Effect of a Boric Acid Corrosive Environment on the Microstructure and Properties of Concrete
Abstract
1. Introduction
2. Materials
2.1. Raw Materials and Preparation of Cement
2.2. Concrete Size and Mix Proportion
2.3. Boric Acid Curing Stage
3. Methods
3.1. Mass Change and Loss Rate of Compressive Strength
3.2. Depth of Neutralization
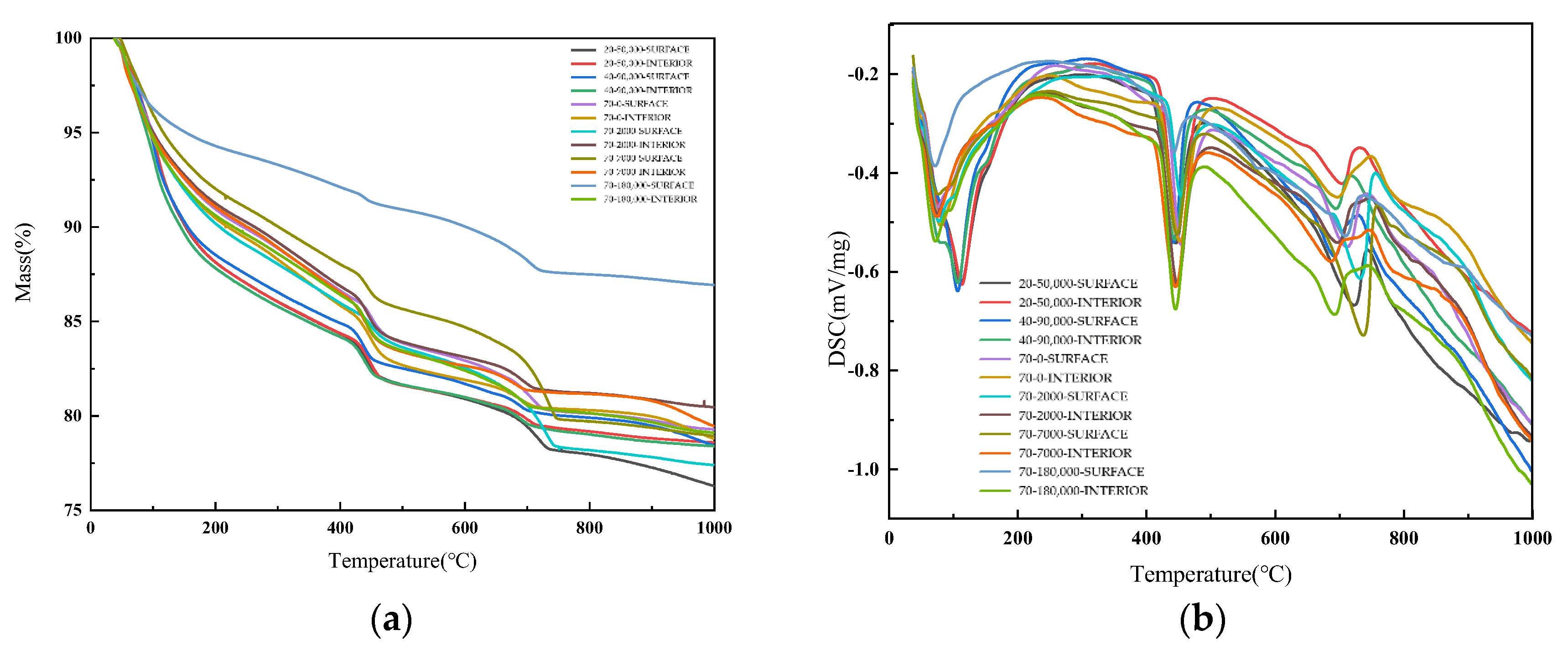
3.3. XRD and TG-DSC Analysis of Mortar
3.4. Pore Structure of Concrete
3.5. Microstructure of Concrete
4. Results and Discussion
