Effect of Carbonation on the Water Resistance of Steel Slag—Magnesium Oxysulfate (MOS) Cement Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Preparation
2.3. Testing Methods
3. Results and Discussion
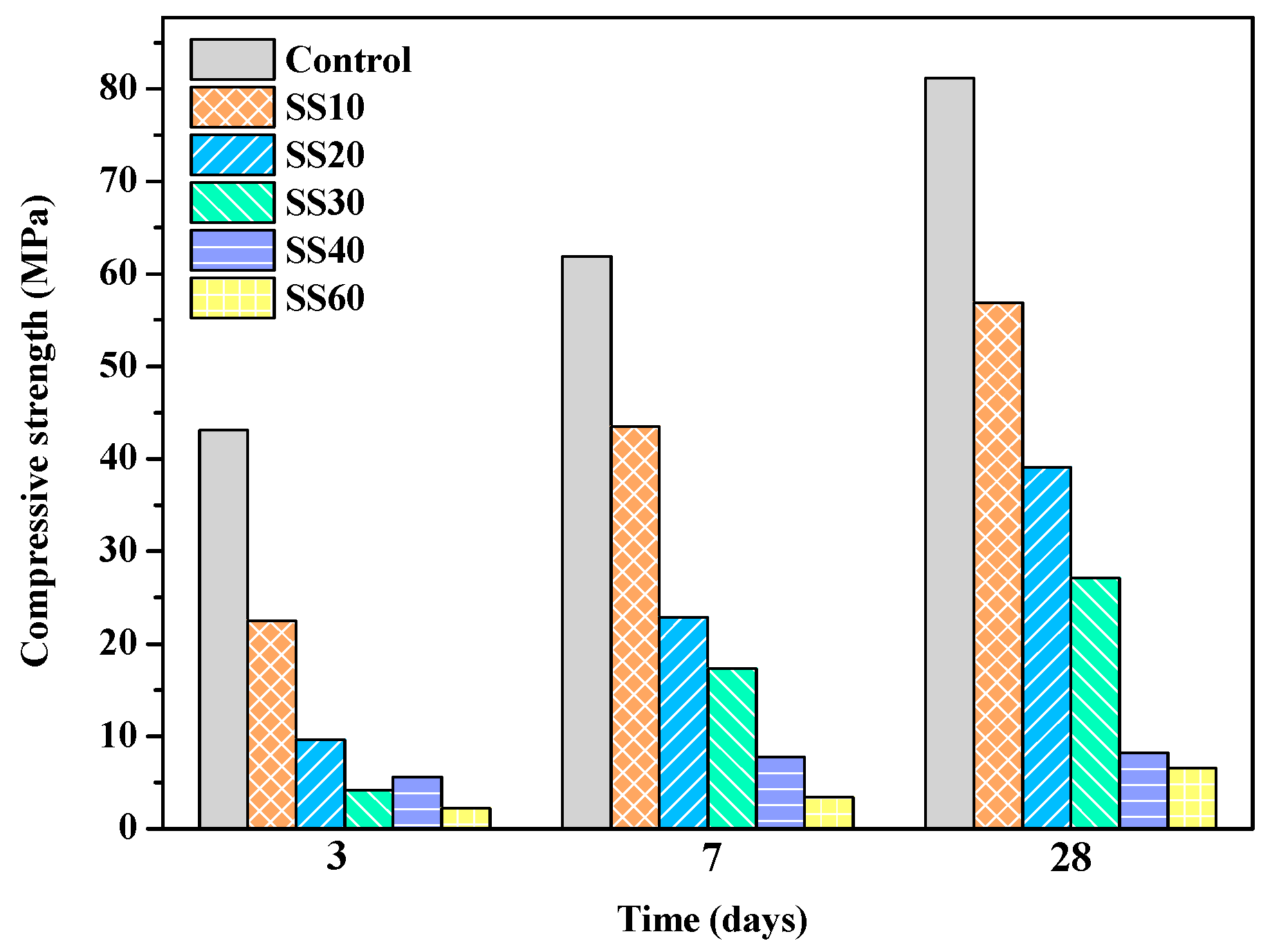
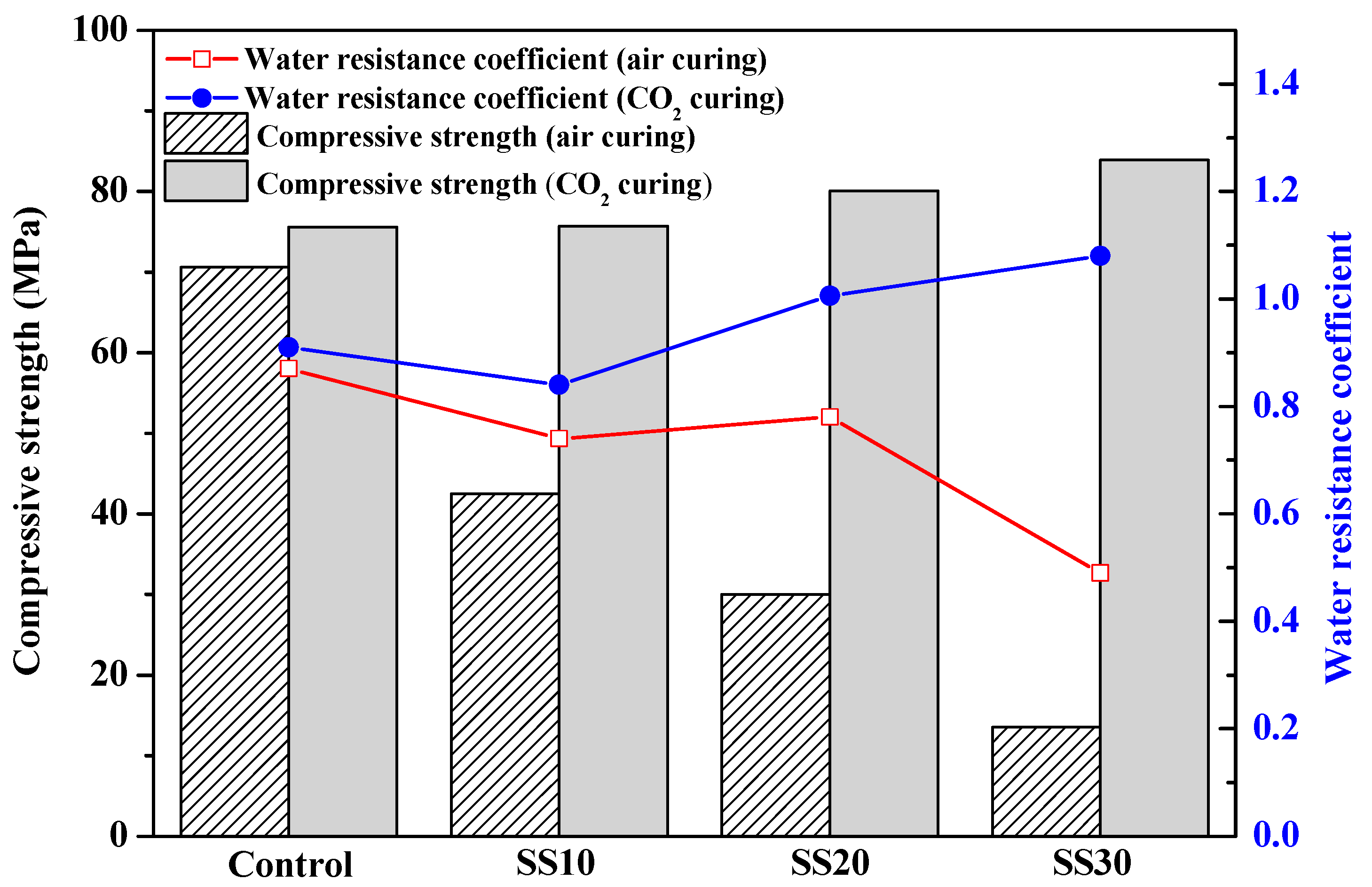
3.1. Mechanical Properties of Samples with and without CO2 Treatment
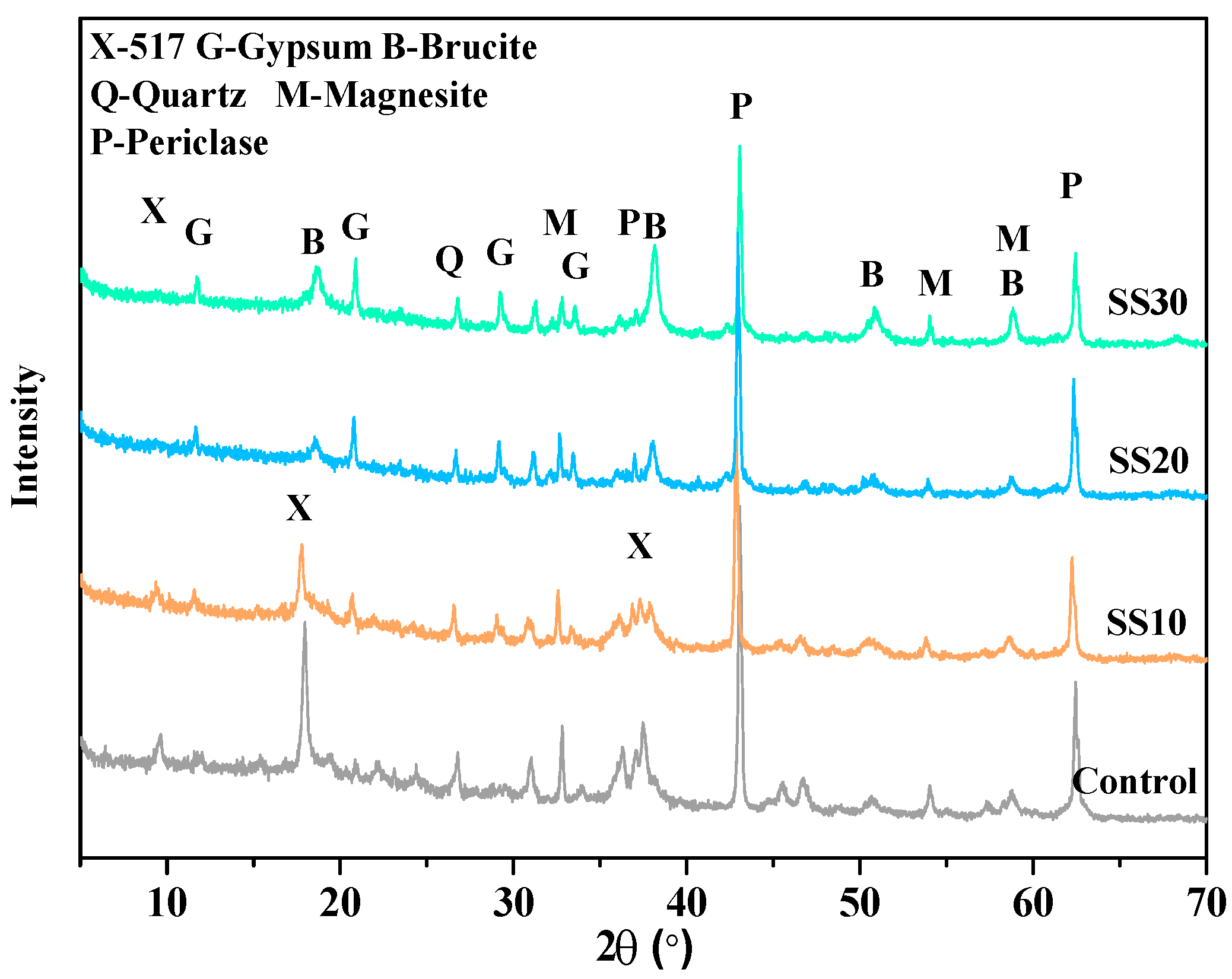
