Improving the Mechanical Properties of Sulfoaluminate Cement-Based Grouting Material by Incorporating Limestone Powder for a Double Fluid System
Abstract
:1. Introduction
2. Materials and Experimental
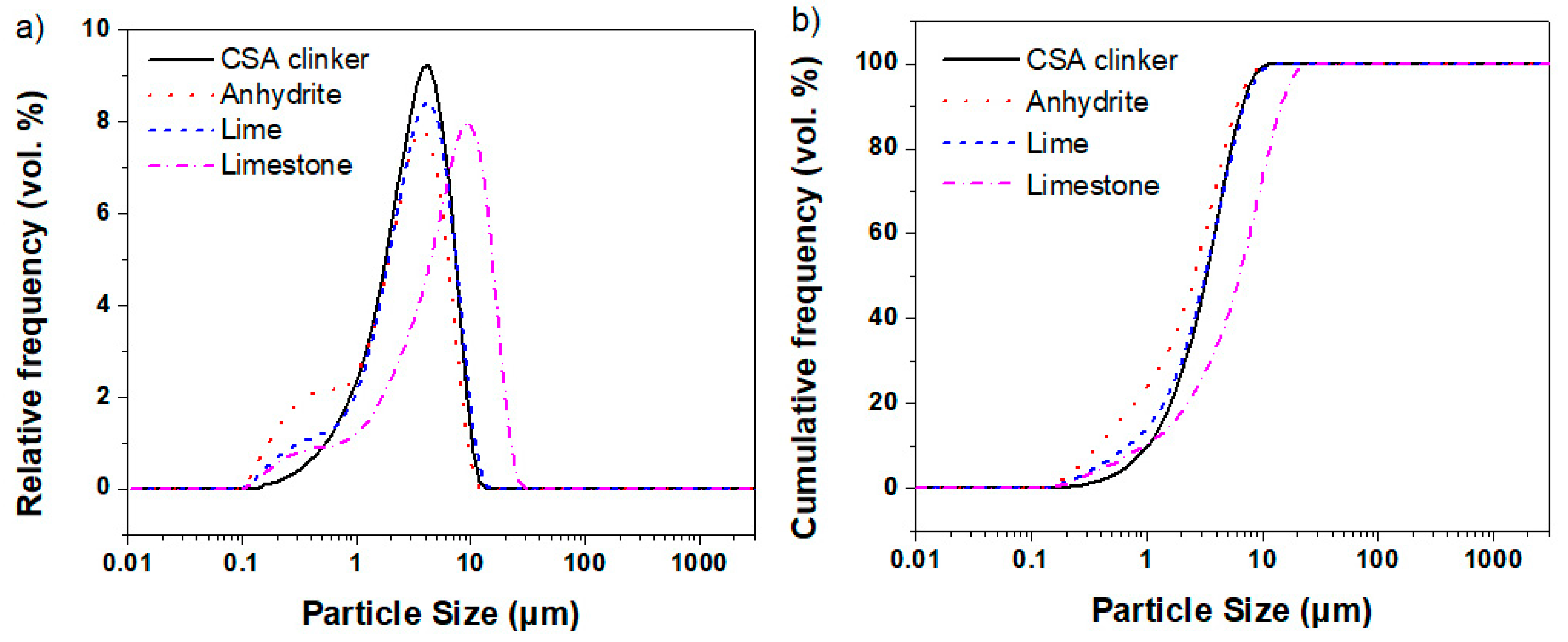
2.1. Raw Materials
