; Nie, S.; Lai, W.; Su, W.; Cui, Z.; Hua, W. Screen-printed poly (3,4-Ethylenedioxythiophene):poly(Styrenesulfonate) grids as ITO-free anodes for flexible organic light-emitting diodes. Adv. Funct. Mater. 2018, 28, 1705955. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on application of PEDOTs and PEDOT: PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Wei, Q.; Mukaida, M.; Naitoh, Y.; Ishida, T. Morphological Change and Mobility Enhancement in PEDOT: PSS by Adding Co-solvents. Adv. Mater. 2013, 25, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Marks, Z.D.; Glugla, D.; Friedlein, J.T.; Shaheen, S.E.; McLeod, R.R.; Kahook, M.Y.; Nair, D.P. Switchable diffractive optics using patterned PEDOT: PSS based electrochromic thin-films. Org. Electron. 2016, 37, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Andersson, P.; Nilsson, D.; Svensson, P.O.; Chen, M.; Malmström, A.; Remonen, T.; Kugler, T.; Berggren, M. Active matrix displays based on all-organic electrochemical smart pixels printed on paper. Adv. Mater. 2002, 14, 1460–1464. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Khan, T.M.; Liu, J.-C.; Hsu, J.; Shim, J.W.; Dindar, A.; Youngblood, J.P.; Moon, R.J.; Kippelen, B. Recyclable organic solar cells on cellulose nanocrystal substrates. Sci. Rep. 2013, 3, srep01536. [Google Scholar] [CrossRef] [Green Version]
- Tobjörk, D.; Österbacka, R. Paper electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef]
- Asadpoordarvish, A.; Sandström, A.; Larsen, C.; Larsen, C.; Bollstrom, R.; Toivakka, M.; Osterbacka, R.; Edman, L. Light-Emitting Paper. Adv. Funct. Mater. 2015, 25, 3238–3245. [Google Scholar] [CrossRef]
- Bollström, R.; Pettersson, F.; Dolietis, P.; Preston, J.; Österbacka, R.; Toivakka, M. Impact of humidity on functionality of on-paper printed electronics. Nanotechnology 2014, 25, 094003. [Google Scholar] [CrossRef]
- Bollström, R.; Tobjörk, D.; Dolietis, P.; Salminen, P.; Preston, J.; Österbacka, R.; Toivakka, M. Printability of functional inks on multilayer curtain coated paper. Chem. Eng. Process. Process Intensif. 2013, 68, 13–20. [Google Scholar] [CrossRef]
- Kong, X.; Chong, X.; Squire, K.; Wang, A.X. Microfluidic diatomite analytical devices for illicit drug sensing withppb-Level sensitivity. Sens. Actuators B Chem. 2018, 259, 587–595. [Google Scholar] [CrossRef]
