Creating Strong Titanium/Titanium Hydride Brown Bodies at Ambient Pressure and Moderate Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. Mold
2.1.2. Experimental Conditions
2.1.3. Firing
2.2. Characterization and Analysis
3. Results
3.1. Visual Observations
3.2. X-Ray Diffraction Phase Analysis
3.3. Microstructural Analysis Using Scanning Electron Microscopy
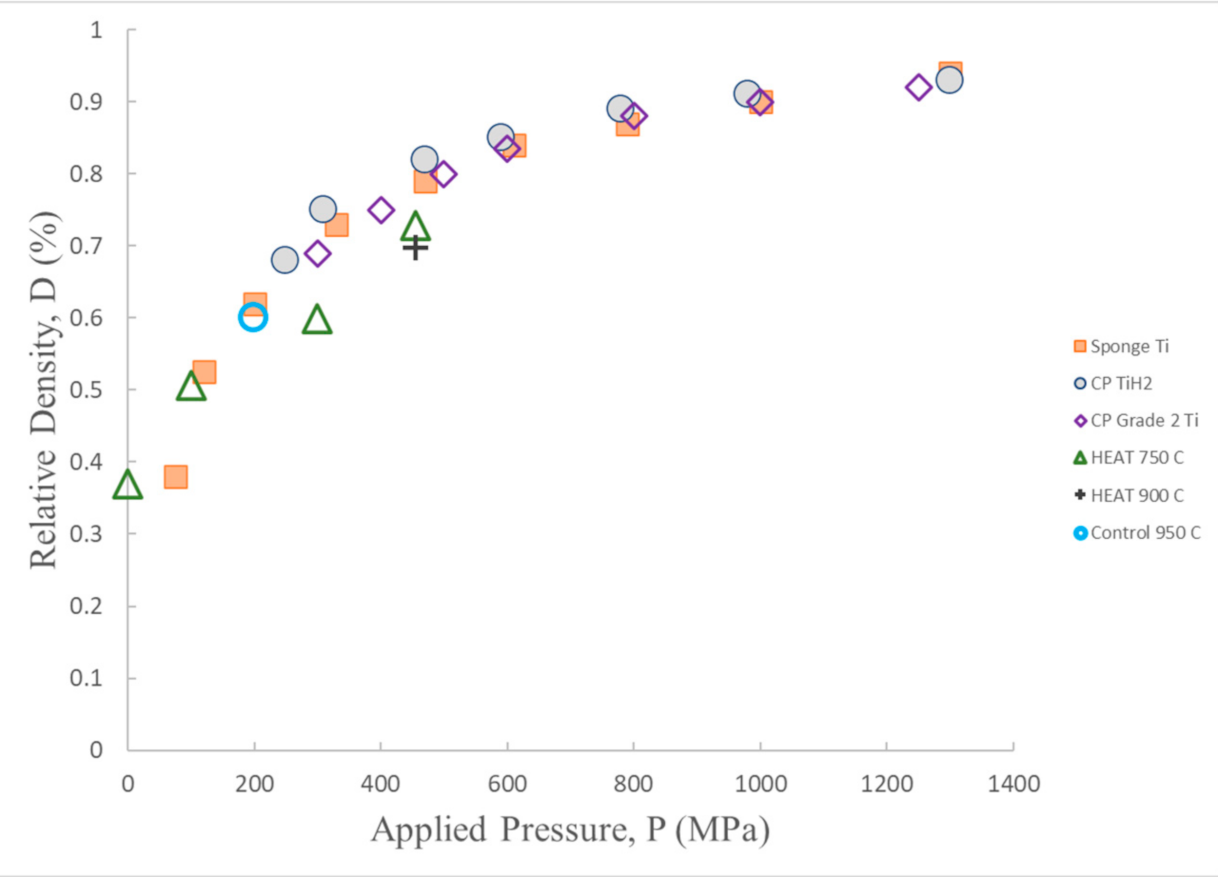
3.4. Density vs. Applied Pressure Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexandrov, D.V. On the theory of Ostwald ripening: Formation of the universal distribution. J. Phys. A 2014, 48, 035103. [Google Scholar] [CrossRef]
- Lifshitz, I.M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Wei, T.C.; Phillips, J. Thermal and catalytic etching: Mechanisms of metal catalysts reconstruction. Adv. Catal. 1996, 41, 359–421. [Google Scholar]
- Wu, N.L.; Phillips, J. Sintering of silica-supported platinum catalysts during ethylene oxidation. J. Catal. 1988, 113, 129–143. [Google Scholar] [CrossRef]
- Phillips, J. System and Methods for Low Temperature Metal Printing. US Patent 10,648,058, 12 May 2020. [Google Scholar]
- Daniels, Z.; Phillips, J. Reduction Expansion Synthesis of Sintered Metal. US Patent 16/712, 18 June 2020. [Google Scholar]
- Zea, H.; Luhrs, C.C.; Phillips, J. Reductive/expansion synthesis of zero valent submicron and nanometal particles. J. Mater. Res. 2014, 26, 672. [Google Scholar] [CrossRef]
- Luhrs, C.C.; Kane, M.; Leseman, Z.; Phillips, J. Novel process for solid state reduction of metal oxides and hydroxides. Metall. Mater. Trans. B 2013, 44, 115. [Google Scholar] [CrossRef]