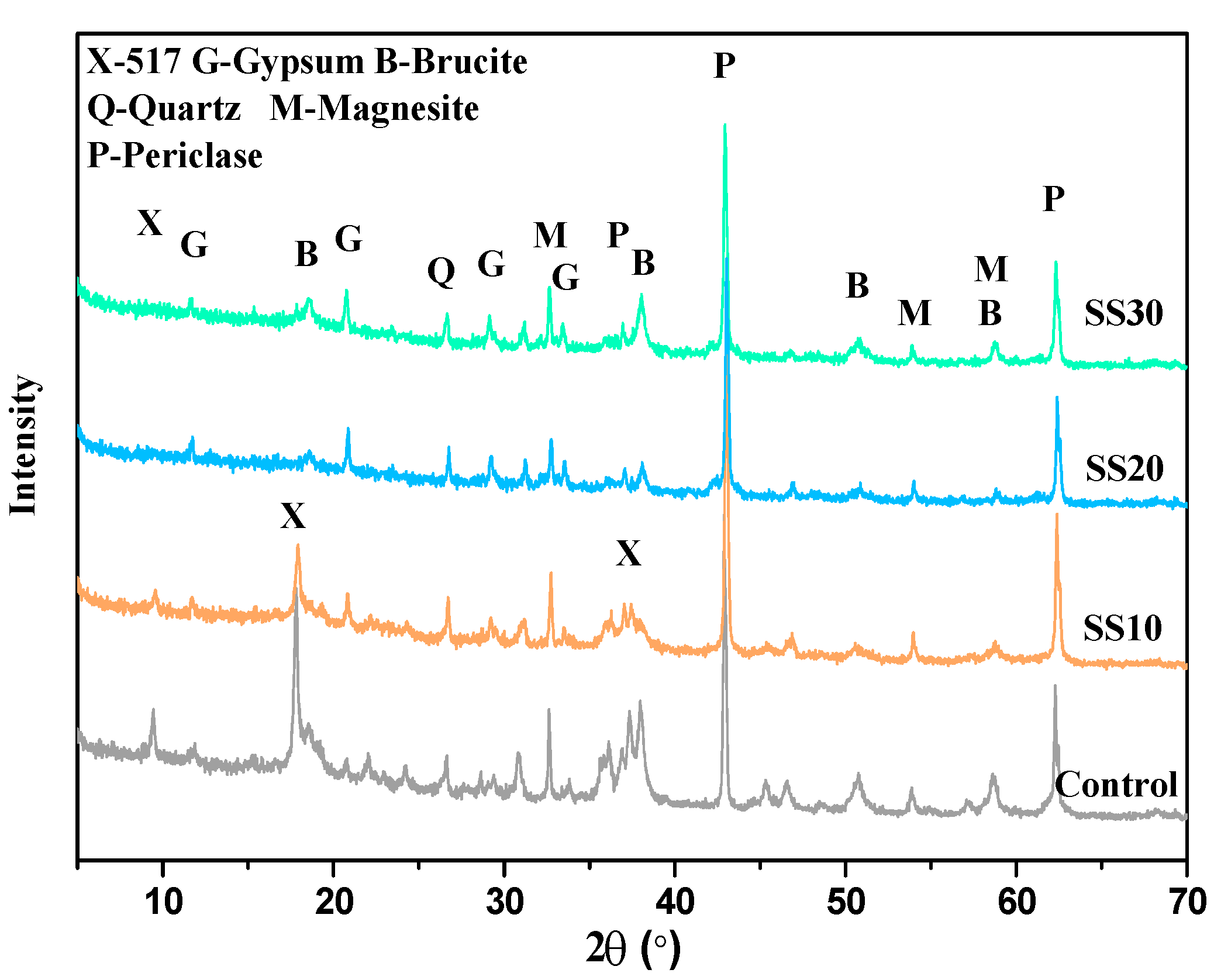
3.2. Hydration Product of Samples with and without CO2 Treatment
3.3. Microstructure of the Samples with and without CO2 Treatment
4. Conclusions
- With an increase in the steel slag content, the compressive strength and the water resistance of MOS cement decreased. The presence of CaO in steel slag increased the pH of the pastes and reacted with SO42- to form gypsum, which reduced the concentration of sulfate ions in slurry, and both of which were not conducive to the formation of 517 phase, so the compressive strength of the pastes decreases.
- The compressive strength of the samples had a significant increase after carbonating, which was mainly due to the promotion of C2S hydration in steel slag after carbonation.