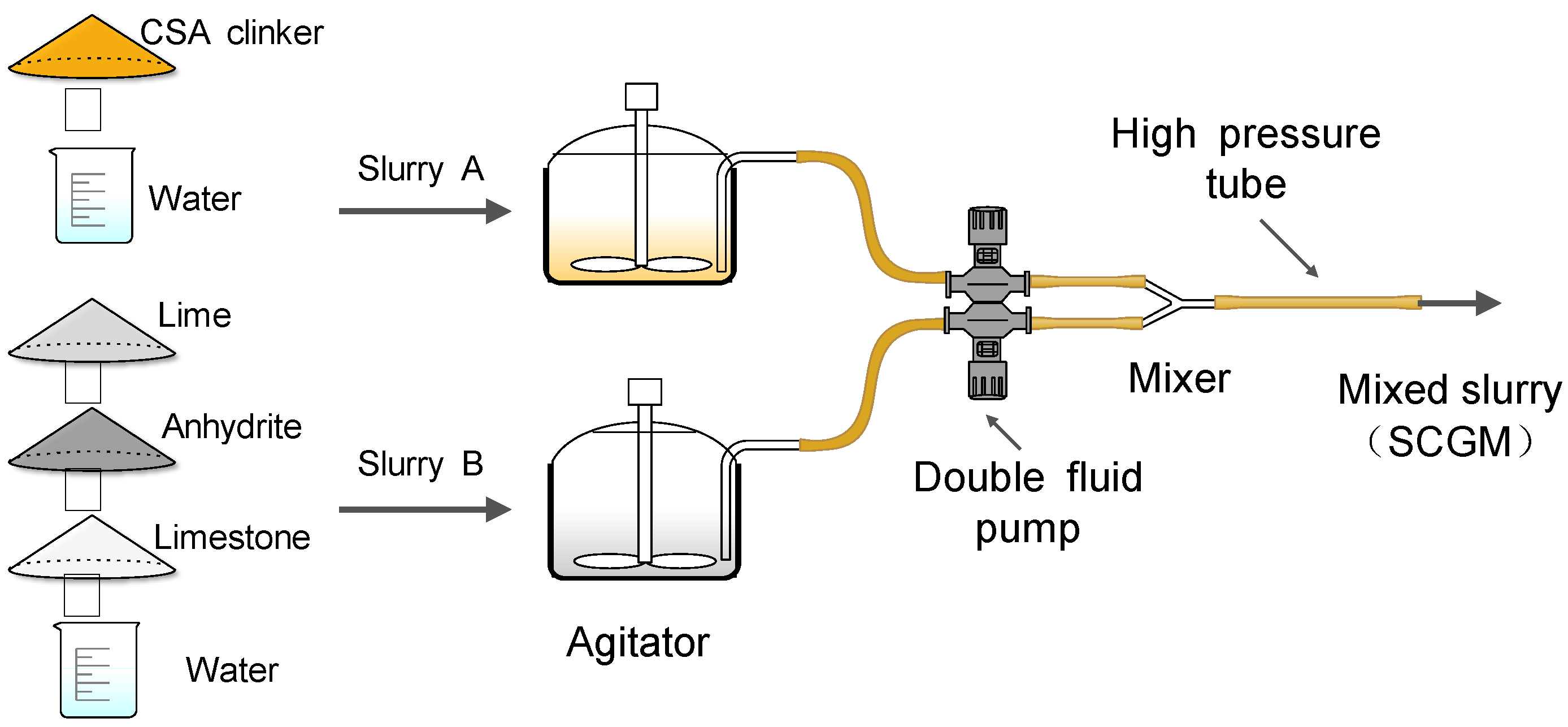
2.2. Mixture Proportion of SCGM
2.3. Test Methods
2.3.1. Flowability and Setting Time Test
2.3.2. Hydration Heat Test
2.3.3. The Compressive Strength of Hardened SCGM
2.3.4. Expansion and Shrinkage Test
2.3.5. Microstructure Analyses