- Luhrs, C.C.; Leseman, Z.; Phillips, J.; Zea, H.R. Generation of Metal and Alloy Micron, Submicron or Nano Particles in Simple, Rapid Process. U.S. Patent 8,709,126, 29 April 2014. [Google Scholar]
- Luhrs, C.C.; Phillips, J. Reductive/Expansion Synthesis of Graphene. U.S. Patent 8,894,886, 25 November 2014. [Google Scholar]
- Lee, T.T.; Adams, R.A.; Luhrs, C.C.; Arora, A.; Pol, V.G.; Wu, C.H.; Phillips, J. High stability tin/carbon battery electrodes produced using reduction expansion synthesis. Carbon 2018, 132, 411. [Google Scholar] [CrossRef]
- Elbaz, L.; Phillips, J.; Artyushkova, K.; More, K.; Brosha, E.L. Evidence of high electrocatalytic activity of molybdenum carbide supported platinum nanorafts. J. Electrochem. Soc. 2015, 162, 681–685. [Google Scholar] [CrossRef]
- Plear, C.; Greenaway, K.; Zea, H.; Wu, C.H.; Luhrs, C.C.; Phillips, J. Novel chemical process for producing chrome coated metal. Materials 2018, 11, 78. [Google Scholar] [CrossRef]
- Phillips, J. Chemical Methods to Create Metal Films on Metal and Ceramic Substrates. US Patent 10,273,582, 30 April 2019. [Google Scholar]
- Daniels, Z.; Rydalch, W.; Ansell, T.; Luhrs, C.C.; Phillips, J. Reduction expansion synthesis of sintered metal. Materials 2019, 12, 2890. [Google Scholar] [CrossRef]
- Rydalch, W. Reduction Expansion Synthesis of Sintered Metal. Master’s Thesis, Naval Postgraduate School, Monterey, CA, USA, 2019. [Google Scholar]
- Lutjering, G.; Williams, J.C. Titanium; Springer: Berlin, Germany, 2007. [Google Scholar]
- Inagki, I.; Takechi, T.; Shirai, Y.; Ariyasu, N. Application and features of titanium for the aerospace industry. Nippon Steel Sumimoto Metal 2014, 106, 22–27. [Google Scholar]