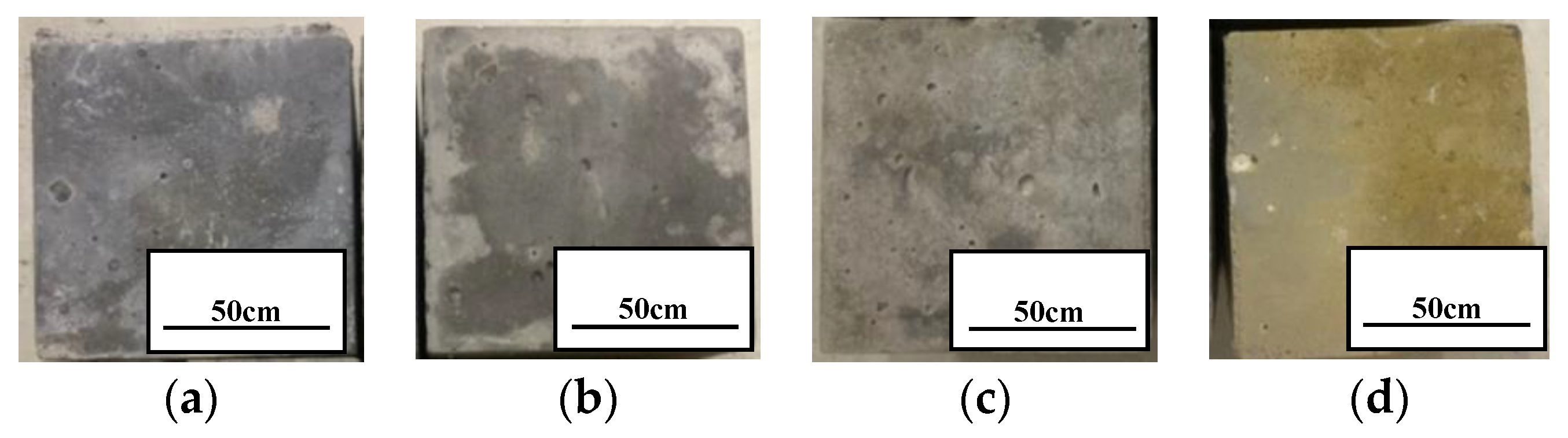
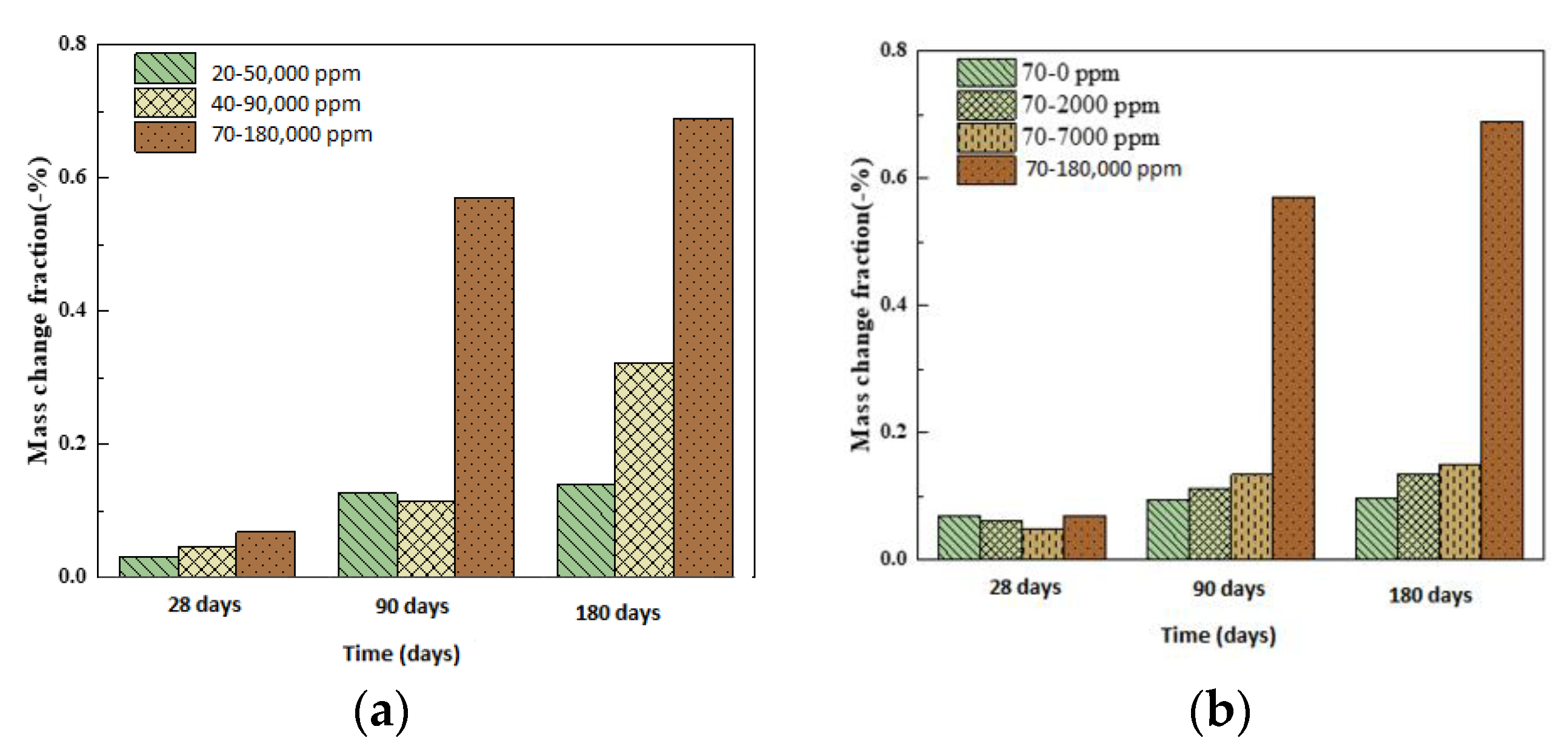
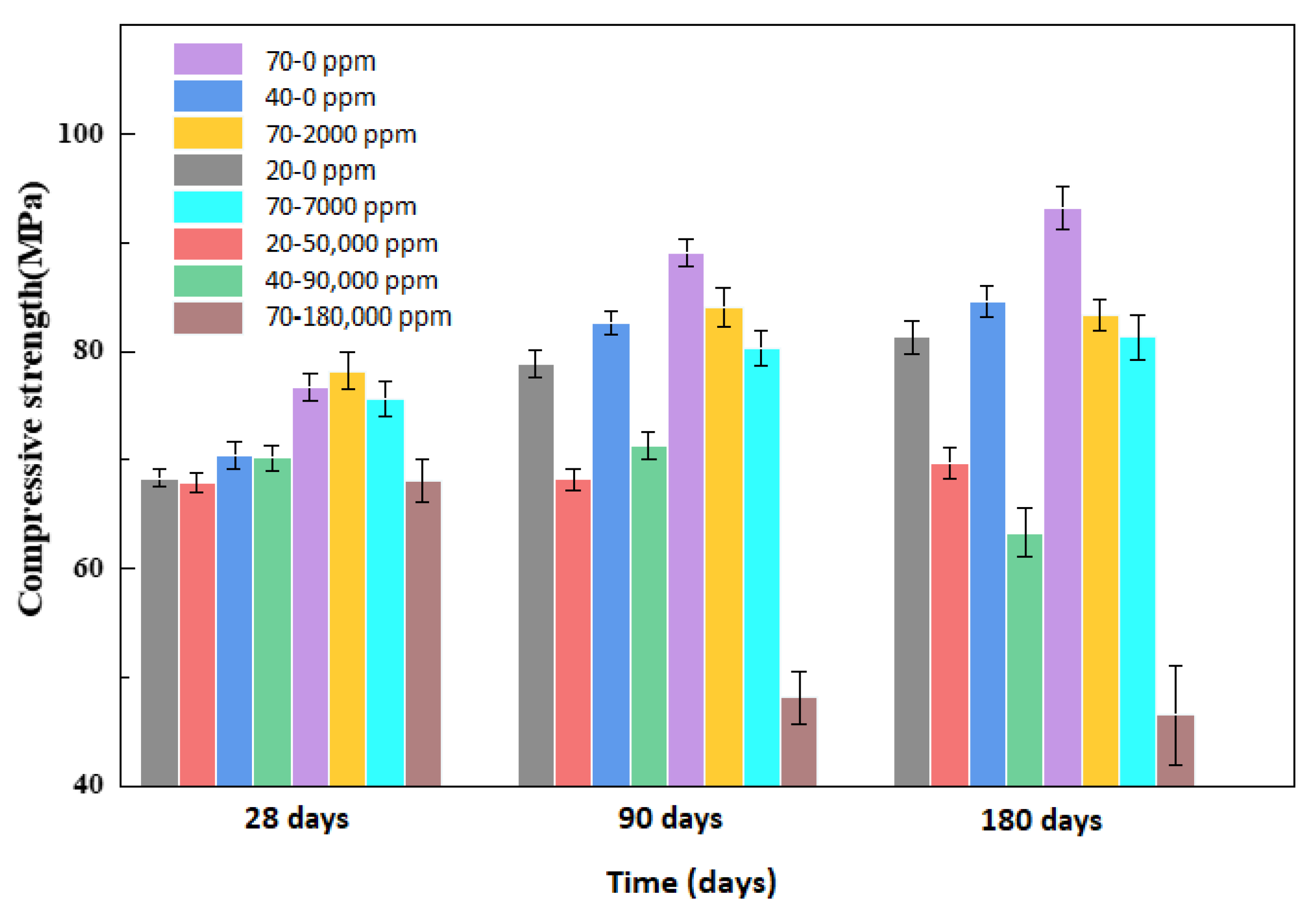
4.1. Mass and Compressive Strength
4.2. Depth of Neutralization
