Core-shell Fe3O4@zeolite NaA as an Adsorbent for Cu2+
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
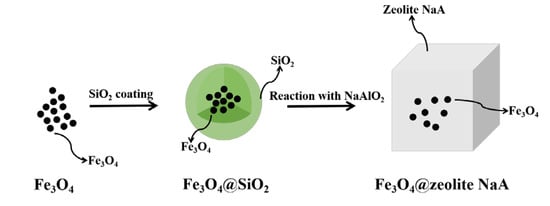
2.2. Preparation of Samples
2.3. Characterization
2.4. Preparation of Heavy Metal Solutions
2.5. Adsorption Experiments
3. Results and Discussion
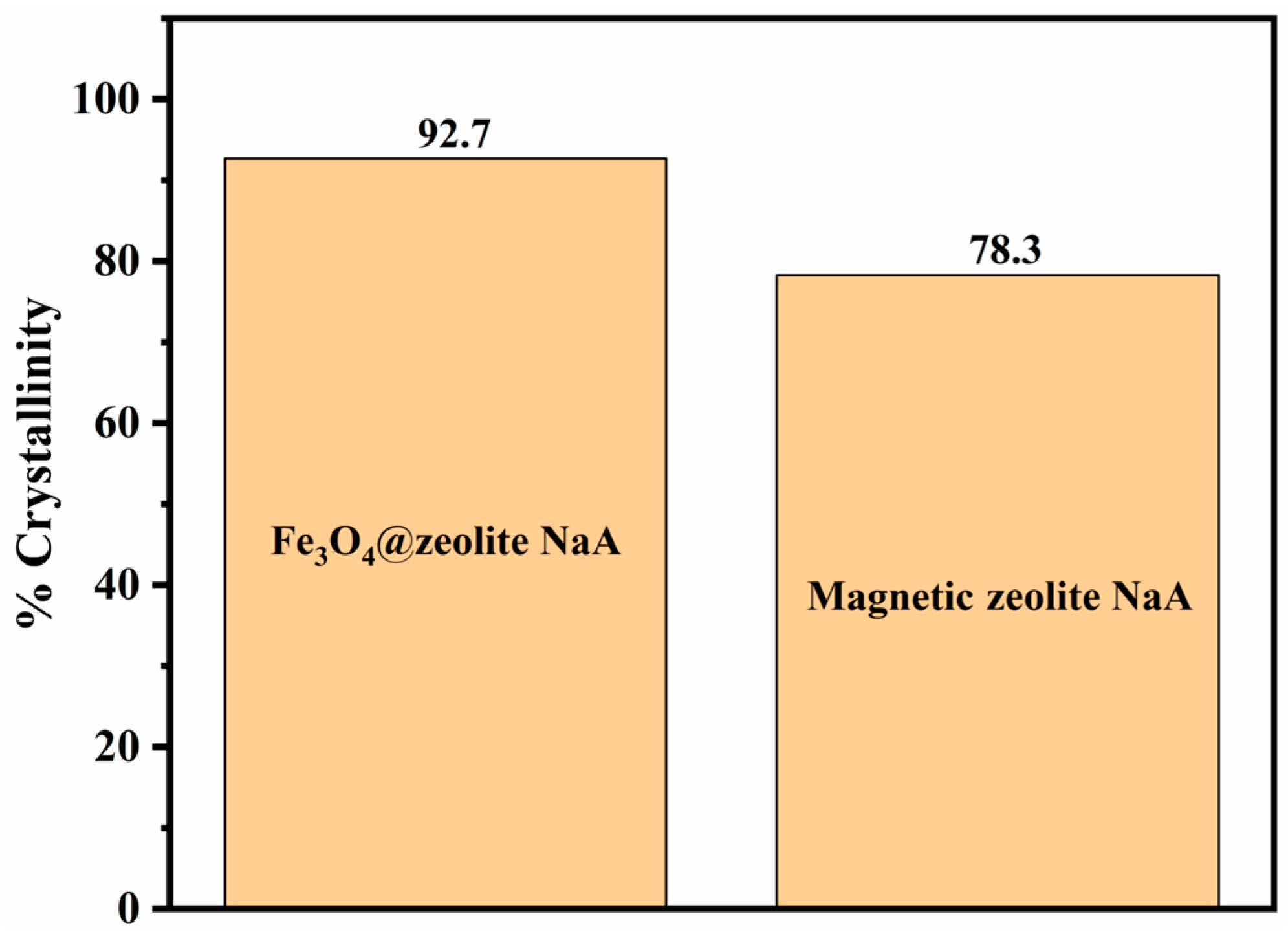
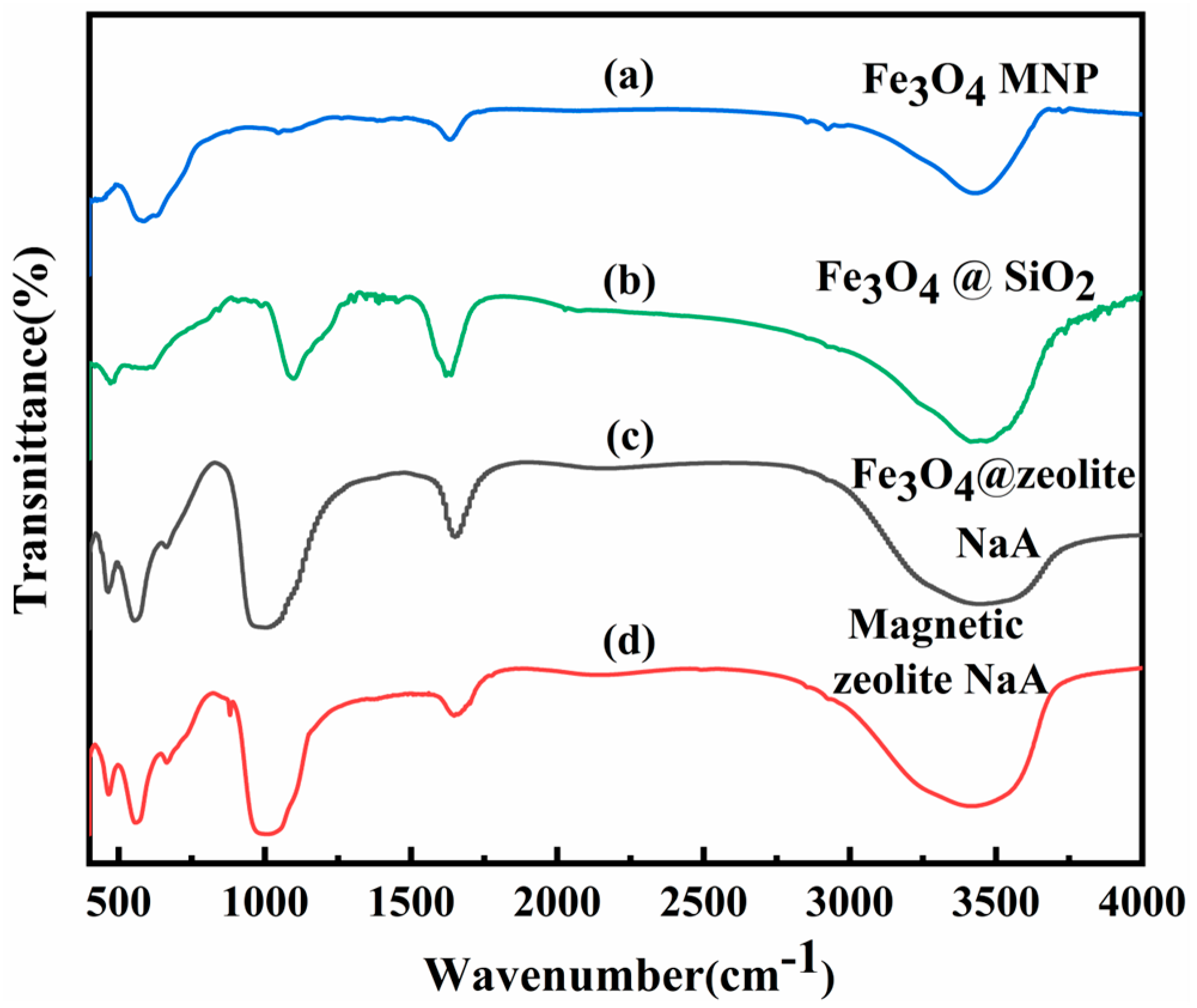
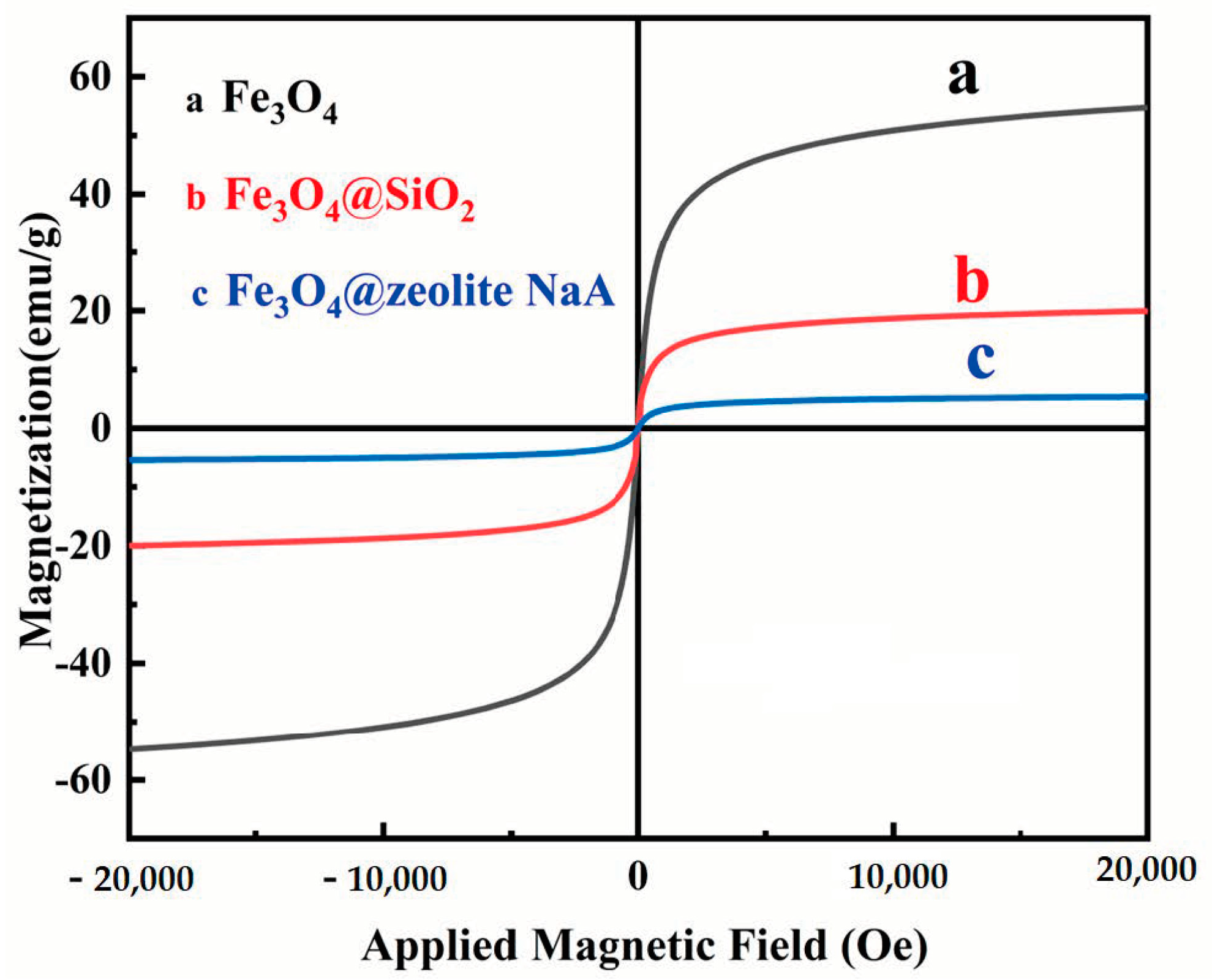
3.1. Characterization of Samples