- Boyer, R. Attributes, characteristics and applications of titanium and its alloys. J. Mater. 2010, 62, 21–24. [Google Scholar] [CrossRef]
- Ewart, P.D. The use of particulate injection molding for fabrication of sports and leisure equipment from titanium metals. Proceedings 2018, 2, 254. [Google Scholar] [CrossRef]
- German, R.M. Progress in titanium metal powder injection molding. Materials 2013, 6, 3641. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Xiao, S.; Chen, Y. Sintering densification of titanium hydride powders. Mater. Manuf. Process. 2017, 32, 517. [Google Scholar] [CrossRef]
- Hedayati, A. Fabrication and Properties of TiH2 and Ti Blends for Powder Metallurgy Ti Products. Master’s Thesis, The University of New South Wales, Wales, UK, 2012. [Google Scholar]
- Ileana, C.; Stefan, G.; Ilie, D.; Claudiu, N. Aspects about sintering behavior of a titanium hydride powder based alloy used for automotive components. Appl. Mech. Mat. 2016, 823, 467. [Google Scholar]
- Lee, D.W.; Lee, H.S.; Park, J.H.; Shin, S.M.; Wang, J.P. Sintering of titanium hydride powder compaction. Proc. Manuf. 2015, 2, 550. [Google Scholar] [CrossRef]
- Phillips, J.; Clausen, B.; Dumesic, J.A. Iron pentacarbonyl decomposition over grafoil production of small metallic iron particles. J. Phys. Chem. 1980, 84, 1814–1822. [Google Scholar] [CrossRef]
- Phillips, J.; Dumesic, J.A. Iron pentacarbonyl decomposition over grafoil: II. Effect of sample outgassing on decomposition kinetics. Appl. Surf. Sci. 1981, 7, 215–230. [Google Scholar] [CrossRef]
- Machaka, R.; Chickwanda, H.K. Analysis of the cold compaction behavior of titanium powders: A comprehensive inter-model comparison study of compaction equations. CrossMark 2015, 46, 4285–4297. [Google Scholar] [CrossRef]