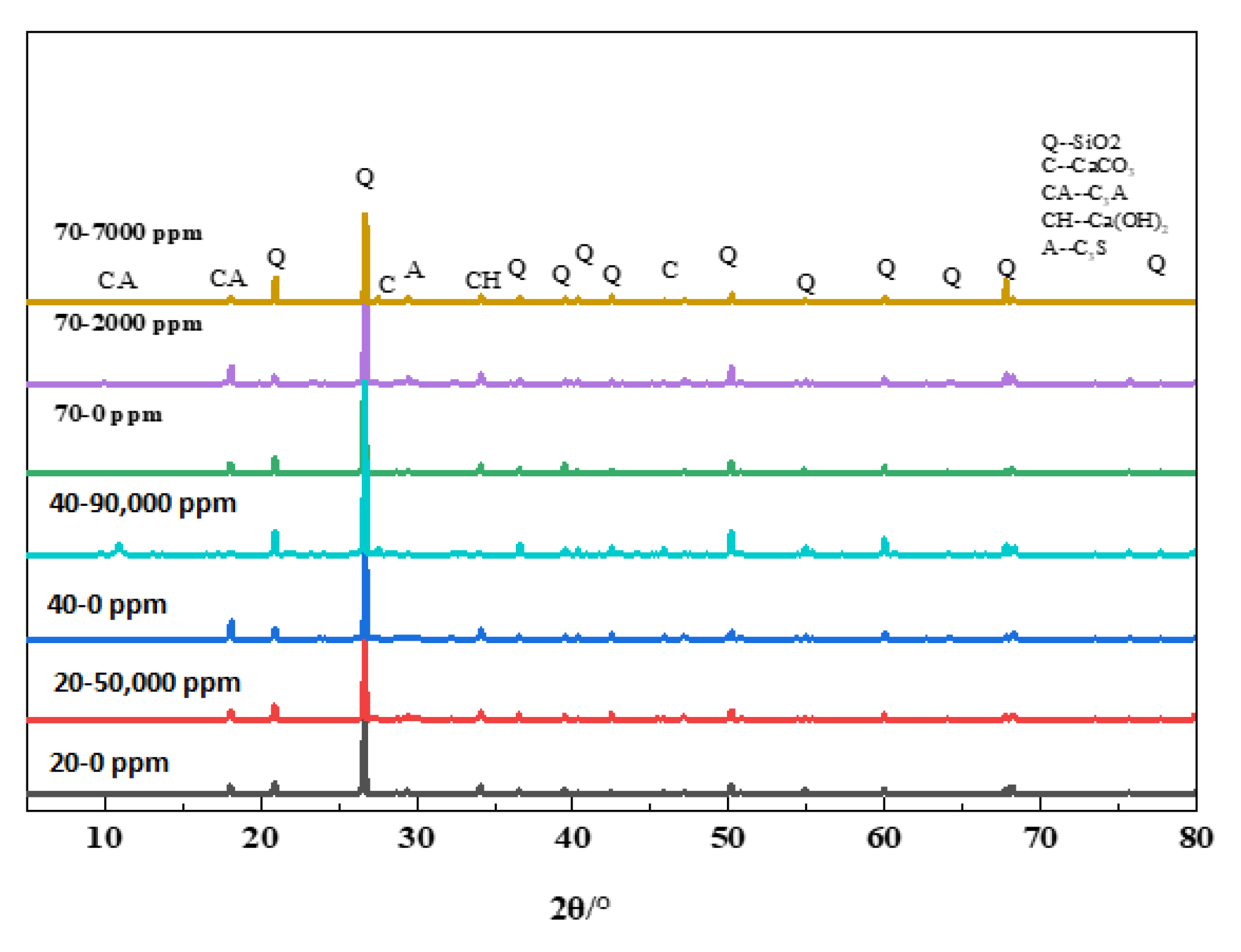
4.3. Mineral Composition Examination
4.4. Pore Structure of Concrete
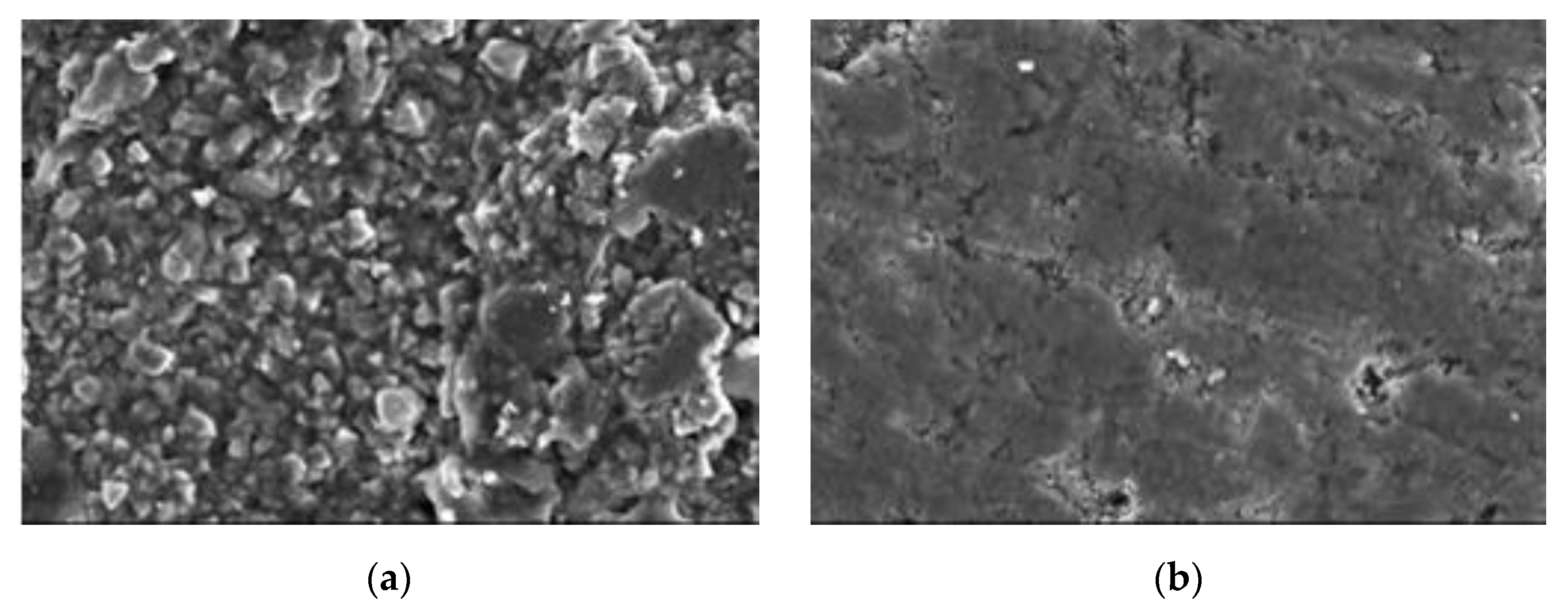