- Kim, Y.H.; Sachse, C.; Machala, M.L.; May, C.; Meskamp, L.M.; Leo, K. Highly conductive PEDOT: PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv. Funct. Mater. 2011, 21, 1076–1081. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Zhang, Z.; Zhang, L. Influence of nano-silica on the properties of inkjet paper coating. Digit. Print. 2019, 199, 96–101. [Google Scholar]
- Levasseur, D.; Mjejri, I.; Rolland, T.; Rougier, A. Color tuning by oxide addition in PEDOT:PSS-based electrochromic devices. Polymers 2019, 11, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]







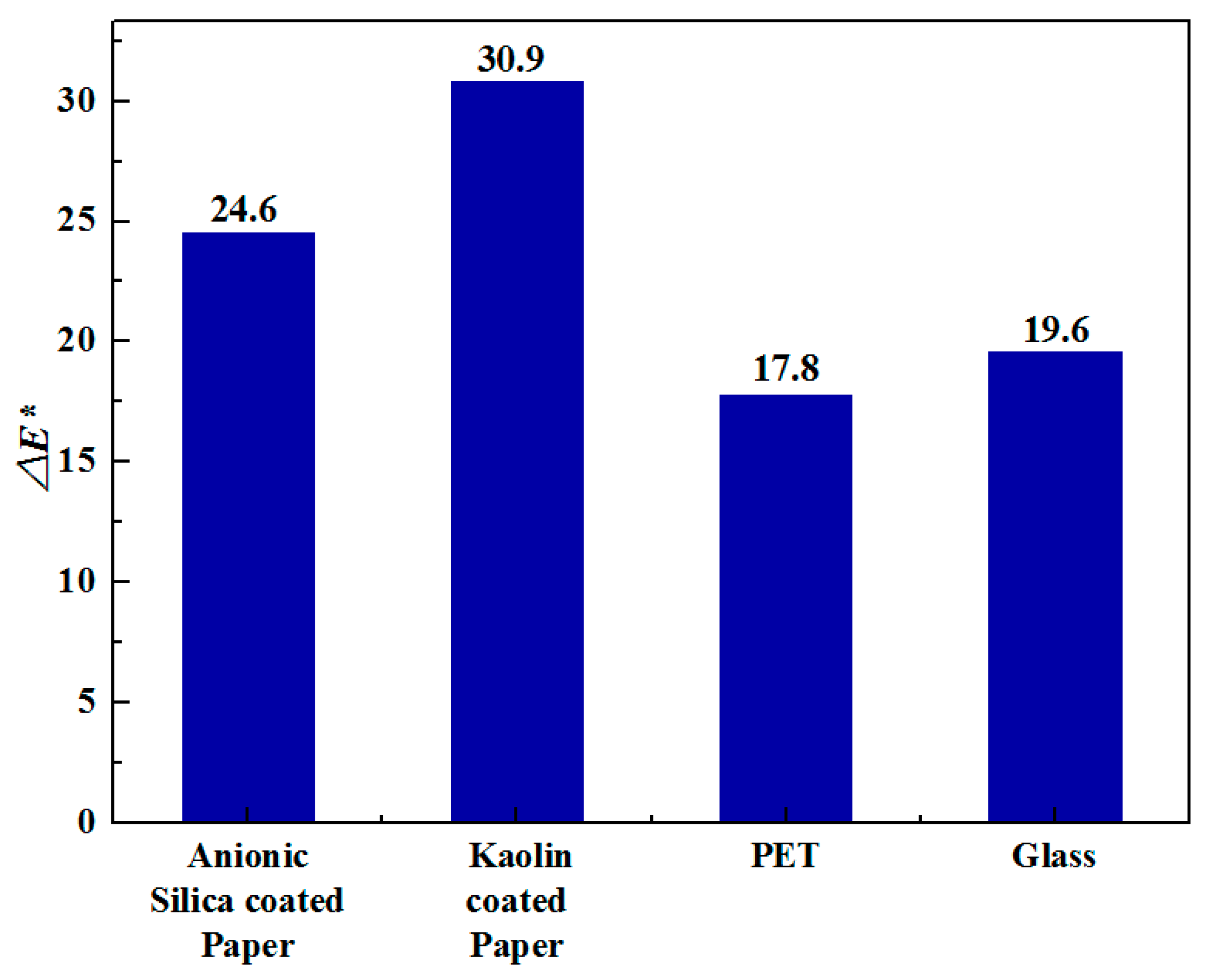
| Substrate | Grammage before Coating/mg·cm−2 | Coated Area/cm2 | Weight of Substrate with Coating/mg | Grammage after Coating/mg·cm−2 | Change in Grammage/mg·cm−2 |
|---|---|---|---|---|---|
| Anionic Silica-coated paper | 14.42 | 2.52 | 36.92 | 14.65 | 0.23 |
| Kaolin-coated paper | 13.43 | 2.99 | 40.67 | 13.60 | 0.17 |
| PET | 14.32 | 2.35 | 33.93 | 14.44 | 0.12 |
| Glass | 250.96 | 5.63 | 1413.52 | 251.07 | 0.11 |
| Electrolyte | Rd/Ω | C/F | Rα/Ω |
|---|---|---|---|
| electrolyte #1 | 1.84 × 106 | 1.13 × 10−7 | 4.795 |
| electrolyte #2 | 2.36 × 107 | 3.18 × 10−9 | 13.625 |
| electrolyte #3 | 7.66 × 104 | 3.15 × 10−6 | - |
| Material | Viscosity/mPa·S | Mass Fraction/% | Temperature/°C |
|---|---|---|---|
| Sorbitol | 900 | 80 | 25 |
| Glycerol | 1500 | 99 | 25 |
| Urea | 1.55 | 50 | 25 |
| electrolyte #1 | 142 | - | 25 |
| electrolyte #2 | 265 | - | 25 |
| electrolyte #3 | 107 | - | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Liu, Y.; Zhang, M.; Pettersson, F.; Toivakka, M. Fabrication of All-Solid Organic Electrochromic Devices on Absorptive Paper Substrates Utilizing a Simplified Lateral Architecture. Materials 2020, 13, 4839. https://doi.org/10.3390/ma13214839
Liu G, Liu Y, Zhang M, Pettersson F, Toivakka M. Fabrication of All-Solid Organic Electrochromic Devices on Absorptive Paper Substrates Utilizing a Simplified Lateral Architecture. Materials. 2020; 13(21):4839. https://doi.org/10.3390/ma13214839
Chicago/Turabian StyleLiu, Guodong, Yu Liu, Meiyun Zhang, Fredrik Pettersson, and Martti Toivakka. 2020. "Fabrication of All-Solid Organic Electrochromic Devices on Absorptive Paper Substrates Utilizing a Simplified Lateral Architecture" Materials 13, no. 21: 4839. https://doi.org/10.3390/ma13214839