- The products (Ca–Mg–C amorphous substance) of carbonation exhibited good water stability as they densified the matrix, thus leading to an improved compressive strength of the MOS cement.
- The HMC substances were formed by carbonation dissolved CO32- when immersed in water, which limited the dissolution of Mg2+ and inhibited MgO hydration forming Mg(OH)2. The HMC substances reacted with MgO to form a stable amorphous substance that filled the cracks and increased the strength after immersion in water.
- Pure MOS cement has low porosity. The hydration of MgO after immersion caused cracking as there was no space for Mg(OH)2 formation. The addition of steel slag increased the porosity of the samples, and the matrix became denser after carbonation and water immersion. Although it still had a few void regions, the average diameter of the pores decreased, enhancing the compressive strength.
- Using steel slag that partially replaced caustic calcined magnesia can reduce CO2 emissions, as an alternative to a sustainable development of Portland cement.
Author Contributions
Funding
Conflicts of Interest
References
- Olivier, J.G.J.; Janssens-Maenhout, G.; Muntean, M.; Peters, J. Trends in Global CO2 Emissions; 2014 Report; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2014; p. 62.
- Gettu, R.; Pillai, R.; Meena, J.P.; Basavaraj, A.; Vinod, D. Considerations of sustainability in the mixture proportioning of concrete for strength and durability. Spec. Publ. 2018, 326, 1–10. [Google Scholar]
- Walling, S.A.; Provis, J.L. Magnesium-based cements: A journey of 150 years, and cements for the future. Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Comparative life cycle assessment of reactive MgO and Portland cement production. J. Clean. Prod. 2017, 137, 258–273. [Google Scholar] [CrossRef]
- Damineli, B.L.; Kemeid, F.M.; Aguiar, P.S.; John, V.M. Measuring the eco-efficiency of cement use. Cem. Concr. Compos. 2010, 32, 555–562. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Gazzaniga, G.; Pastore, T. An Empathetic Added Sustainability Index (EASI) for cementitious based construction materials. J. Clean. Prod. 2019, 220, 475–482. [Google Scholar] [CrossRef]
- Zhou, X.M.; Li, Z.J. Light-weight wood-magnesium oxychloride cement composite building products made by extrusion. Constr. Build. Mater. 2012, 27, 382–389. [Google Scholar] [CrossRef] [Green Version]