X‑ray Diffractometry (XRD)
Thermogravimetric Analysis (TGA)
Scanning Electron Microscopy (SEM)
Mercury Intrusion Porosimetry (MIP)
3. Results and Discussion
3.1. Fresh Properties of SCGM
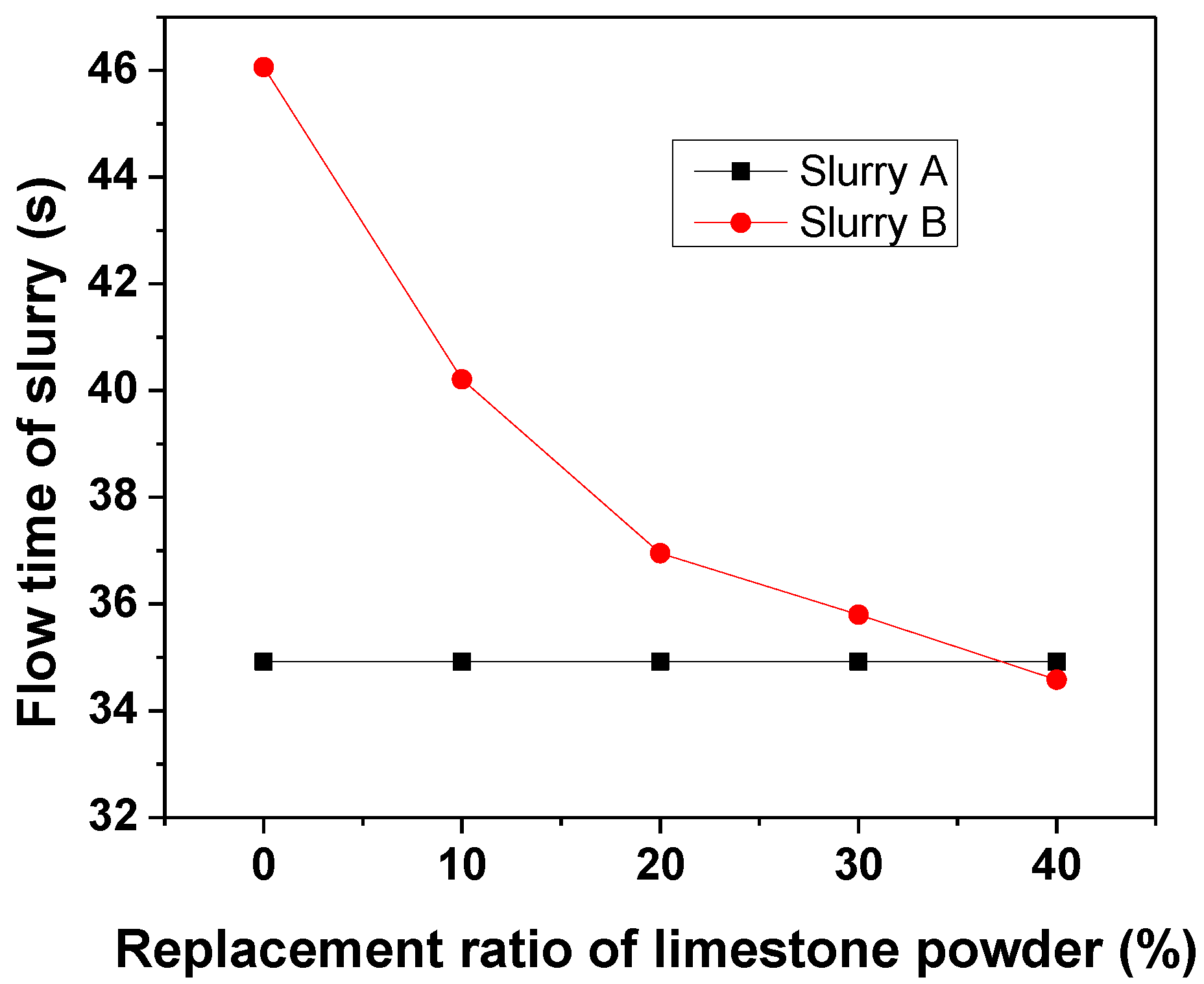
3.1.1. Flowability of Slurry A and Slurry B