3.2. Optimization of Adsorption Parameters
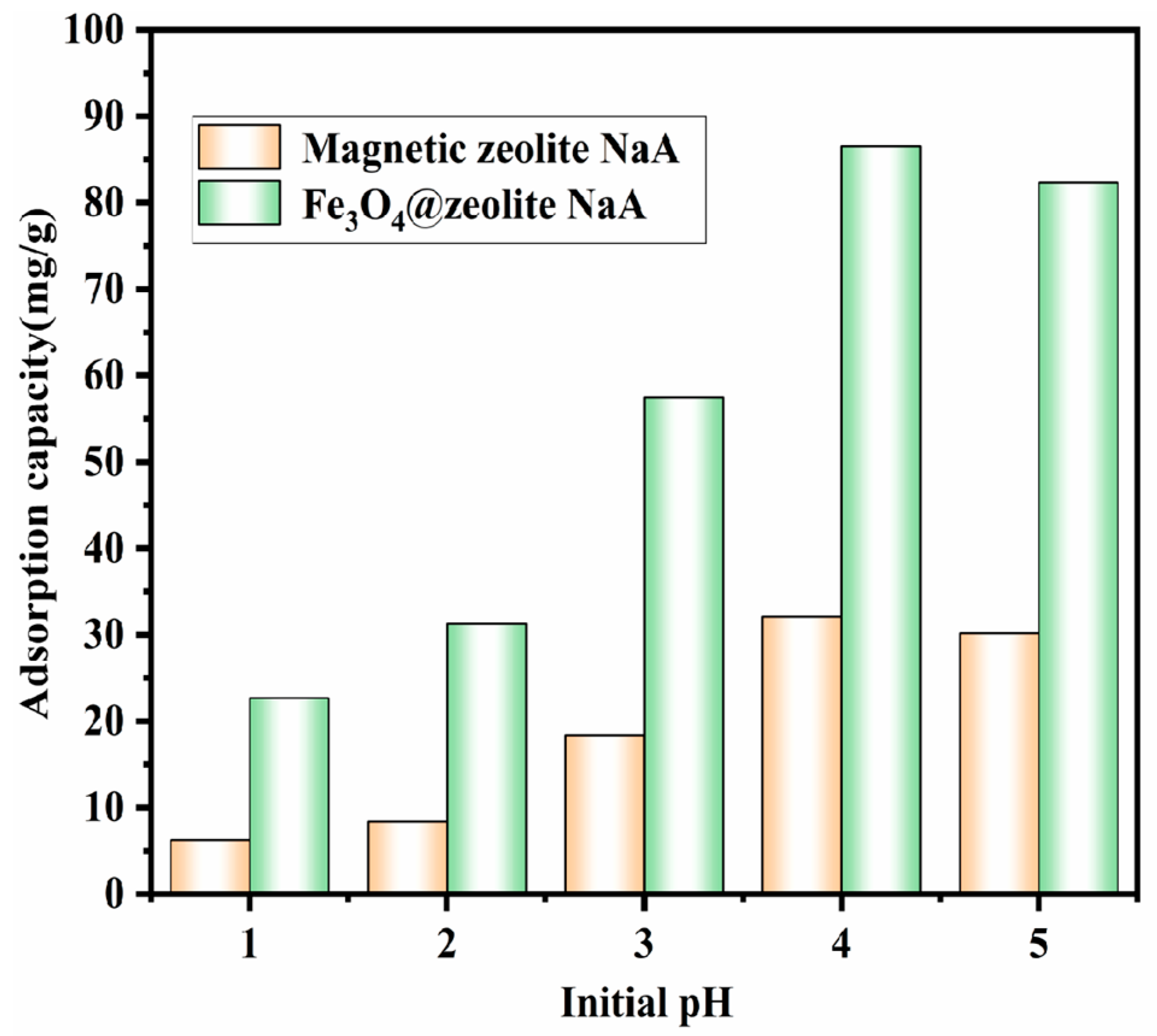
3.2.1. Effect of pH
3.2.2. Effect of Adsorbent Does on Cu2+ Adsorption