- Dang, L.; Nai, X.Y.; Dong, Y.P.; Li, W. Functional group effect on flame retardancy, thermal, and mechanical properties of organophosphorus-based magnesium oxysulfate whiskers as a flame retardant in polypropylene. RSC Adv. 2017, 7, 21655–21665. [Google Scholar] [CrossRef] [Green Version]
- Runcevski, T.; Wu, C.Y.; Yu, H.F.; Yang, B.; Dinnebier, R.E. Structural characterization of a new magnesium oxysulfate hydrate cement phase and its surface reactions with atmospheric carbon dioxide. J. Am. Cream. Soc. 2013, 96, 3609–3616. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Xu, J.H.; Cui, C.Y.; Yu, C.Y.; Chang, J.; Hu, Z.Q.; Bi, W.L. Stability and phase transition of 5·1·7 phase in alkaline solutions. Constr. Build. Mater. 2020, 258, 119683. [Google Scholar] [CrossRef]
- Wang, N.; Yu, H.F.; Bi, W.L.; Tan, Y.S.; Zhang, N.; Wu, C.Y.; Ma, H.Y.; Hua, S. Effects of sodium citrate and citric acid on the properties of magnesium oxysulfate cement. Constr. Build. Mater. 2018, 169, 697–704. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chen, W.H.; Zhang, H.F.; Yu, H.F.; Zhang, W.Y.; Jiang, N.S.; Liu, L.X. The hydration mechanism and performance of Modified magnesium oxysulfate cement by tartaric acid. Constr. Build. Mater. 2017, 144, 516–524. [Google Scholar] [CrossRef]
- Wu, C.Y.; Yu, H.F.; Zhang, H.F.; Dong, J.M.; Wen, J.; Tan, Y.S. Effects of phosphoric acid and phosphates on magnesium oxysulfate cement. Mate. Struct. 2015, 48, 907–917. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.J.; Chen, T.F. Recycling of raw rice husk to manufacture magnesium oxysulfate cement based lightweight building materials. J. Clean. Prod. 2018, 191, 220–232. [Google Scholar] [CrossRef]
- Eubank, W.R. Calcination Studies of Magnesium Oxides. J. Am. Ceram. Soc. 1951, 34, 225–229. [Google Scholar] [CrossRef]
- Deng, D. The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cem. Concr. Res. 2003, 33, 1311–1317. [Google Scholar] [CrossRef]
- Guo, J.L.; Bao, Y.P.; Wang, M. Steel slag in China: Treatment, recycling, and management. Waste Manag. 2018, 78, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lu, Y.; Dong, J.; Gan, L.; Tong, Z. Roles of mineralogical phases in aqueous carbonation of steelmaking slag. Metals 2016, 6, 117. [Google Scholar] [CrossRef] [Green Version]
- Palankar, N.; Shankar, R.A.U.; Mithun, B.M.; Muhammad, B. Durability studies on eco-friendly concrete mixes incorporating steel slag as coarse aggregates. J. Clean. Prod. 2016, 129, 437–448. [Google Scholar] [CrossRef]