3.1.2. Setting Time
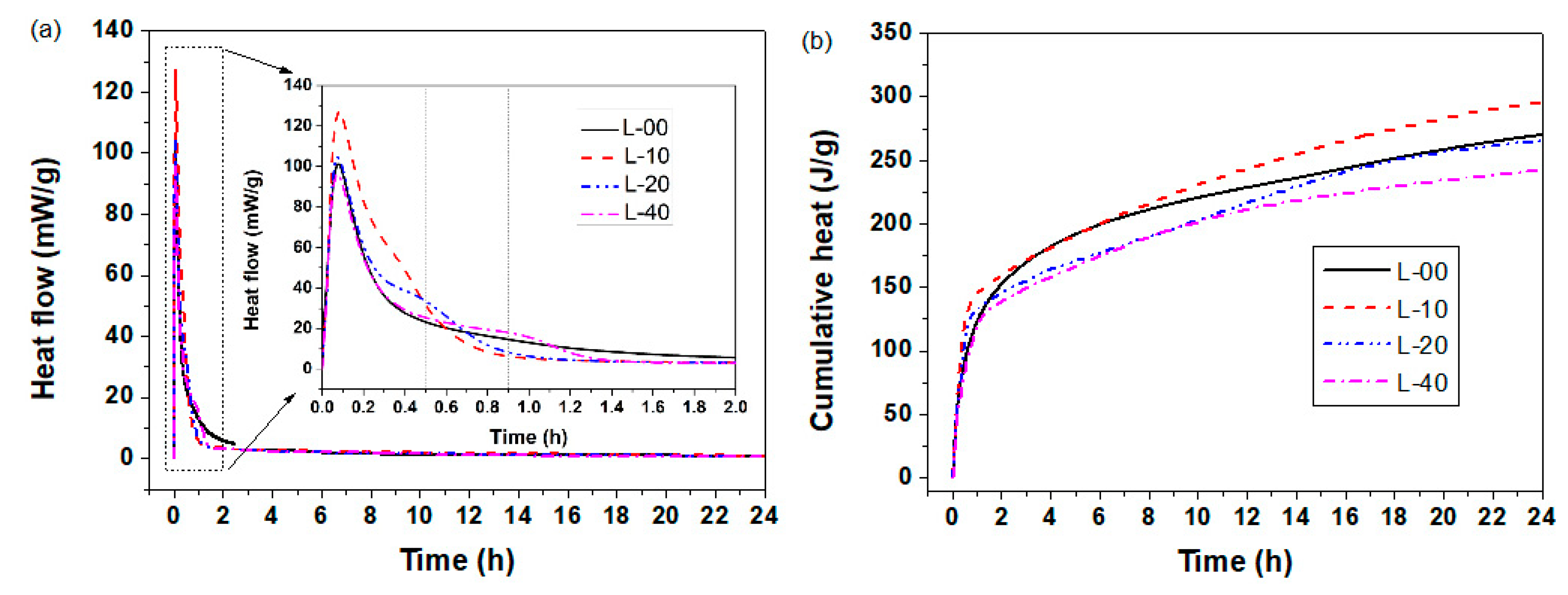
3.1.3. Heat of Hydration
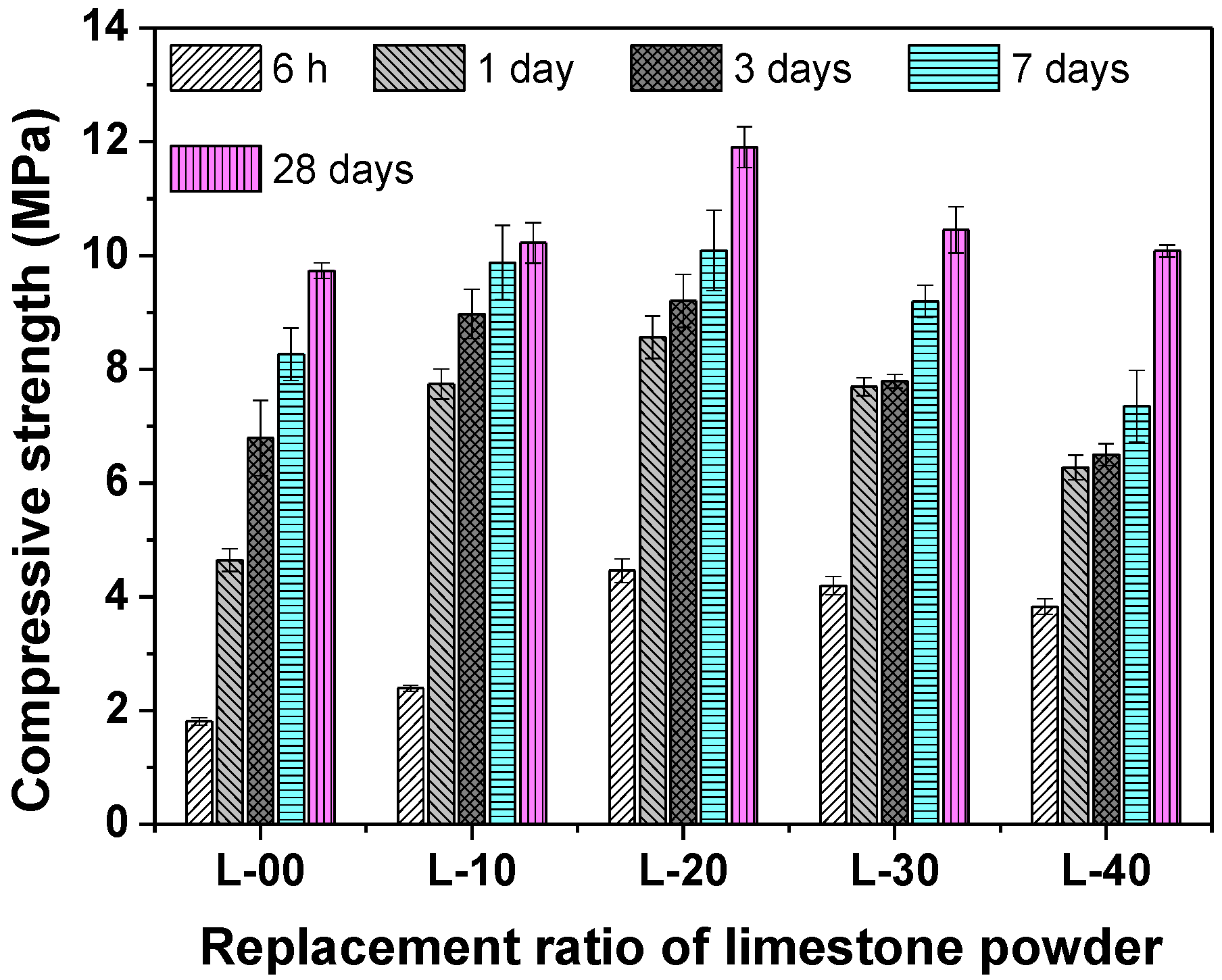
3.2. The Compressive Strength Development
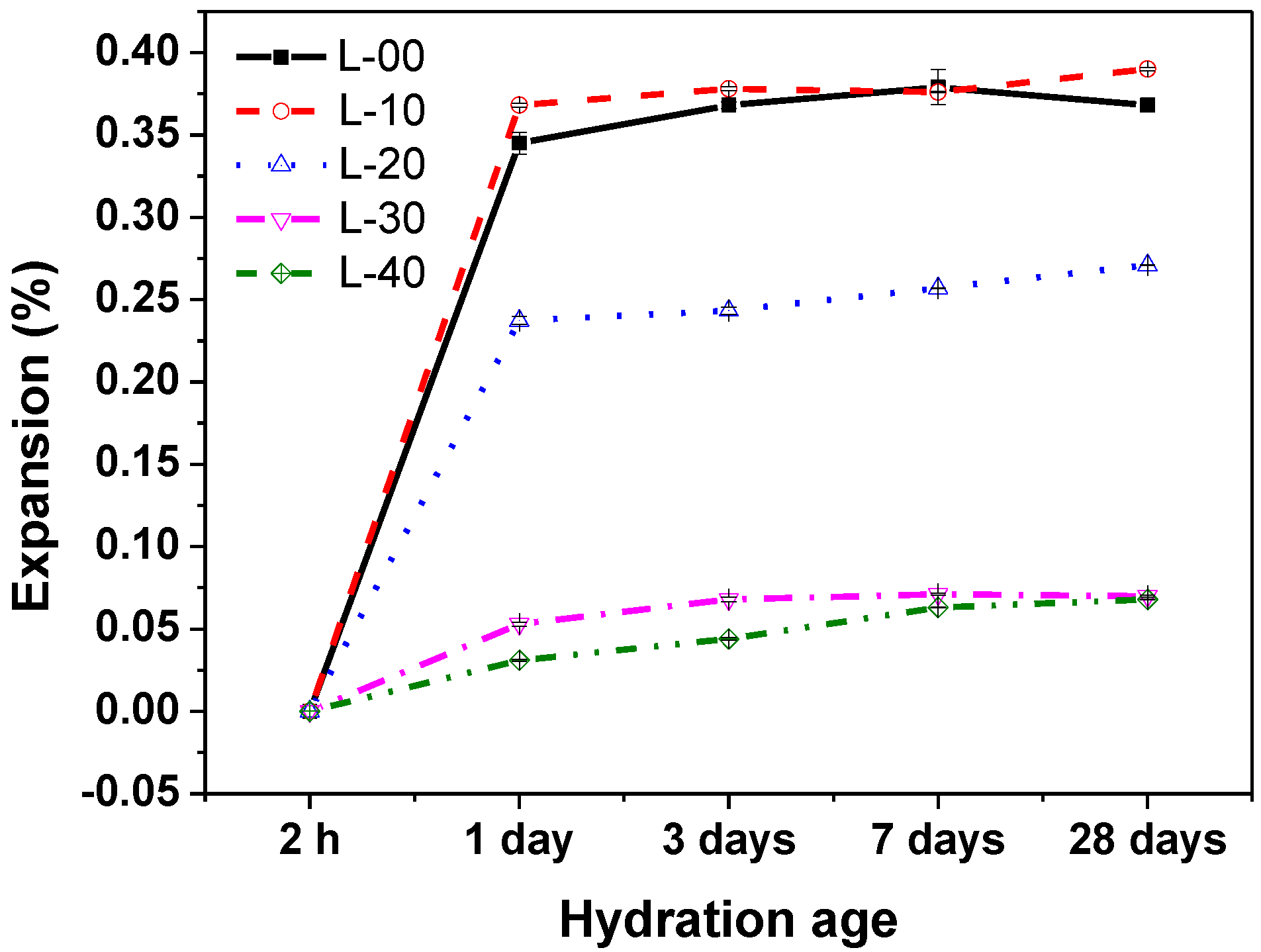
3.3. Expansion Behavior of SCGM
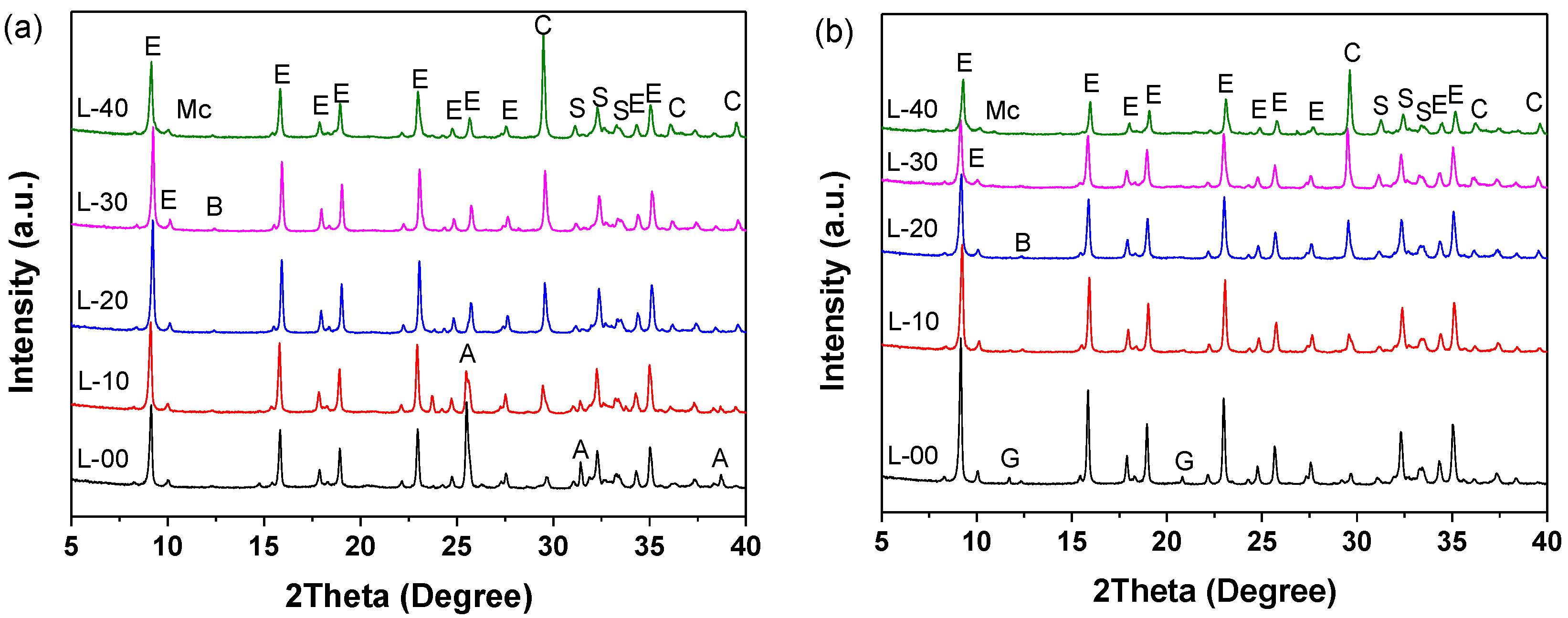