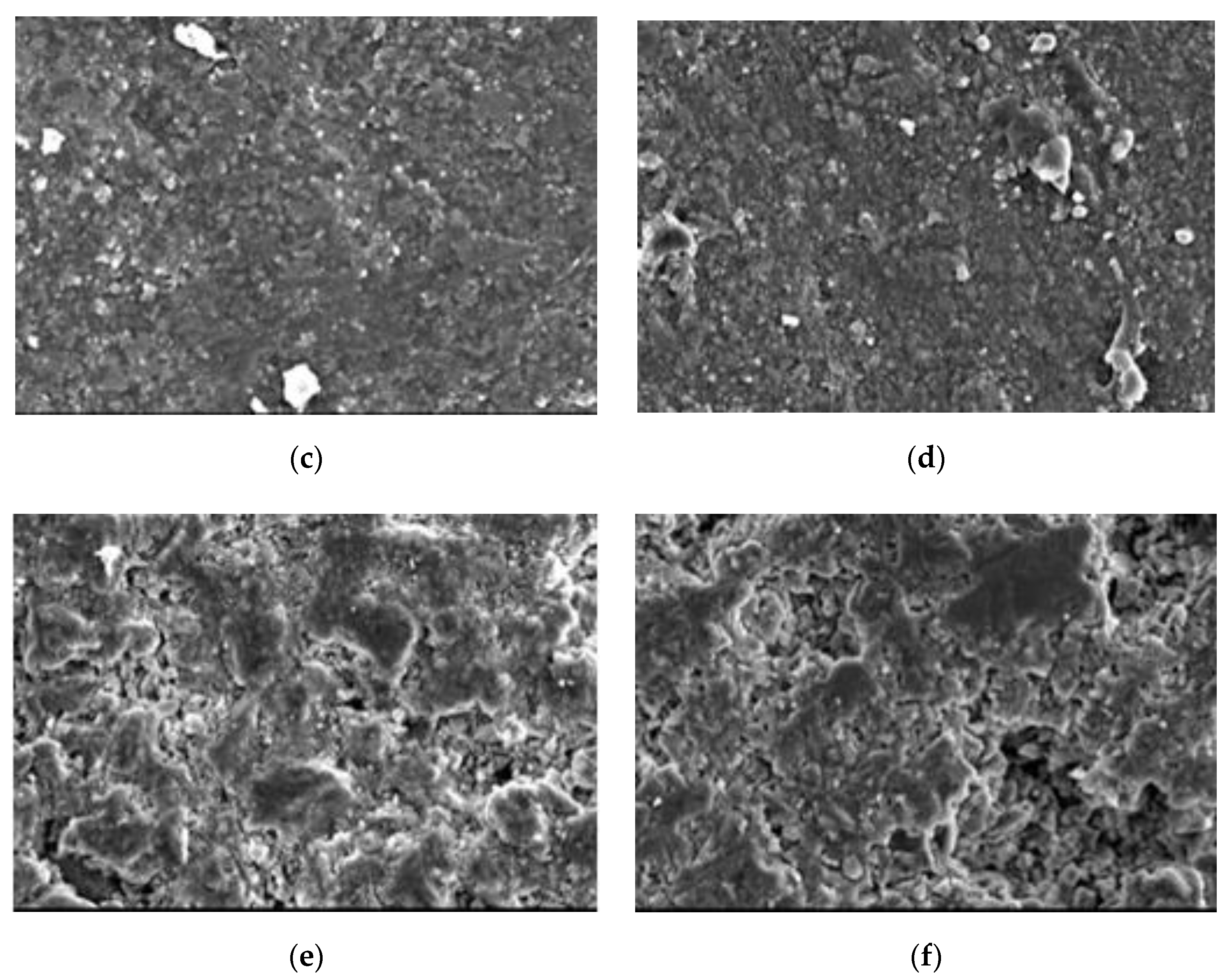
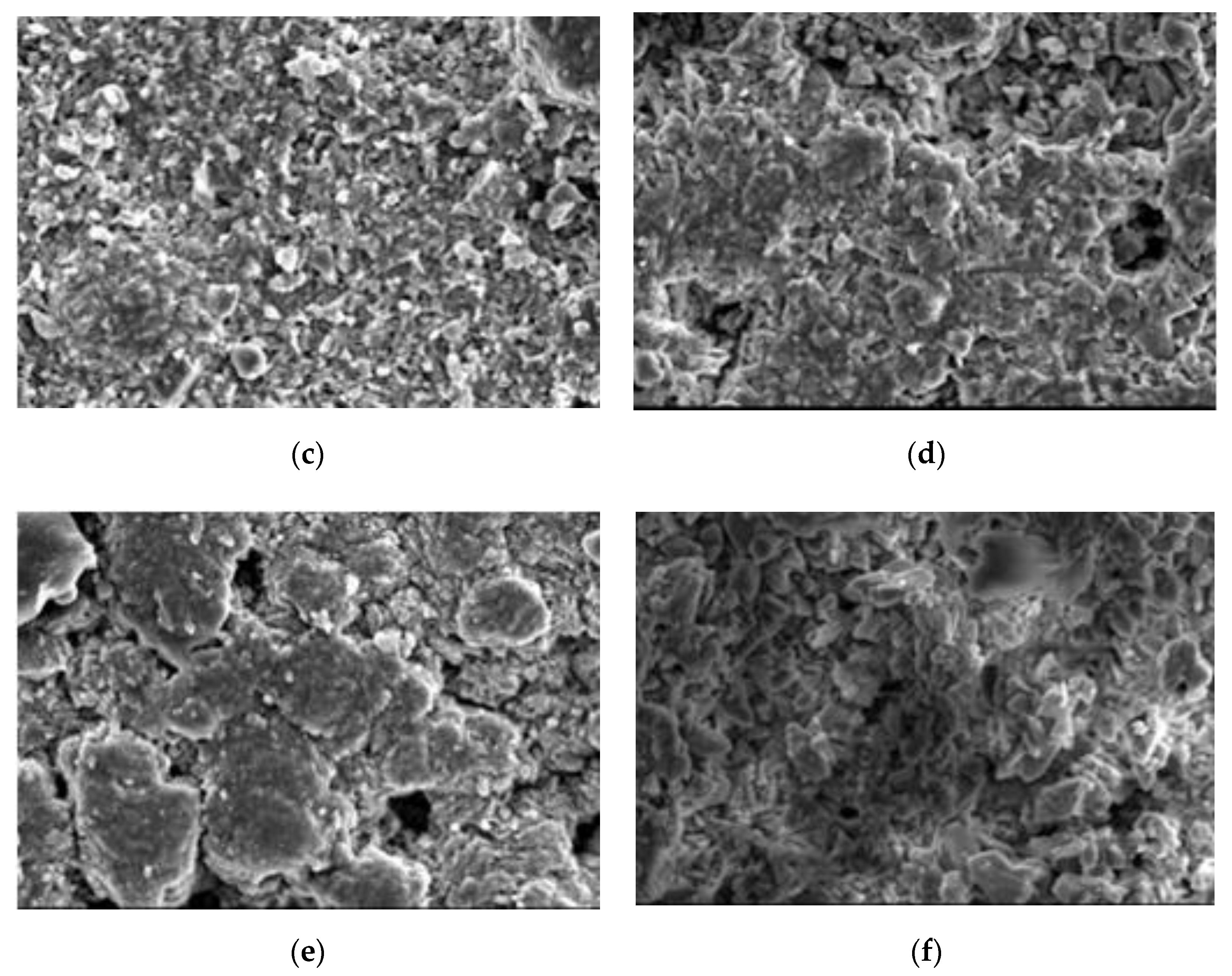
4.5. Scanning Electron Microscope
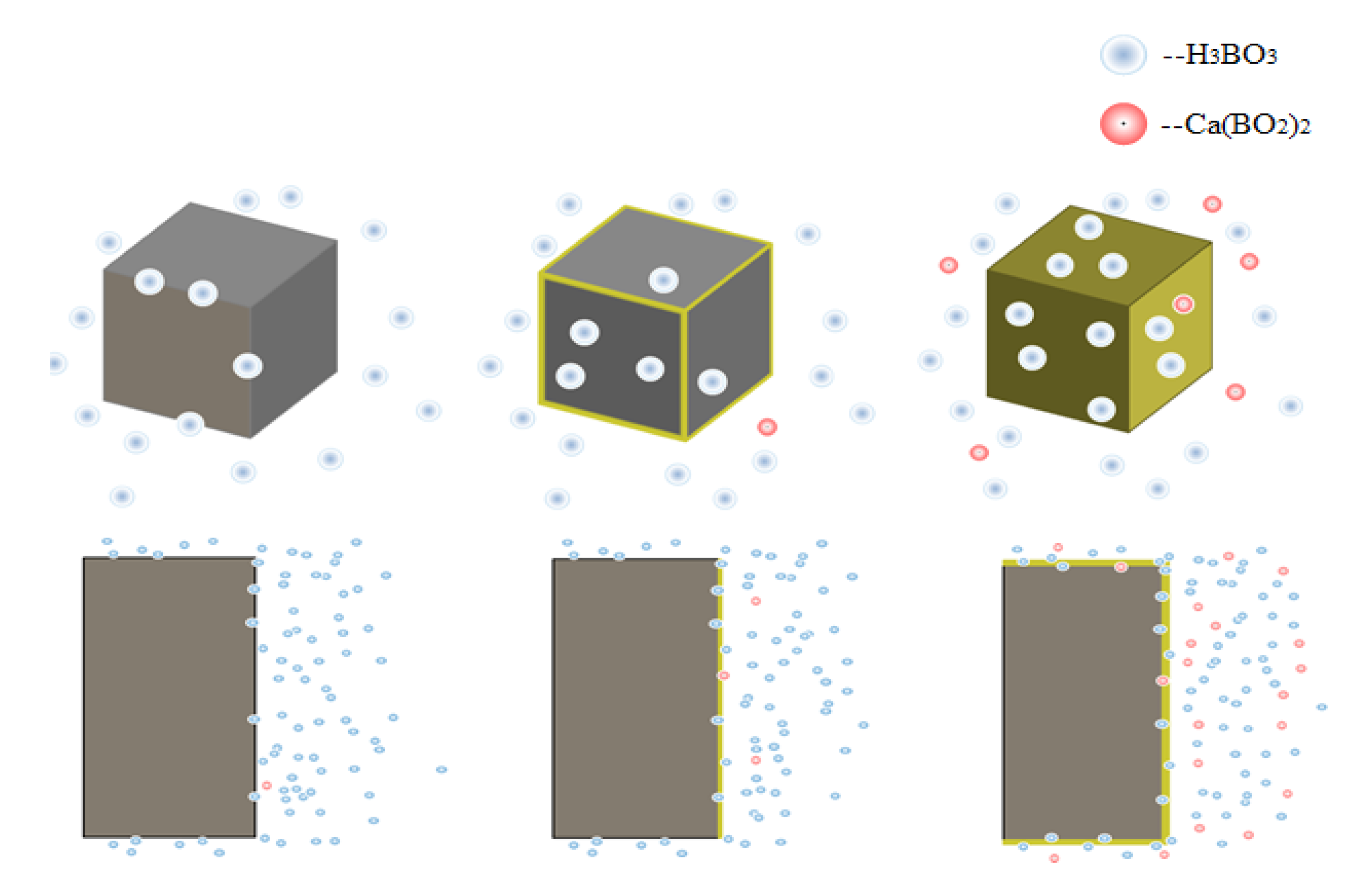
4.6. Discussion
5. Conclusions
- i.