- Roslan, N.H.; Ismail, M.; Abdul-Majid, Z.; Ghoreishiamiri, S.; Muhammad, B. Performance of steel slag and steel sludge in concrete. Constr. Build. Mater. 2016, 104, 16–24. [Google Scholar] [CrossRef]
- Berger, R.L.; Young, J.F.; Leung, K. Acceleration of Hydration of Calcium Silicates by Carbon Dioxide Treatment. Nat. Phys. Sci. 1972, 240, 16–18. [Google Scholar] [CrossRef]
- Ghouleh, Z.; Guthrie, R.I.; Shao, Y. Production of carbonate aggregates using steel slag and carbon dioxide for carbon-negative concrete. J. CO2 Util. 2017, 18, 125–138. [Google Scholar] [CrossRef]
- Mo, L.W.; Panesar, D.K. Effects of accelerated carbonation on the microstructure of Portland cement pastes containing reactive MgO. Cem. Concr. Res. 2012, 42, 769–777. [Google Scholar] [CrossRef]
- Hänchen, M.; Prigiobbe, V.; Baciocchi, R.; Mazzotti, M. Precipitation in the Mgcarbonate system-effects of temperature and CO2 pressure. Chem. Eng. Sci. 2008, 63, 1012–1028. [Google Scholar] [CrossRef]
- Ba, M.F.; Xue, T.; He, Z.M.; Wang, H.; Liu, J.Z. Carbonation of magnesium oxysulfate cement and its influence on mechanical performance. Constr. Build. Mater. 2019, 223, 1030–1037. [Google Scholar] [CrossRef]
- Li, Q.Y.; Zhang, L.C.; Gao, X.J.; Zhang, J.Y. Effect of pulverized fuel ash, ground granulated blast-furnace slag and CO2 curing on performance of magnesium oxysulfate cement. Constr. Build. Mater. 2020, 230, 116990. [Google Scholar] [CrossRef]
- Kuenzel, C.; Zhang, F.; Ferrándiz-Mas, V.; Cheeseman, C.R.; Gartner, E.M. The mechanism of hydration of MgO-hydromagnesite blends. Cem. Concr. Res. 2018, 103, 123–129. [Google Scholar] [CrossRef]
- Davies, P.J.; Bubela, B. The transformation of nesquehonite into hydromagnesite. Chem. Geol. 1973, 12, 289–300. [Google Scholar] [CrossRef]
- Dong, J.M.; Yu, H.F.; Zhang, L.M. Study on experimental conditions of hydration methods of determining active magnesium oxide content. Int. J. Salt Lake Res. 2010, 18, 38–41. [Google Scholar]
- Gualtieri, A.F. Accuracy of XRPD QPA using the combined Rietveld-RIR method. J. Appl. Crystallogr. 2000, 33, 267–278. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Influence of particle size on the carbonation of stainless steel slag for CO2 storage. Energy Procedia 2009, 1, 4859–4866. [Google Scholar] [CrossRef] [Green Version]
- Salomão, R.; Bittencourt, L.R.M.; Pandolfelli, V.C. A novel approach for magnesia hydration assessment in refractory castables. Ceram. Int. 2007, 33, 803–810. [Google Scholar] [CrossRef]