3.4. Microstructure Analysis
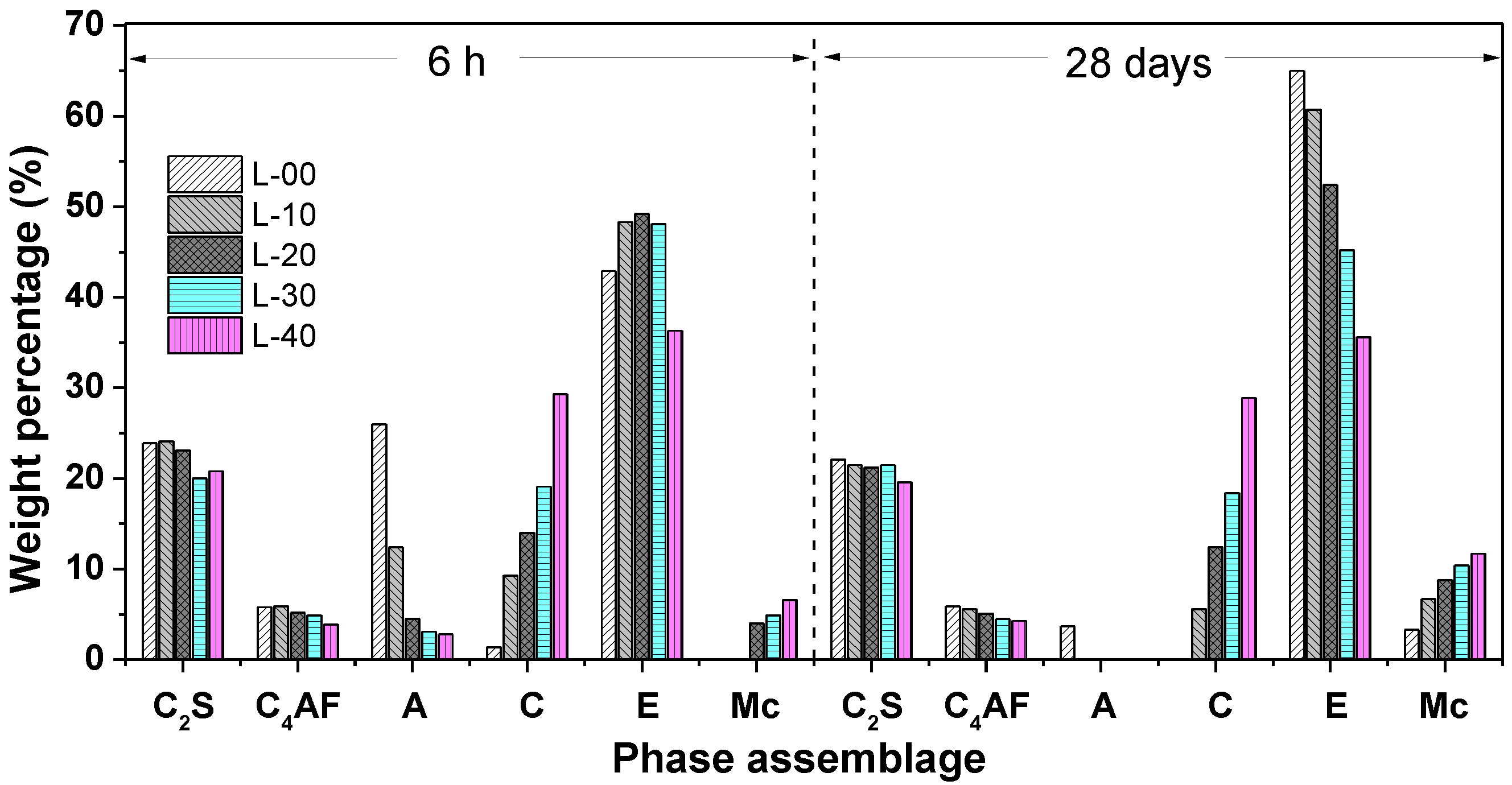
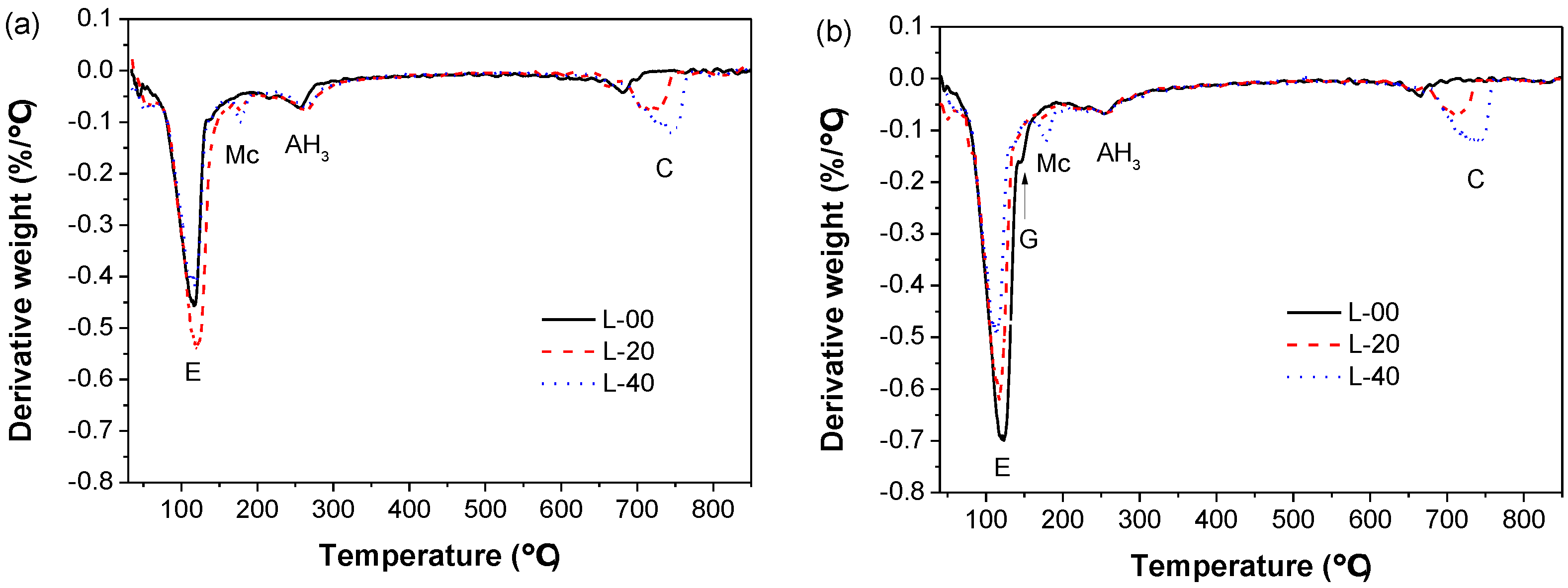
3.4.1. Phase Assemblage of Hardened SCGM
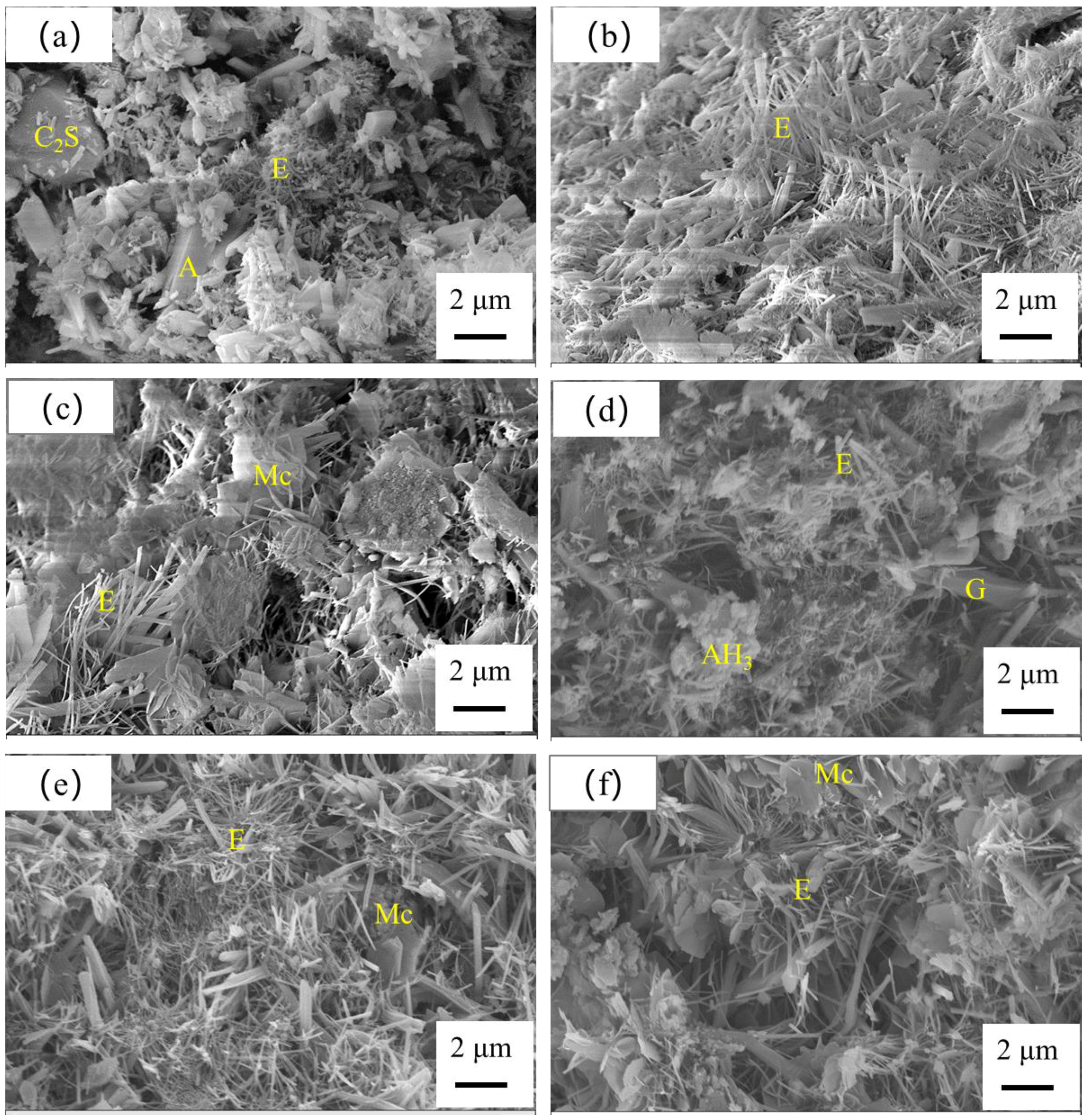
3.4.2. SEM Observation
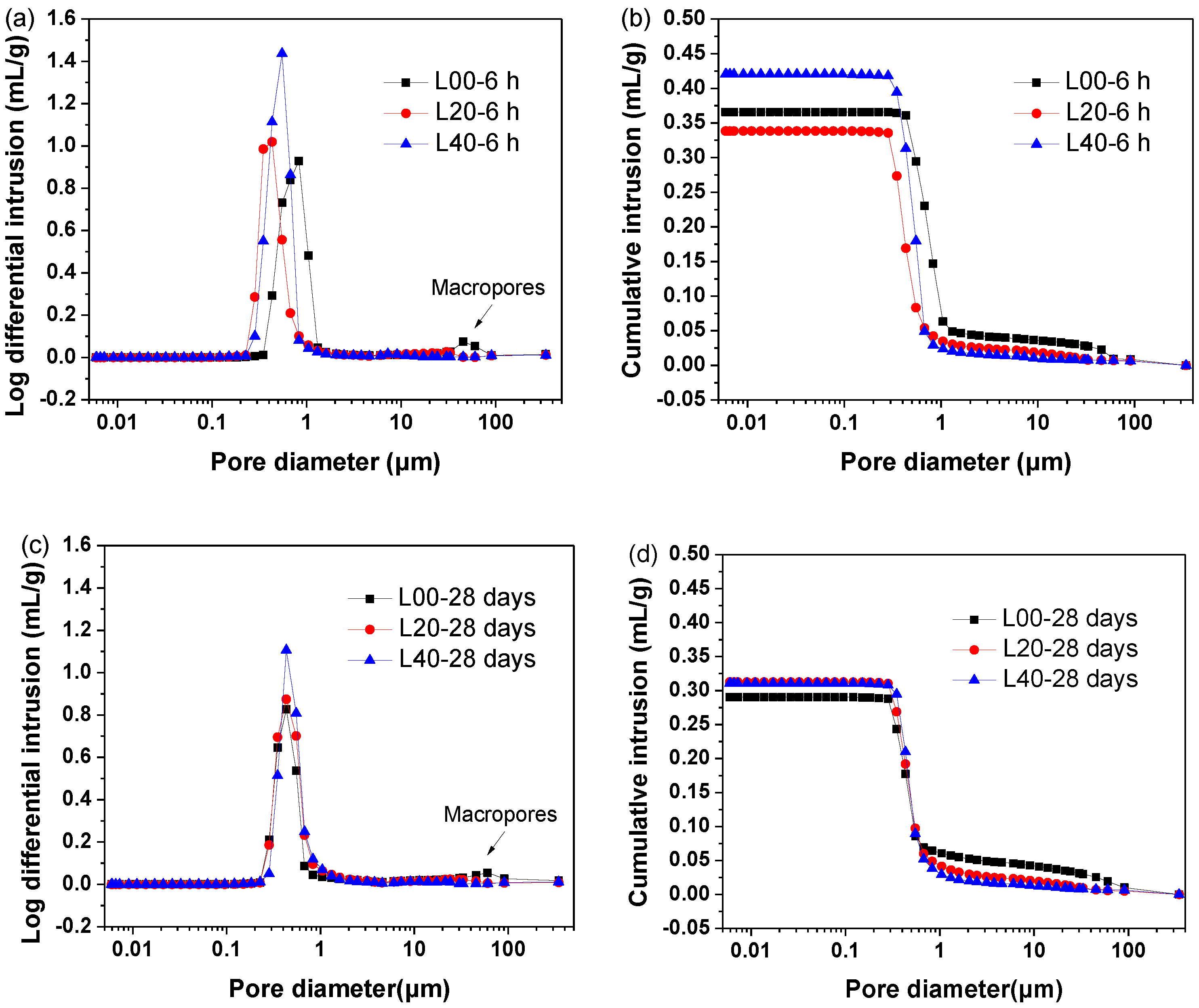