- Machaka, R.; Chickwanda, H.K. An experimental evaluation of the gerdemann–jablonski compaction equation. Metall. Mater. Trans. A 2015, 46, 2194–2200. [Google Scholar] [CrossRef]
- Frykholm, R.; Brash, B. Press and sintering of titanium. Key Eng. Mater. 2016, 704, 369. [Google Scholar] [CrossRef]
- Esen, Z.; Tarhan Bor, E.; Bor, S. Characterization of loose powder sintered porous titanium and Ti6Al4V alloy. Turk. J. Eng. Environ. Sci. 2009, 33, 207–219. [Google Scholar]
- San-Martin, A.; Manchester, F.D. The H-Ti (hydrogen-titanium) system. Bull. Alloy Phase Diagr. 1987, 8, 30–42. [Google Scholar] [CrossRef]
- Denquin, A.; Naka, S. Phase transformation mechanisms involved in two-phase TiAl-based alloys–I. Lamellar structure formation. Pergamon 1996, 44, 343–352. [Google Scholar]
- Paneth, F.; Hofeditz, W. Über die Darstellung von freiem Methyl. Chem. Eur. 1929, 62, 1335. [Google Scholar] [CrossRef]
- Paneth, F.; Lautsch, W. Isolation of radical ethyl. Nature 1930, 125, 564. [Google Scholar] [CrossRef]
- Rice, F.O.; Glasebrook, A.L. The thermal decomposition of organic compounds from the standpoint of free radicals. XI. The methylene radical. J. Am. Chem. Soc. 1934, 56, 2381. [Google Scholar] [CrossRef]
- Pearson, T.G.; Purcell, R.H.; Saigh, G.H. Methylene. J. Chem. Soc. 1938, 82, 409. [Google Scholar] [CrossRef]
- Pearson, T.G.; Robinson, P.L.; Stoddart, E.M. The behavior of metals, particularly lead and bismuth, in atomic hydrogen, and attempts to prepare atomic hydrogen from hydrides. Proc. R. Soc. Lond. 1933, 142, 275–285. [Google Scholar]