- Humbert, P.S.; Castro-Gomes, J.P.; Savastano, H. Clinker-free CO2 cured steel slag based binder: Optimal conditions and potential applications. Constr. Build. Mater. 2019, 210, 413–421. [Google Scholar] [CrossRef]
- Sawada, Y.; Yamaguchi, J.; Sakurai, O.; Uematsu, K.; Mizutani, N.; Kato, M. Thermal decomposition of basic magcarbonates under high-pressure gas atmospheres. Thermochim. Acta 1979, 32, 277–291. [Google Scholar] [CrossRef]
- Liu, W.; Peng, X.; Liu, W. Effect mechanism of the iso-propanol substituent on amine collectors in the flotation of quartz and magnesite. Powder. Technol. 2020, 360, 1117–1125. [Google Scholar] [CrossRef]
- Bruni, S.; Cariati, F.; Fermo, P.; Pozzi, A.; Toniolo, L. Characterization of ancient magnesian mortars coming from northern Italy. Thermochim. Acta 1998, 321, 161–165. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. Impact of hydrated magnesium carbonate additives on the carbonation of reactive MgO cements. Cem. Concr. Res. 2013, 54, 87–97. [Google Scholar] [CrossRef]
- García-González, C.A.; Andrade, C.; Alonso, M.C.; Fraile, J.; López-Periago, A.; Domingo, C. Modification of composition and microstructure of Portland cement pastes as a result of natural and supercritical carbonation procedures. Ind. Eng. Chem. Res. 2006, 45, 4985–4992. [Google Scholar] [CrossRef]
- Mo, L.W.; Panesar, D.K. Accelerated carbonation—A potential approach to sequester CO2 in cement paste containing slag and reactive MgO. Cem. Concr. Compos. 2013, 43, 69–77. [Google Scholar] [CrossRef]
- Hummel, W.; Berner, U.; Curti, E.; Pearson, F.J.; Thoenen, T. Nagra/PSI chemical thermodynamic data base 01/01. Radiochim. Acta 2002, 90, 805–813. [Google Scholar] [CrossRef] [Green Version]
- Helgeson, H.C.; Delany, J.M.; Nesbitt, H.W.; Bird, D.K. Summary and critique of the thermodynamic properties of rock-forming minerals. Am. J. Sci. 1978, 278, 1–229. [Google Scholar]




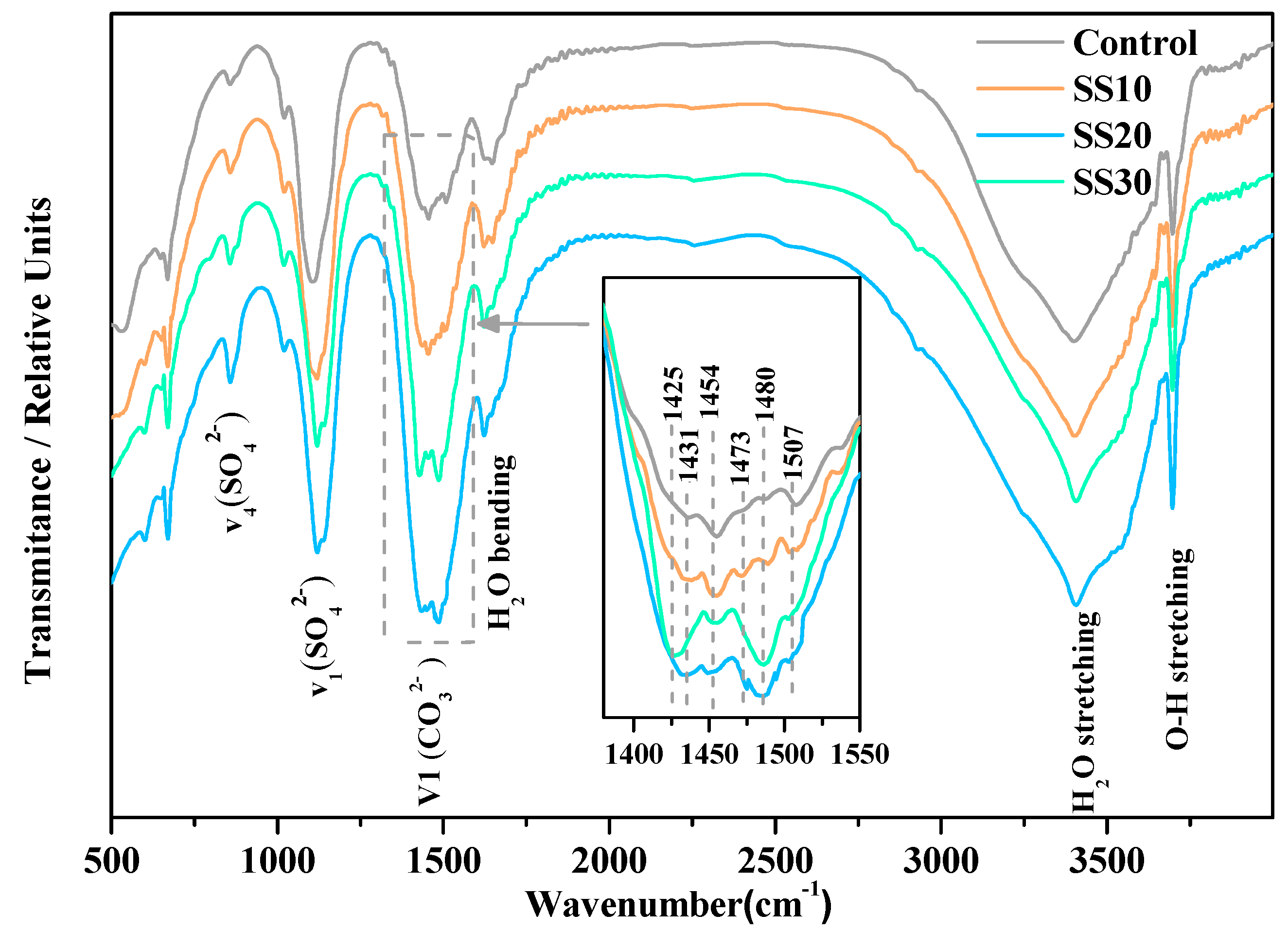


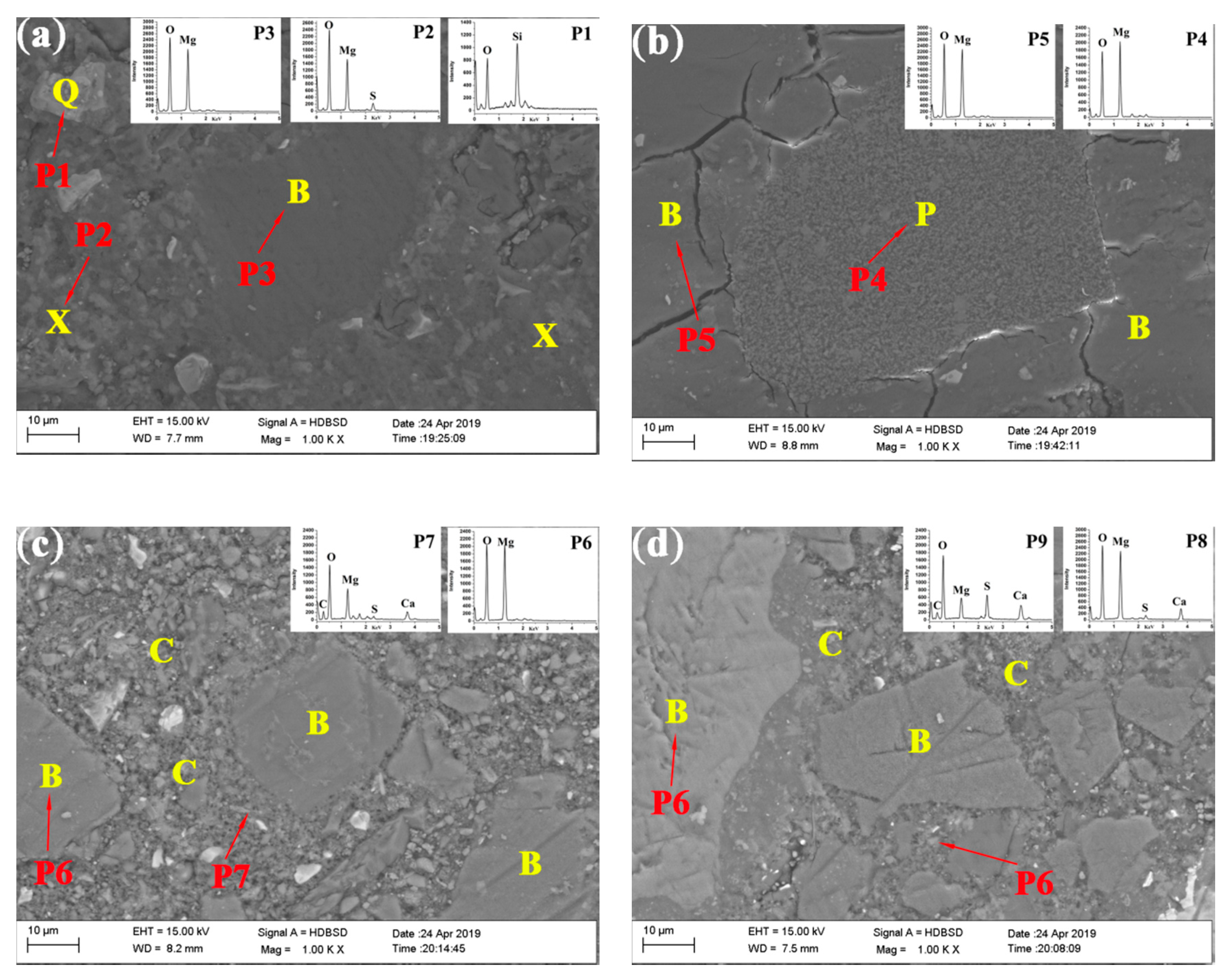

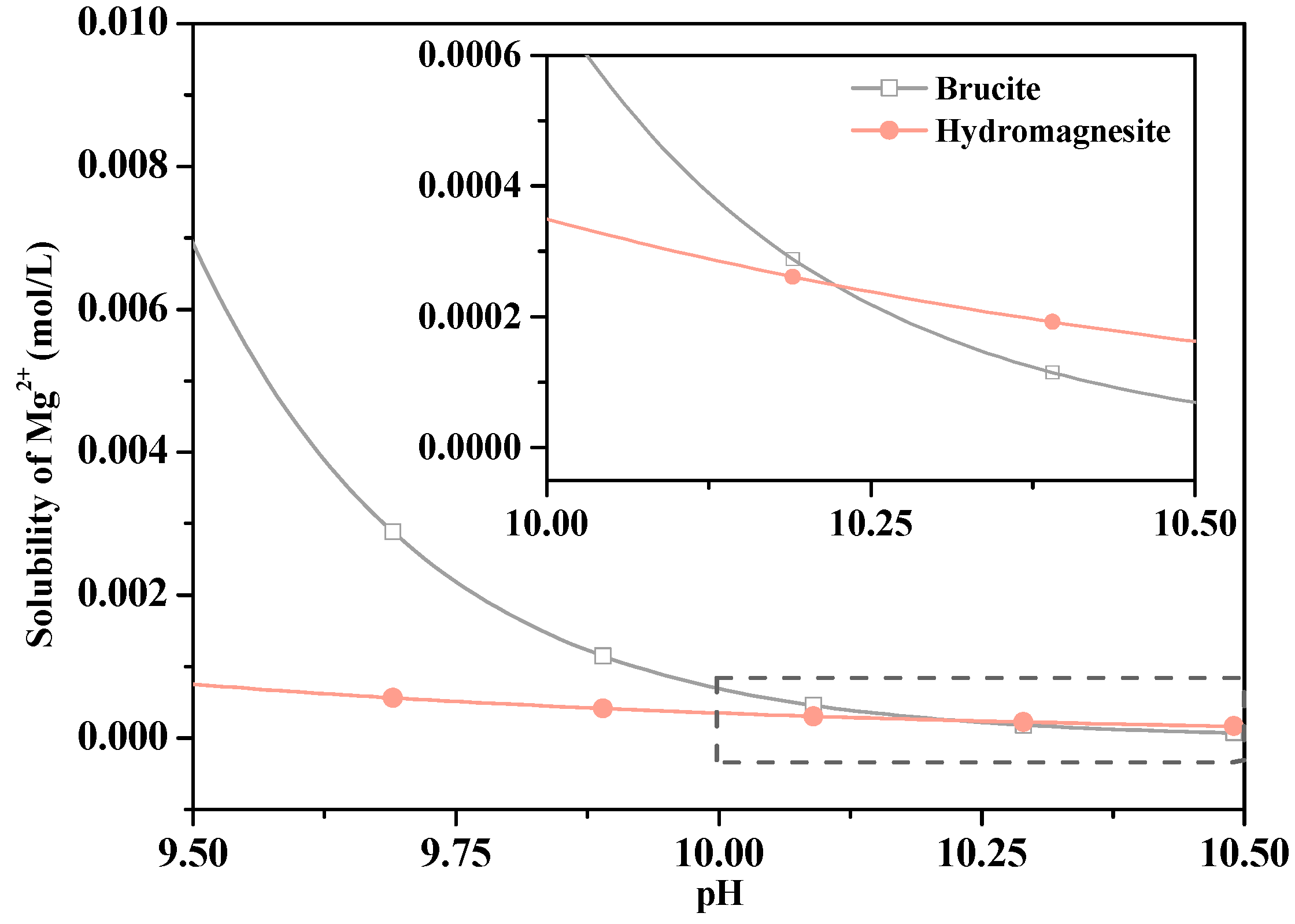
| Content (wt.%) | Caustic Calcined Magnesia | Steel Slag |
|---|---|---|
| MgO | 85.6 | 9.19 |
| CaO | 2.46 | 37.85 |
| Fe2O3 | 1.60 | 25.00 |
| Al2O3 | 0.23 | 5.13 |
| SiO2 | 5.56 | 18.20 |
| LOI | 4.50 | 1.80 |
| Density (g/cm3) | 2.94 | 3.45 |
| Water absorption (wt.%) | 20.17 | 4.87 |
| Specific surface area (m2/g) | 25.3 | 15.7 |