- At the maximum allowable concentration of boric acid, 7000 ppm, even boric acid does not cause obvious performance loss to the outer wall of the pressurized water reactor at 20, 40, and 70 °C, and boric acid is safe for the external concrete structure of a pressurized water reactor under permissible operating conditions.
- ii.
- We simulated the 70 °C and 180,000 ppm scenario and found that, under a high-temperature and high-curing-concentration environment, the mass loss rate of 70–180,000 ppm was 0.69% at 180 days under conservation conditions, and its loss rate of compressive strength was 27.8% at 180 days. The surface corrosion of the concrete was serious. CH% in the surface was 3.12%, the neutralization depth was 1.5 mm, and the porosity of the harmful pores in the concrete surface was much higher than that of the rest of the specimens. The deterioration of the concrete surface caused by a high concentration of boric acid at a high temperature was the main reason for this loss of properties. Such a large loss rate of compressive strength is extremely dangerous in practical engineering.
- iii.
- Temperature and the concentration of boric acid are two important factors that cause concrete corrosion. The corrosion of concrete becomes more serious as the temperature and concentration of boric acid rise. However, at the early stage of the experiment, the hydration reaction of cement was stronger than the corrosion of boric acid at a high temperature, so the influence of the initial concentration was smaller than that of temperature. With the growth of the phase, the hydration reaction ended, and the influence of boric acid concentration began to dominate.
- iv.
- Ca(BO2)2 was detected on the surface of the 70–180,000 ppm concrete specimen at 180 days. This was not detected for any of the other specimens. As the concentration of boric acid increased, the porosity of harmful pores in the paste increased. The corrosion area on the surface of the paste gradually increased, along with corrosion depth. By combining this with the mass loss rate, it can be concluded that most of the substances produced during boric acid corrosion were dissolved into the acid conservation solution.
- v.
- Some highly active auxiliary cementites such as silica ash accelerate the hydration of cement, thus giving cement hydration in concrete an advantage over boric acid in the neutralization process. The addition of silica ash into cement is the focus of the next stage of our research.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gang, S.; Hong, Y. Leak detection technology of boric acid corrosion for nuclear power plant. Total. Cor. Con. 2016, 1, 16–19. [Google Scholar]
- Kam, D.H.; Choi, Y.J.; Jeong, Y.H. Boric acid and boiling time effects on critical heat flux for corrosive and non-corrosive materials. Ann. Nucl. Energy 2020, 136, 106999. [Google Scholar] [CrossRef]
- Jhonson, J. Who’s watching the reactors? Che. Eng. News. 2010, 81, 27–31. [Google Scholar] [CrossRef]
- Queral, C.; Gonzalez-Cadelo, J.; Jimenez, G.; Villalba, E. Accident management actions in an upper-head small-break loss-of-coolant accident injection failed. Nucl. Technol. 2011, 175, 572–593. [Google Scholar] [CrossRef]
- Hua, R.; Lin, Y.; Jiwa, L.; Yonghuan, W. Raesearch on the corrosion effect on the durability of concrete structure of a nuclear power plant by boric acid. Indu. Constr. 2017, 47, 35–38. [Google Scholar]
- Wheeler, D.M.; Posivak, E.; Freitag, A.; Geddes, B. Reactor pressure vessel head packaging and disposal. In Proceedings of the Office of Scientific and Technical Information Technical Reports, Tucson, AZ, USA, 26 February 2003. [Google Scholar]
- Qi, P.; Cui, H.; Feng, M.; Shao, W.; Liao, S.; Li, W. Preliminary study on detection technology of the cladding weld of spent fuel storage pool. In AIP Conference Proceedings; AIP Publishing LLC: College Park, MD, USA, 2018; Volume 1949, p. 230022. [Google Scholar] [CrossRef]
- Renger, S.; Alt, S.; Gocht, U.; Kästner, W.; Seeliger, A.; Kryk, H.; Harm, U. Multiscaled Experimental Investigations of Corrosion and Precipitation Processes After Loss-of-Coolant Accidents in Pressurized Water Reactors. Nucl. Technol. 2018, 205, 248–261. [Google Scholar] [CrossRef]