- Hiraoka, H. Selective removal of metal atoms in hydrogen reactive ion-etching. J. Vac. Sci. Techol. 1986, 4, 345. [Google Scholar] [CrossRef]
- Chou, C.H.; Phillips, J. Tin foil reconstruction in hydrogen plasma. J. Vac. Sci. Technol. A 1990, 8, 3941. [Google Scholar] [CrossRef]
- Wu, N.L.; Phillips, J. Catalytic etching of platinum during ethylene oxidation. J. Phys. Chem. 1985, 89, 591–600. [Google Scholar] [CrossRef]
- Wu, N.L.; Phillips, J. Reaction-enhanced sintering of platinum thin films during ethylene oxidation. J. Appl. Phys. 1986, 59, 769. [Google Scholar] [CrossRef]
- Hess, J.M.; Phillips, J. Catalytic etching of Pt/Rh guazes. J. Catal. 1992, 136, 149. [Google Scholar] [CrossRef]
- Dean, V.W.; Frenklach, M.; Phillips, J. Catalytic etching of platinum foils and thin films in hydrogen-oxygen mixtures. J. Phys. Chem. 1988, 92, 5731–5738. [Google Scholar] [CrossRef]
- Kaess, U.; Majera, G.; Stolla, M.; Peterson, T.; Barnes, R. Hydrogen and deuterium diffusion in titanium dihydrides/dideuterides. J. Alloys Compd. 1997, 259, 74. [Google Scholar] [CrossRef]
- Weigle, J.C.; Phillips, J. Modeling hydrogen spillover in dual bed catalytic reactors. AIChE 2004, 50, 821. [Google Scholar] [CrossRef]
- Weigle, J.C.; Phillips, J. Novel dual bed reactors: Utilization of hydrogen spillover in reactor design. Langmuir 2004, 20, 1189–1193. [Google Scholar] [CrossRef]
- Change, H.; Heck, R.; Phillips, J. Catalytic synergism in physical mixtures. Langmuir 1996, 12, 2756. [Google Scholar] [CrossRef]