| Component | Content (wt.%) | Rwp | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MgO | MgCO3 | SiO2 | C2S | C4AF | CaO | FeO | ACn | ||
| Caustic calcined Magnesia | 80 | 9.9 | 1.6 | - | - | - | - | 8.5 | 5.766 |
| Steel slag | 5.9 | - | - | 27.9 | 17.5 | 3.9 | 2.6 | 42.5 | 7.183 |
| Sample No. | Mixture Design (wt.%) | ||||
|---|---|---|---|---|---|
| Steel Slag | Caustic Calcined Magnesia | MgSO4·7H2O | Water | Citric Acid | |
| Control | 0 | 100 | 50 | 50 | 0.5 |
| SS10 | 10 | 90 | 45 | 50 | 0.5 |
| SS20 | 20 | 80 | 40 | 50 | 0.5 |
| SS30 | 30 | 70 | 35 | 50 | 0.5 |
| SS40 | 40 | 60 | 30 | 50 | 0.5 |
| SS60 | 60 | 40 | 20 | 50 | 0.5 |
| Sample | Period (days) | Phase Content (wt.%) | Rwp(%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 517 Phase | Periclase | Brucite | Magnesite | Quartz | Calcite | Gypsum | C2S | C4AF | ACn | |||
| Control | 3 | 19.8 | 28.1 | 5.3 | 5.6 | 0.9 | - | - | - | - | 40.3 | 7.079 |
| 28 | 20.1 | 13.3 | 15.7 | 6.3 | 0.8 | - | - | - | - | 43.8 | 7.568 | |
| 28a | 22.9 | 7.7 | 18.9 | 6.1 | 1.1 | - | - | - | - | 43.3 | 8.598 | |
| SS10 | 3 | 13.6 | 22.1 | 2.4 | 8.8 | 0.7 | 0.7 | 1.5 | 2.1 | - | 48.1 | 7.134 |
| 28 | 15.9 | 15.1 | 8.7 | 8.1 | 0.6 | 1.1 | 1.9 | 0.5 | - | 48.1 | 6.994 | |
| 28a | 15.6 | 7.9 | 17.0 | 6.7 | 0.4 | 0.5 | 2.1 | 0.1 | - | 49.7 | 9.164 | |
| SS20 | 3 | 0.3 | 26.1 | 6.6 | 7.6 | 0.8 | 1.3 | 1.7 | 4.8 | 6.3 | 44.5 | 7.129 |
| 28 | 0.5 | 20.2 | 10.7 | 7.1 | 1.1 | 1.6 | 3.1 | 2.3 | 2.3 | 51.1 | 8.567 | |
| 28a | 3.7 | 19.1 | 13.1 | 7.2 | 0.7 | 0.6 | 2.7 | 0.9 | 2.1 | 49.9 | 8.131 | |
| SS30 | 3 | - | 24.1 | 5.7 | 6.2 | 0.6 | 0.9 | 3.2 | 9.3 | 5.1 | 44.9 | 7.213 |
| 28 | 0.5 | 18.8 | 7.8 | 6.7 | 0.3 | 1.2 | 5.2 | 3.2 | 3.3 | 53.0 | 7.466 | |
| 28 a | 1.1 | 18.7 | 8.7 | 5.6 | 0.5 | 1.4 | 5.1 | 2.3 | 1.1 | 55.3 | 8.586 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Guan, Y.; Chang, J.; Bi, W.; Zhang, T. Effect of Carbonation on the Water Resistance of Steel Slag—Magnesium Oxysulfate (MOS) Cement Blends. Materials 2020, 13, 5006. https://doi.org/10.3390/ma13215006
Hu Z, Guan Y, Chang J, Bi W, Zhang T. Effect of Carbonation on the Water Resistance of Steel Slag—Magnesium Oxysulfate (MOS) Cement Blends. Materials. 2020; 13(21):5006. https://doi.org/10.3390/ma13215006
Chicago/Turabian StyleHu, Zhiqi, Yan Guan, Jun Chang, Wanli Bi, and Tingting Zhang. 2020. "Effect of Carbonation on the Water Resistance of Steel Slag—Magnesium Oxysulfate (MOS) Cement Blends" Materials 13, no. 21: 5006. https://doi.org/10.3390/ma13215006