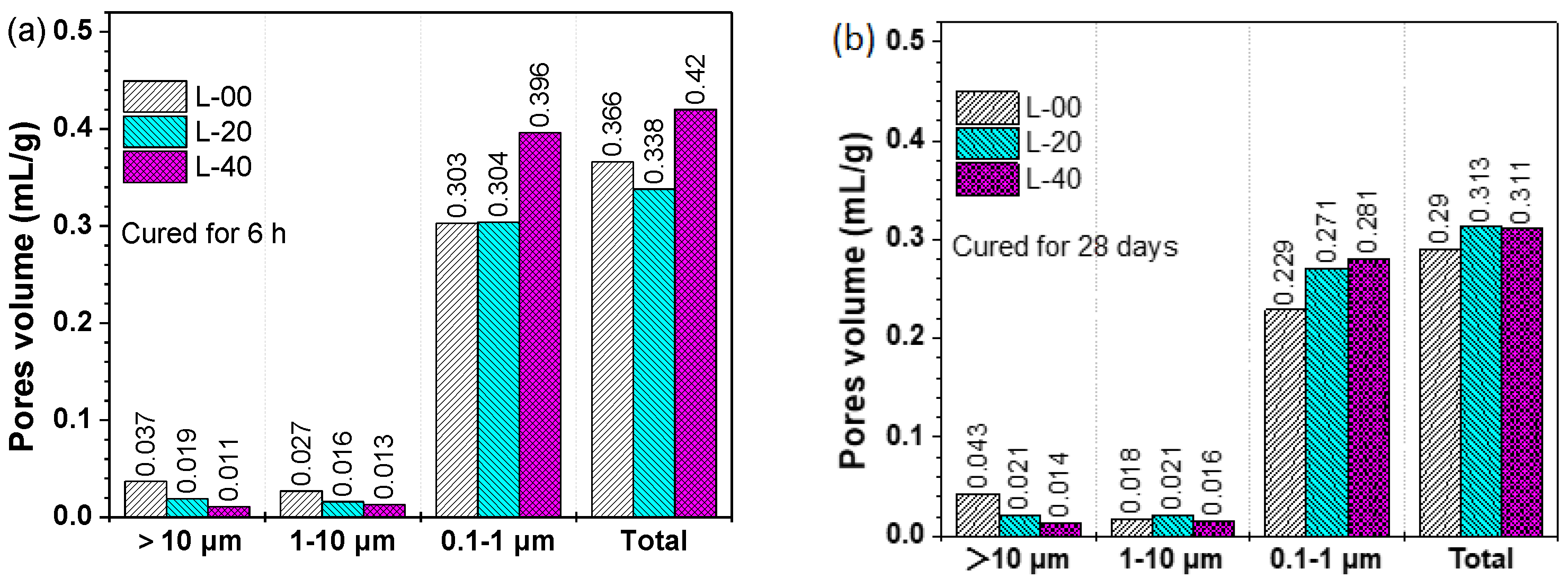
3.4.3. Porosity Evolution
4. Conclusions
- (1)
- The replacement of anhydrite by limestone in slurry B would improve its flowability, shorten the setting time, and accelerate the hydration reaction of the mixed grouting material.