- Chang, H.; Phillips, J. Catalytic synergism in physical mixtures of supported iron-cerium and supported nobel metal for hydroisomerization of 1,3-butadiene. Langmuir 1977, 13, 477. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Radovic, L.R.; Xia, B.; Phillips, J. Low-temperature generation of basic carbon surfaces by hydrogen spillover. J. Phys. Chem. 1996, 100, 17243–17248. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Sun, P.; Wang, H. Hydrogen sintering of titanium to produce high density fine grain titanium alloys. Adv. Eng. Mater. 2012, 14, 383–384. [Google Scholar] [CrossRef]
- Beranoagirre, A.; Olvera, D.; López de Lacalle, L.N. Milling of gamma titanium–aluminum alloys. Int. J. Adv. Manuf. Technol. 2012, 62, 83–88. [Google Scholar] [CrossRef]
- Beranoagirre, A.; Urbikain, G.; Calleja, A.; Lopez de Lacalle, L.N. Drilling process in.-TiAl intermetallic alloys. Materials 2018, 11, 2379. [Google Scholar] [CrossRef]
- Banhout, J. Manufacture, characterization and applications of cellular metal and metal foams. Prog. Mater. Sci. 2001, 46, 559. [Google Scholar]
- Salimon, A.; Brechet, Y.; Ashby, M.E.; Greer, A.L. Potential applications for steel and titanium metal foam. J. Mater. Sci. 2005, 40, 5793. [Google Scholar] [CrossRef]
- Evans, A.G.; Hutchinson, J.W.; Ashby, M.F. Cellular metals. Sol. State Mater. Sci. 1998, 3, 288–303. [Google Scholar] [CrossRef]
- Tuchinsky, L.; Loutfy, R. Titanium Foams for Medical Applications. Materials & Processes for Medical Devices Conference, Anaheim, CA, USA, 10 September 2003; pp. 377–381. [Google Scholar]
- Zhao, C.Y.; Kim, T.; Lu, T.J.; Hodson, J.P. Thermal transport in high porosity cellular metal foams. J. Therm. Heat Trans. 2004, 18, 309–317. [Google Scholar] [CrossRef]