- Kang, S.-S.; Hwang, S.S.; Kim, H.; Lim, Y.S.; Kim, J.-S. The experience and analysis of vent pipe PWSCC (primary water stress corrosion cracking) in PWR vessel head penetration. Nucl. Eng. Des. 2014, 269, 291–298. [Google Scholar] [CrossRef]
- Jin, X.Y.; Jin, N.G.; Tian, Y. Study on the Behavior and Durability of Reinforced Concrete in Boric Acid Environment. Key Eng. Mater. 2008, 400, 441–446. [Google Scholar] [CrossRef]
- Zhan, Y.; Li, Q.; Jin, X.Y.; Tian, Y.; Jin, N.G. Behavior of Reinforced Concrete Subjected to Boric Acid Corrosion of Nuclear Power Plant. Adv. Mater. Res. 2011, 242–246. [Google Scholar] [CrossRef]
- Demirbaş, A.; Karslioǧlu, S.; Karslioglu, S. The effect of boric acid sludges containing borogypsum on properties of cement. Cem. Concr. Res. 1995, 25, 1381–1384. [Google Scholar] [CrossRef]
- Pabalan, R.; Chiang, K.-T.K. Experimental Study and Reactive Transport Modeling of Boric Acid Leaching of Concrete. In Proceedings of the EPJ Web of Conferences; EDP Sciences: Les Ulis, France, 2013; Volume 56, p. 1009. [Google Scholar]
- Pabalan, R.; Yang, L.; Chiang, K. Boric Acid Corrosion of Concrete Rebar. Eur. Phys. J. Conf. 2013, 56, 06005. [Google Scholar]
- Chung-Hsien, L.; Su, K.-H. A Cleanup Machine Using Reverse Osmosis Method to Remove Silica from Boric Acid Tank at Maanshan Nuclear Power Station. Nucl. Technol. 2008, 162, 333–341. [Google Scholar] [CrossRef]
- El-Sakhawy, N.R.; El-Dien, H.S.; Ahmed, M.E.; Bendary, K.A. Influence of curing on durability performance of concrete. Mag. Concr. Res. 1999, 51, 309–315. [Google Scholar] [CrossRef]
- Li, B.; Cai, L. Acidification prediction model of concrete based on grey system. JCSS 2013, 41, 1375–1380. [Google Scholar] [CrossRef]
- Niu, J.; Niu, D. Experimental study on the neutralization of fly ash concrete attacked by acid rain. In Proceedings of the 2011 International Conference on Electric Technology and Civil Engineering (ICETCE); Institute of Electrical and Electronics Engineers (IEEE): Lushan, China, 22–24 April 2011; pp. 3162–3165. [Google Scholar] [CrossRef]
- Huang, B.; Qian, C. Experiment study of chemo-mechanical coupling behavior of leached concrete. Constr. Build. Mater. 2011, 25, 2649–2654. [Google Scholar] [CrossRef]
- Chen, W.; Huang, B.; Yuan, Y.; Deng, M. Deterioration Process of Concrete Exposed to Internal Sulfate Attack. Materials 2020, 13, 1336. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Bhattacharjee, B. Porosity, pore size distribution and in situ strength of concrete. Cem. Concr. Res. 2003, 33, 155–164. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Han, S.F.; Shi, Z.H.; Ai, Y.X.; Huang, Z.Y.; Zhang, Y.B.; Chen, Y.L.; Shang, J.L.; Tian, G.F.; Li, X.C.; et al. Standard for Evaluation of Concrete Compressive Strength; GB/T 50107-2010; CABR: Beijing, China, 31 May 2010; pp. 5–9. [Google Scholar]





| Type | Chemical Compositions (%) | Loss on Ignition (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | R2O | ||
| PC | 19.43 | 4.73 | 2.96 | 64.00 | 2.35 | 2.58 | 0.71 | 2.81 |
| FA | 50.10 | 29.27 | 6.95 | 3.40 | 1.11 | 1.15 | 1.37 | - |
| Cement | Water | Fine Aggregate | Coarse Aggregate (5–16 mm) | Coarse Aggregate (16–31.5 mm) | Fly Ash | Water Reducer | Slump (mm) |
|---|---|---|---|---|---|---|---|
| 415 | 160 | 688 | 538 | 538 | 62 | 5.7 | 165 |
| CH% | 20–50,000 | 40–90,000 | 70-0 | 70–2000 | 70-7000 | 70–180,000 |
|---|---|---|---|---|---|---|
| Surface | 10.02 | 9.66 | 9.55 | 7.65 | 7.89 | 3.12 |
| Interior | 10.72 | 9.74 | 12.1 | 11.34 | 11.91 | 10.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Huang, B.; Mao, Z.; Deng, M.; Cao, H. Effect of a Boric Acid Corrosive Environment on the Microstructure and Properties of Concrete. Materials 2020, 13, 5036. https://doi.org/10.3390/ma13215036
Wang Y, Huang B, Mao Z, Deng M, Cao H. Effect of a Boric Acid Corrosive Environment on the Microstructure and Properties of Concrete. Materials. 2020; 13(21):5036. https://doi.org/10.3390/ma13215036
Chicago/Turabian StyleWang, Yu, Bei Huang, Zhongyang Mao, Min Deng, and Huan Cao. 2020. "Effect of a Boric Acid Corrosive Environment on the Microstructure and Properties of Concrete" Materials 13, no. 21: 5036. https://doi.org/10.3390/ma13215036
APA StyleWang, Y., Huang, B., Mao, Z., Deng, M., & Cao, H. (2020). Effect of a Boric Acid Corrosive Environment on the Microstructure and Properties of Concrete. Materials, 13(21), 5036. https://doi.org/10.3390/ma13215036