- (2)
- Replacing anhydrite with less than 20% limestone, both the early and later compressive strengths were increased significantly compared with that of the L-00 sample. Further increasing the replacement rate to 40%, the later compressive strength was decreased, while the early compressive strength was still increased.
- (3)
- The L-20 sample achieved the maximum compressive strength. Compared with L-00, the compressive strength for 6 h, 1 day, 3 days, 7 days, and 28 days was increased by 146.41%, 84.29%, 35.54%, 22.11%, and 22.33%, respectively.
- (4)
- L-00 samples showed an expansion rate of 0.35% by 1 day, and the expansion rate of L-10 was similar to that of L-00. However, when the replacement rate of limestone powder was further increased, the expansion level was decreased.
- (5)
- The primary hydrates phase assemblage of SCGM was ettringite, Mc and AH3. In the early ages, limestone powder would promote the formation of ettringite due to its nucleation effects. But the total amount of ettringite formed in the later ages was reduced as increasing the replacement rate of limestone powder.
- (6)
- Enhancement of the hardening performance of SCGM by limestone powder was attributed to the increased ettringite amount, the densified microstructure, and the refined porosity.
- (7)
- Moreover, the replacement rate of anhydrite by limestone powder may also affect the toughness or fracture behavior of the SCGM [40], and the related works will be conducted in the future.
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Guan, X.; Zhang, X.; Ge, P.; Hu, X.; Zou, D. Influence of superfine ettringite on the properties of sulphoaluminate cement-based grouting materials. Constr. Build. Mater. 2018, 166, 723–731. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Liu, X.; Guan, X.; Zhang, C.; Niu, Y. Flexible and stretchable polyurethane/waterglass grouting material. Constr. Build. Mater. 2017, 138, 240–246. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, X.; Li, H.; Liu, X. Performance and hydration study of ultra-fine sulfoaluminate cement-based double liquid grouting material. Constr. Build. Mater. 2017, 132, 262–270. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Wang, J.; Guan, X. Effects of Aluminum Sulfate and Quicklime/Fluorgypsum Ratio on the Properties of Calcium Sulfoaluminate (CSA) Cement-Based Double Liquid Grouting Materials. Materials 2019, 12, 1222. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Hu, X.-M.; Wu, M.-Y.; Zhao, Y.-Y.; Yu, C. Effects of different catalysts on the structure and properties of polyurethane/water glass grouting materials. J. Appl. Polym. Sci. 2018, 135, 46460. [Google Scholar] [CrossRef]
- Yang, Z.-P.; Zhang, X.; Liu, X.; Guan, X.; Zhang, C.; Niu, Y. Polyglycerol-based organic-inorganic hybrid adhesive with high early strength. Mater. Des. 2017, 117, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Li, Q.; Peng, W.; Zhang, F.; Cao, J.; Wang, H. Development of cement-based self-stress composite grouting material for reinforcing rock mass and engineering application. Constr. Build. Mater. 2019, 201, 314–327. [Google Scholar] [CrossRef]
- Pascual-Muñoz, P.; Indacoechea-Vega, I.; Zamora-Barraza, D.; Castro-Fresno, D. Experimental analysis of enhanced cement-sand-based geothermal grouting materials. Constr. Build. Mater. 2018, 185, 481–488. [Google Scholar] [CrossRef]
- Sha, F.; Li, S.C.; Liu, R.-T.; Li, Z.; Zhang, Q. Experimental study on performance of cement-based grouts admixed with fly ash, bentonite, superplasticizer and water glass. Constr. Build. Mater. 2018, 161, 282–291. [Google Scholar] [CrossRef]
- Pantazopoulos, I.; Markou, I.; Christodoulou, D.; Droudakis, A.; Atmatzidis, D.; Antiohos, S.; Chaniotakis, E. Development of microfine cement grouts by pulverizing ordinary cements. Cem. Concr. Compos. 2012, 34, 593–603. [Google Scholar] [CrossRef]
- Li, J.; Chang, J. Effect of crystal/amorphous ratio on mechanical properties in a C4A3$-C2S hydration system with or without gypsum addition. Constr. Build. Mater. 2019, 208, 36–45. [Google Scholar] [CrossRef]
- Chang, J.; Li, J.; Han, J.; Zhang, T. Traces of CH in a C4A3$-C2S hydration system. Constr. Build. Mater. 2019, 197, 641–651. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, J.; Zhao, J.; Fang, Y. Nanostructural characterization of Al(OH)3 formed during the hydration of calcium sulfoaluminate cement. J. Am. Ceram. Soc. 2018, 101, 4262–4274. [Google Scholar] [CrossRef]
- Li, H.; Yang, K.; Guan, X. Effect of water-to-binder ratio on the properties of CSA cement-based grouting materials with LiAl-LDH. Adv. Compos. Lett. 2020, 29, 2090887. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, X.; Wang, X.; Ma, X.; Li, Z.; Xu, Z.; Jin, B. Microstructure and Properties of Sulfoaluminate Cement-Based Grouting Materials: Effect of Calcium Sulfate Variety. Adv. Mater. Sci. Eng. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Guan, X.; Li, H.; Luo, S.; Liu, X.; Zhang, J. Influence of LiAl-layered double hydroxides with 3D micro-nano structures on the properties of calcium sulphoaluminate cement clinker. Cem. Concr. Compos. 2016, 70, 15–23. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Guan, X.; Zou, D. Properties of calcium sulfoaluminate cement-based grouting materials with LiAl-layered double hydroxides slurries. Adv. Compos. Lett. 2020, 29, 2092652. [Google Scholar] [CrossRef]
- Li, H.; Yang, K.; Guan, X. Properties of sulfoaluminate cement-based grouting materials modified with LiAl-layered double hydroxides in the presence of PCE superplasticizer. Constr. Build. Mater. 2019, 226, 399–405. [Google Scholar] [CrossRef]
- Li, C.; Jiang, L.; Li, S. Effect of limestone powder addition on threshold chloride concentration for steel corrosion in reinforced concrete. Cem. Concr. Res. 2020, 131, 106018. [Google Scholar] [CrossRef]