- Nazar, A.; Abate, K.M.; Kumar, A.; Jeng-Ywan, J. A state-of-the-art review of types, design, optimization and additive manufacturing of cellular structures. Int. J. Adv. Manuf. Technol. 2019, 104, 3489. [Google Scholar] [CrossRef]
- Kashef, S.; Asgari, A.; Hilditch, T.B.; Yan, W.; Goel, V.K.; Hodgson, P.D. Fatigue crack growth behavior of titanium foams for medical applications. Mater. Sci. Eng. A 2011, 528, 1602–1607. [Google Scholar] [CrossRef]
- Kashef, S.; Asgari, A.; Hilditch, T.B.; Yan, W.; Goel, V.K.; Hodgson, P.D. Fracture toughness of titanium foams for medical applications. Mater. Sci. Eng. A 2010, 527, 7689–7693. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tassou, S.A.; Lu, T.J. Analytical considerations of thermal radiation in cellular metal foams with open cells. Int. J. Heat Mass Trans. 2008, 51, 929–940. [Google Scholar] [CrossRef]
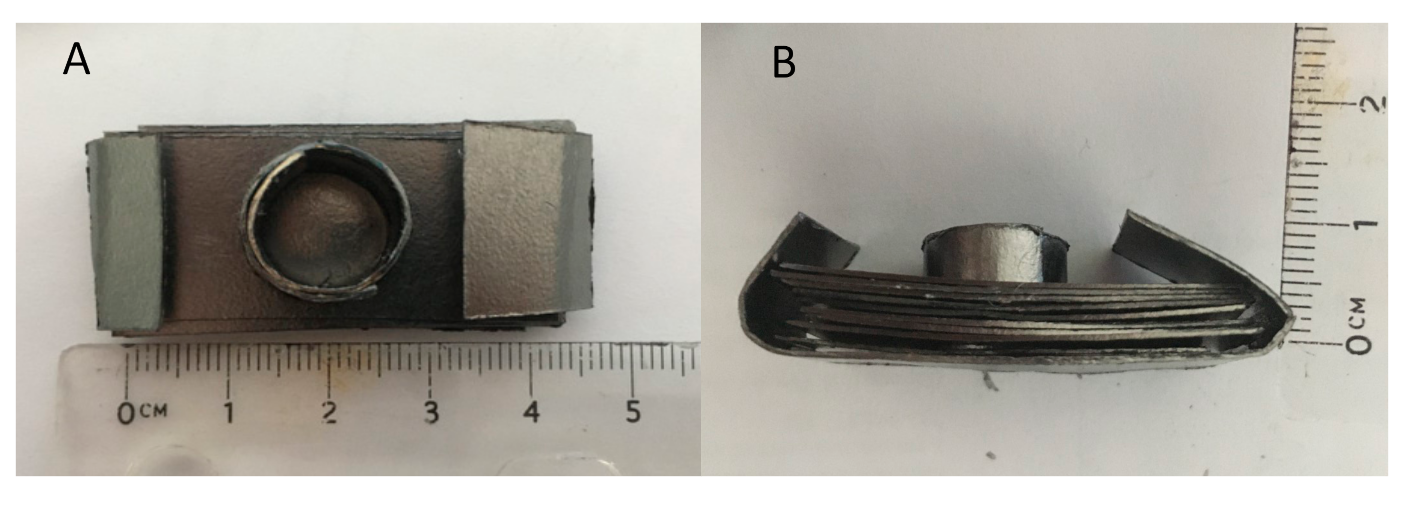
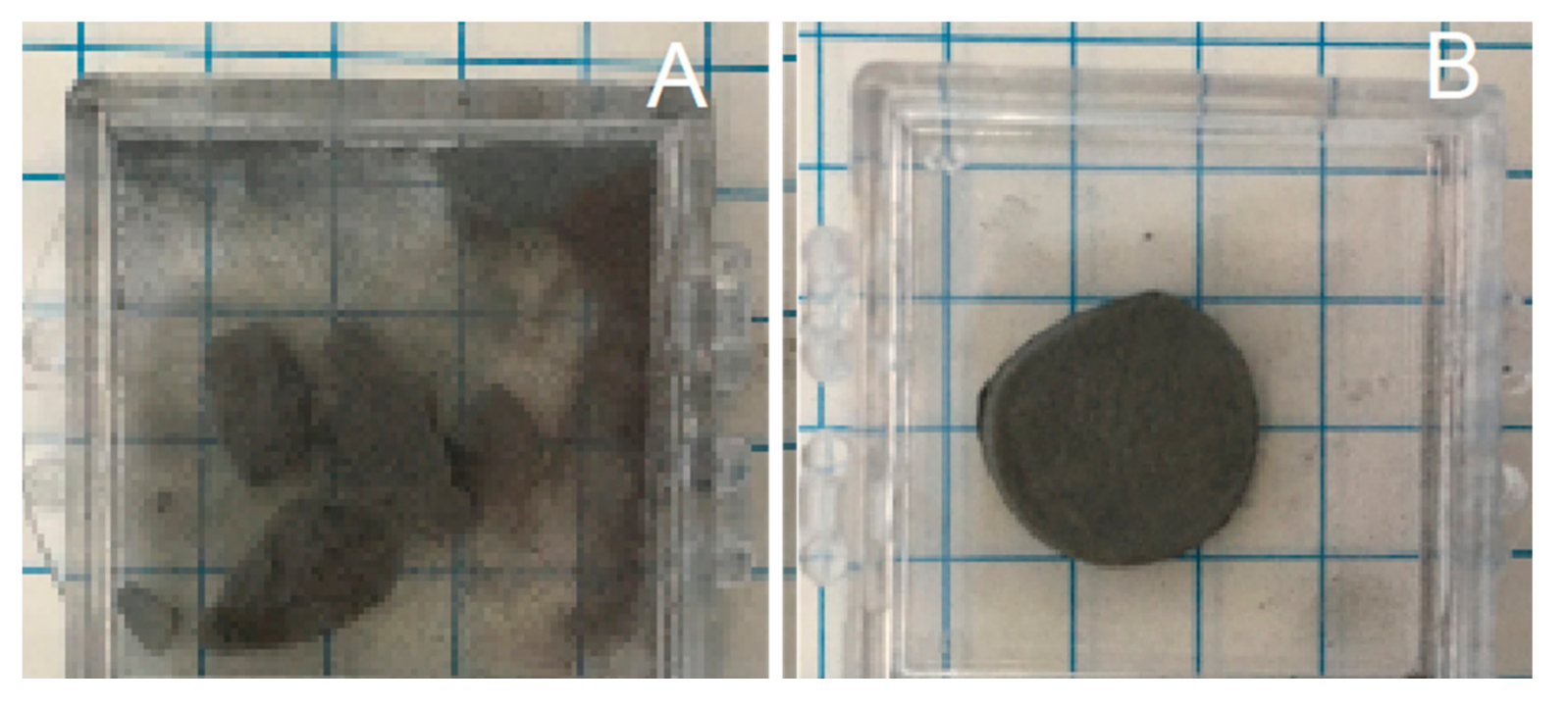

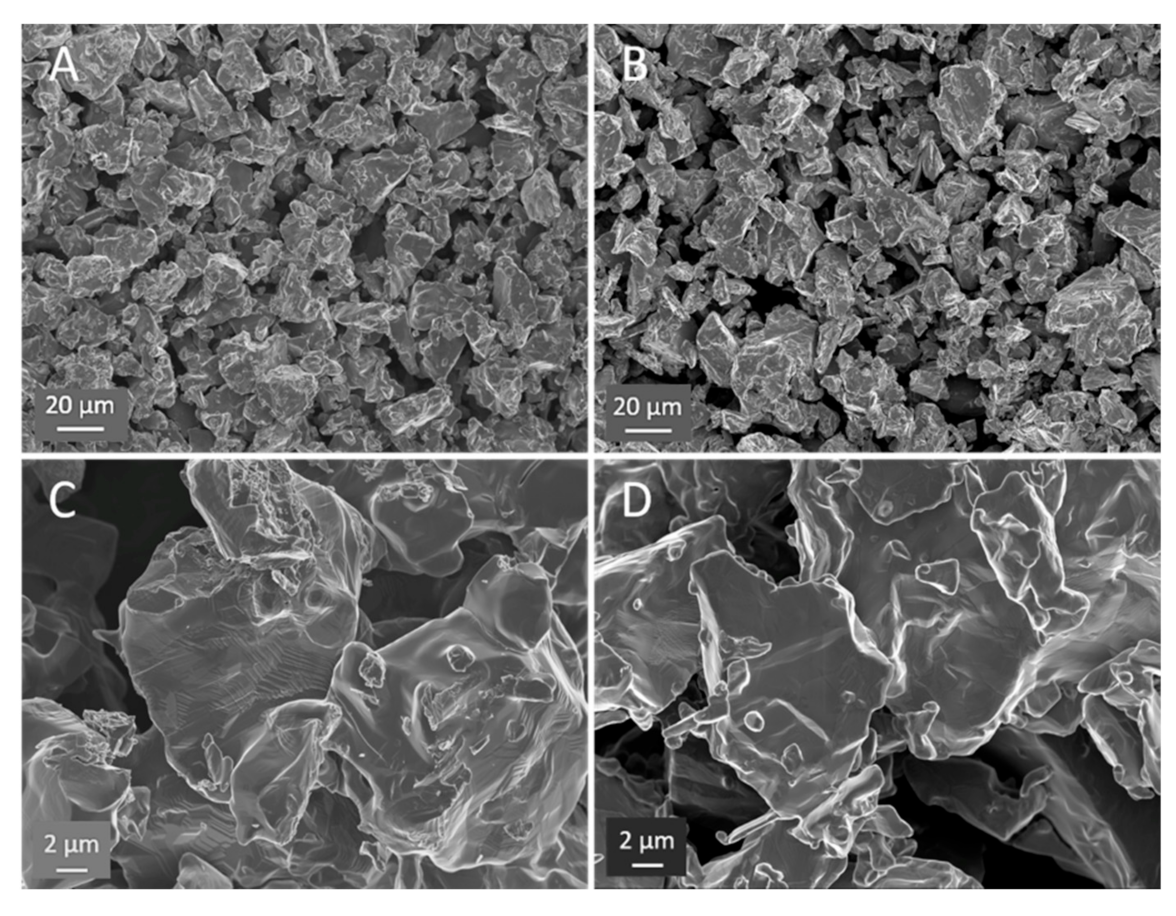

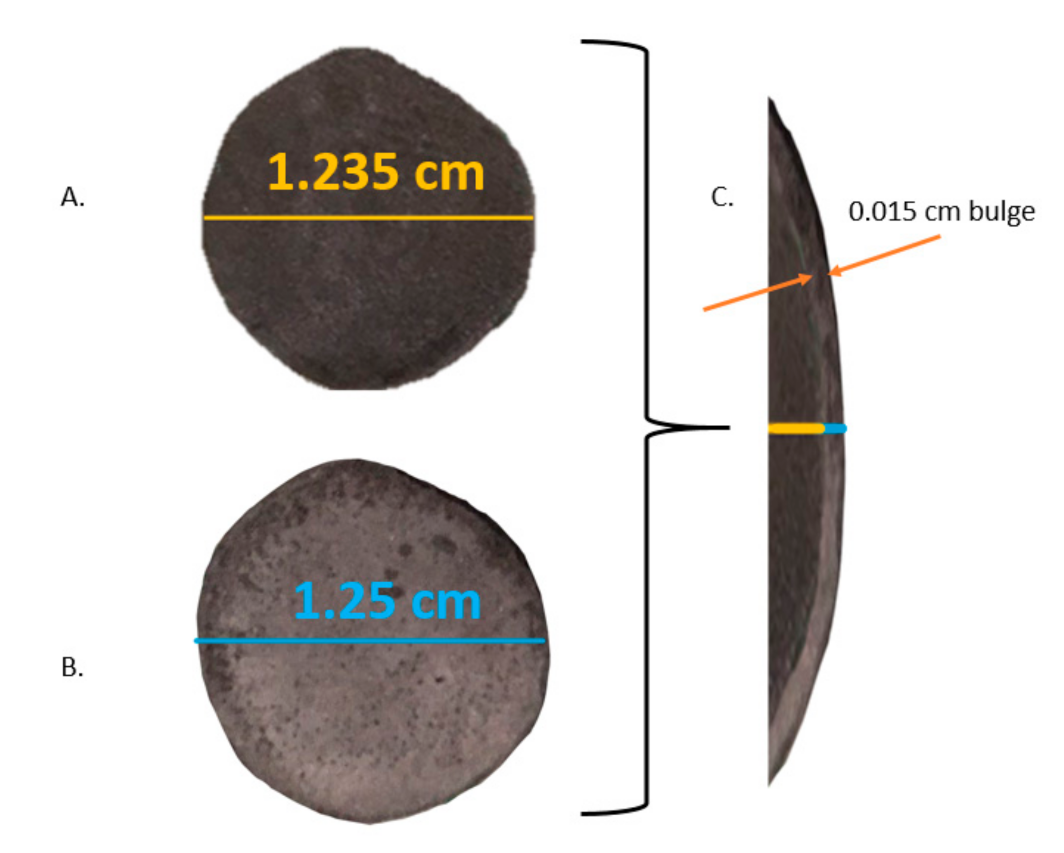

| Test | Firing Temp (°C) | Flow | Key Visual Observations—Raw | Key Visual Observations— Post-(5000 Atm) Compression Test |
|---|---|---|---|---|
| 1 | 650 | Ar/H2 | Solid | Metallic Bonded Solid/modest shape change/SBB |
| 2 | 650 | Ar | Powder | - |
| 3 | 650 | Ar | Powder | - |
| 4 | 650 | Ar/H2 | Solid | Metallic Bonded Solid/modest shape change (5000 Atm)/SBB |
| 5 | 550 | Ar/H2 | Unstable solid | - |
| 6 | 750 | Ar/H2 | Solid | Metallic Bonded Solid/modest shape change(5000 Atm)/SBB |
| 7 | 750 | Ar | Powder | Only powder after compression (5000 Atm). |
| 8 | 850 | Ar/H2 | Solid | Metallic Bonded Solid/modest shape change (5000 Atm)/SBB |
| 9 | 950 | Ar/H2 | Solid | Metallic Bonded Solid/modest shape change(5000 Atm)/SBB |
| 10 | 850 | Ar | Solid | Only powder remains |
| 11 | 850 | Ar | Solid | Retest of 10, only powder remained (confirmation) |
| 12 | 750 | Ar/H2 | Solid | Metallic Bonded Solid/modest shape change (1000 Atm)/SBB |
| 13 | 750 | Ar/H2 | Solid | Metallic Bonded Solid/modest shape change (3000 Atm)/SBB |
| 14 | 900 | Ar/H2 | Solid | Metallic Bonded Solid/modest shape change(5000 Atm)/SBB |
| 15 | 950 | Ar | Solid | Cracked Solid (1800 Atm) |
| Test | Firing Temp (°C) | Density (g/cm3) | Fraction Solid |
|---|---|---|---|
| 1 | 650 | 1.44 | 0.32 |
| 6 | 750 | 1.786 | 0.396 |
| 8 | 850 | 1.919 | 0.426 |
| 12 | 750 | 1.659 | 0.368 |
| 13 | 750 | 1.652 | 0.367 |
| 14 | 900 | 1.668 | 0.370 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, J.; Janssen, A.; Y. Ansell, T.; C. Luhrs, C. Creating Strong Titanium/Titanium Hydride Brown Bodies at Ambient Pressure and Moderate Temperatures. Materials 2020, 13, 5008. https://doi.org/10.3390/ma13215008
Phillips J, Janssen A, Y. Ansell T, C. Luhrs C. Creating Strong Titanium/Titanium Hydride Brown Bodies at Ambient Pressure and Moderate Temperatures. Materials. 2020; 13(21):5008. https://doi.org/10.3390/ma13215008
Chicago/Turabian StylePhillips, Jonathan, Anthony Janssen, Troy Y. Ansell, and Claudia C. Luhrs. 2020. "Creating Strong Titanium/Titanium Hydride Brown Bodies at Ambient Pressure and Moderate Temperatures" Materials 13, no. 21: 5008. https://doi.org/10.3390/ma13215008
APA StylePhillips, J., Janssen, A., Y. Ansell, T., & C. Luhrs, C. (2020). Creating Strong Titanium/Titanium Hydride Brown Bodies at Ambient Pressure and Moderate Temperatures. Materials, 13(21), 5008. https://doi.org/10.3390/ma13215008