- De Weerdt, K.; Ben Haha, M.; Le Saout, G.; Kjellsen, K.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K.L. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Müller, C.J. Beneficial use of limestone filler with calcium sulphoaluminate cement. Constr. Build. Mater. 2012, 26, 619–627. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.-C.; Moon, J. Effect of Calcium Carbonate Fineness on Calcium Sulfoaluminate-Belite Cement. Materials 2017, 10, 900. [Google Scholar] [CrossRef] [Green Version]
- Yao, Y.; Wang, W.; Ge, Z.; Ren, C.; Yao, X.; Wu, S. Hydration study and characteristic analysis of a sulfoaluminate high-performance cementitious material made with industrial solid wastes. Cem. Concr. Compos. 2020, 112, 103687. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Influence of pH on the chloride binding capacity of Limestone Calcined Clay Cements (LC3). Cem. Concr. Res. 2020, 131, 106031. [Google Scholar] [CrossRef]
- Martin, L.H.; Winnefeld, F.; Müller, C.J.; Lothenbach, B. Contribution of limestone to the hydration of calcium sulfoaluminate cement. Cem. Concr. Compos. 2015, 62, 204–211. [Google Scholar] [CrossRef]
- Chen, I.A.; Hargis, C.W.; Juenger, M.C. Understanding expansion in calcium sulfoaluminate–belite cements. Cem. Concr. Res. 2012, 42, 51–60. [Google Scholar] [CrossRef]
- Xu, J.; Chen, J.; Lu, D.; Xu, Z.; Hooton, R. Effect of dolomite powder on the hydration and properties of calcium sulfoaluminate cements with different gypsum contents. Constr. Build. Mater. 2019, 225, 302–310. [Google Scholar] [CrossRef]
- Krishnan, S.; Emmanuel, A.C.; Bishnoi, S. Hydration and phase assemblage of ternary cements with calcined clay and limestone. Constr. Build. Mater. 2019, 222, 64–72. [Google Scholar] [CrossRef]
- Huang, Y.; Pei, Y.; Qian, J.; Gao, X.; Liang, J.; Duan, G.; Zhao, P.; Lu, L.; Cheng, X. Bauxite free iron rich calcium sulfoaluminate cement: Preparation, hydration and properties. Constr. Build. Mater. 2020, 249, 118774. [Google Scholar] [CrossRef]
- Borštnar, M.; Daneu, N.; Dolenec, S.; Kramar, S. Phase development and hydration kinetics of belite-calcium sulfoaluminate cements at different curing temperatures. Ceram. Int. 2020. [CrossRef]
- García-Maté, M.; De La Torre, A.G.; León-Reina, L.; Losilla, E.R.; Aranda, M.A.; Santacruz, I. Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement. Cem. Concr. Compos. 2015, 55, 53–61. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Provis, J.L.; Tsang, D.C.; Poon, C.S. Accelerated carbonation of reactive MgO and Portland cement blends under flowing CO2 gas. Cem. Concr. Compos. 2020, 106, 103489. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S.; Shi, C.J.; Jian, Z.B.; Xing, X.D.; Poon, C.-S.; Jun, S.C. Mechanism for rapid hardening of cement pastes under coupled CO2-water curing regime. Cem. Concr. Compos. 2019, 97, 78–88. [Google Scholar] [CrossRef]
- Gao, D.; Meng, Y.; Yang, L.; Tang, J.; Lv, M. Effect of ground granulated blast furnace slag on the properties of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 227, 116665. [Google Scholar] [CrossRef]
- Li, P.; Ma, Z.; Zhang, Z.; Li, X.; Lu, X.; Hou, P.; Du, P. Effect of Gypsum on Hydration and Hardening Properties of Alite Modified Calcium Sulfoaluminate Cement. Materials 2019, 12, 3131. [Google Scholar] [CrossRef] [Green Version]
- De Domenico, D.; Faleschini, F.; Pellegrino, C.; Ricciardi, G. Structural behavior of RC beams containing EAF slag as recycled aggregate: Numerical versus experimental results. Constr. Build. Mater. 2018, 171, 321–337. [Google Scholar] [CrossRef]
| Component | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | TiO2 | SO3 | Loss |
|---|---|---|---|---|---|---|---|---|
| CSA Clinker | 41.53 | 6.36 | 38.27 | 1.27 | 1.15 | 1.77 | 8.88 | 0.17 |
| Lime | 64.72 | 3.46 | 0.67 | 0.48 | 2.1 | — | — | 27.58 |
| Anhydrite | 38.63 | 2.44 | 0.23 | 0.18 | 2.64 | 0.64 | 50.11 | 4.84 |
| Limestone | 54.45 | 1.12 | 0.47 | 0.09 | 0.54 | 0.02 | 0.15 | 43.12 |
| β‑C2S | C4AF | f‑SO3 | f-CaO | CaO·TiO2 | |
|---|---|---|---|---|---|
| 74.54 | 18.25 | 3.86 | 0.81 | 2.02 | 3.01 |
| Mix No. | Slurry A | Slurry B | ||||
|---|---|---|---|---|---|---|
| CSA Clinker | Water | Lime | Anhydrite | Limestone | Water | |
| L‑00 | 500 | 600 | 100 | 400 | 0 | 600 |
| L‑10 | 500 | 600 | 100 | 360 | 40 | 600 |
| L‑20 | 500 | 600 | 100 | 320 | 80 | 600 |
| L‑30 | 500 | 600 | 100 | 280 | 120 | 600 |
| L‑40 | 500 | 600 | 100 | 240 | 160 | 600 |
| Mix No. | Initial Setting Time (min) | Final Setting Time (min) | ||
|---|---|---|---|---|
| Absolute | Relative | Absolute | Relative | |
| L-00 | 5.0 | 100.0 | 7.5 | 100.0 |
| L-10 | 4.5 | 90.0 | 6.0 | 80.0 |
| L-20 | 2.7 | 54.0 | 5.5 | 73.3 |
| L-30 | 2.5 | 50.0 | 5.0 | 66.7 |
| L-40 | 1.4 | 28.0 | 4.8 | 64.0 |
| Mix No. | Porosity (%) | Average Pore Diameter (µm) | Total Intrusion Volume (mL/g) | Bulk Density (g/mL) |
|---|---|---|---|---|
| L-00-6h | 31.71 | 0.77428 | 0.3659 | 0.8665 |
| L-20-6h | 29.78 | 0.46227 | 0.3384 | 0.8799 |
| L-40-6h | 34.79 | 0.51232 | 0.4203 | 0.8276 |
| L-00-28d | 30.29 | 0.52180 | 0.2904 | 1.0432 |
| L-20-28d | 29.64 | 0.49943 | 0.3127 | 0.9481 |
| L-40-28d | 27.23 | 0.50505 | 0.3107 | 0.8766 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, S.; Xuan, D.; Guan, X.; Zhang, H. Improving the Mechanical Properties of Sulfoaluminate Cement-Based Grouting Material by Incorporating Limestone Powder for a Double Fluid System. Materials 2020, 13, 4854. https://doi.org/10.3390/ma13214854
Wang Y, Liu S, Xuan D, Guan X, Zhang H. Improving the Mechanical Properties of Sulfoaluminate Cement-Based Grouting Material by Incorporating Limestone Powder for a Double Fluid System. Materials. 2020; 13(21):4854. https://doi.org/10.3390/ma13214854
Chicago/Turabian StyleWang, Yanfeng, Songhui Liu, Dongxing Xuan, Xuemao Guan, and Haibo Zhang. 2020. "Improving the Mechanical Properties of Sulfoaluminate Cement-Based Grouting Material by Incorporating Limestone Powder for a Double Fluid System" Materials 13, no. 21: 4854. https://doi.org/10.3390/ma13214854