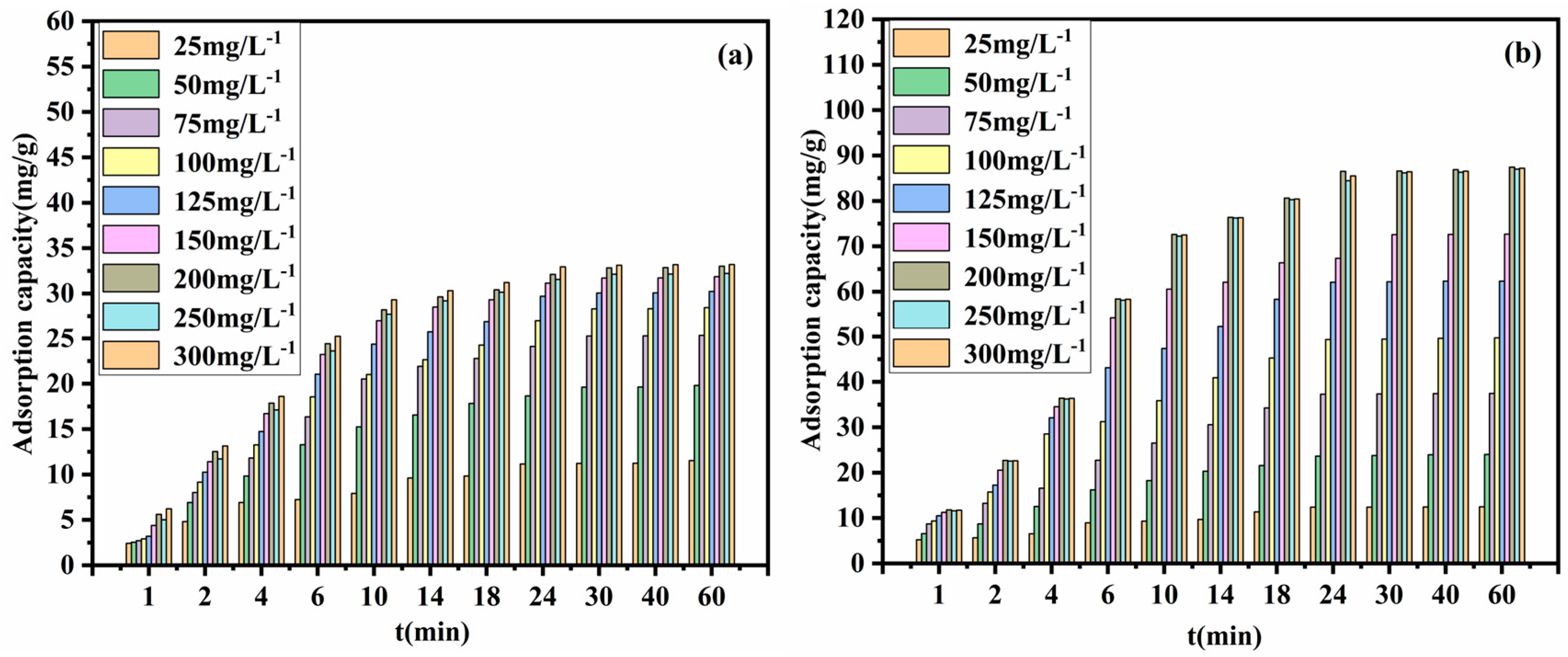
3.2.3. Effect of Contact Time on Cu2+ Adsorption
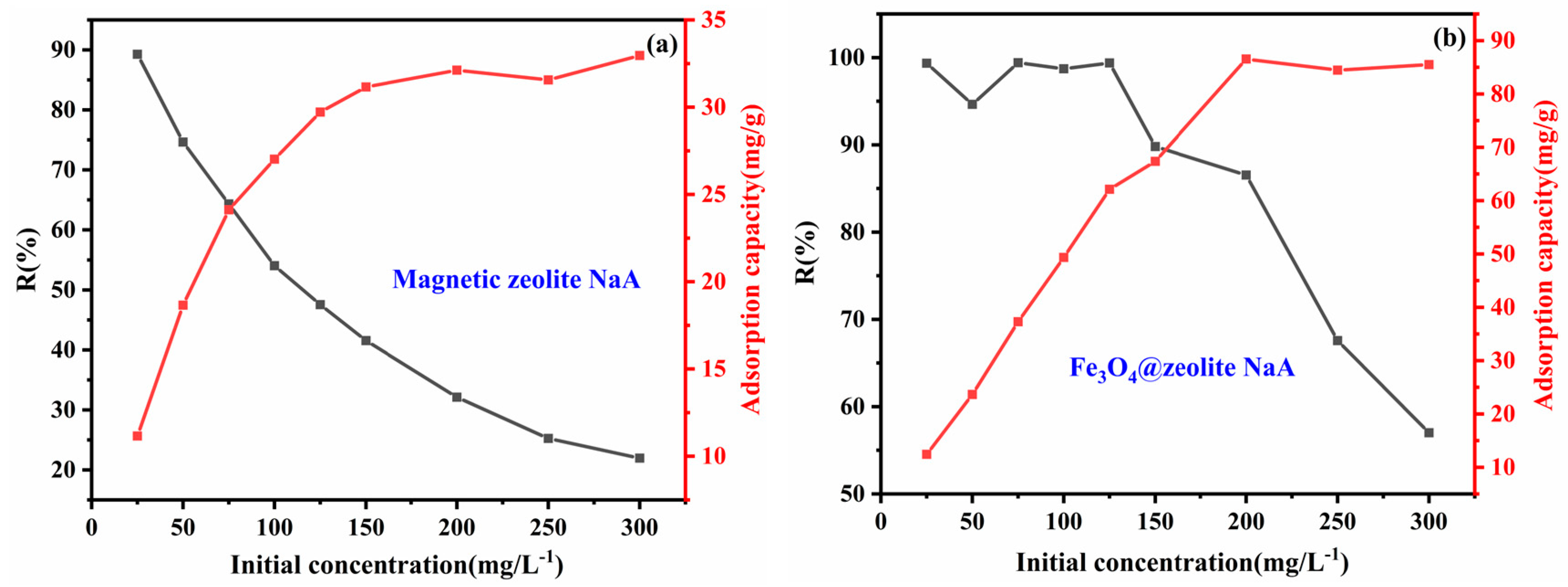
3.2.4. Effect of Initial Concentration
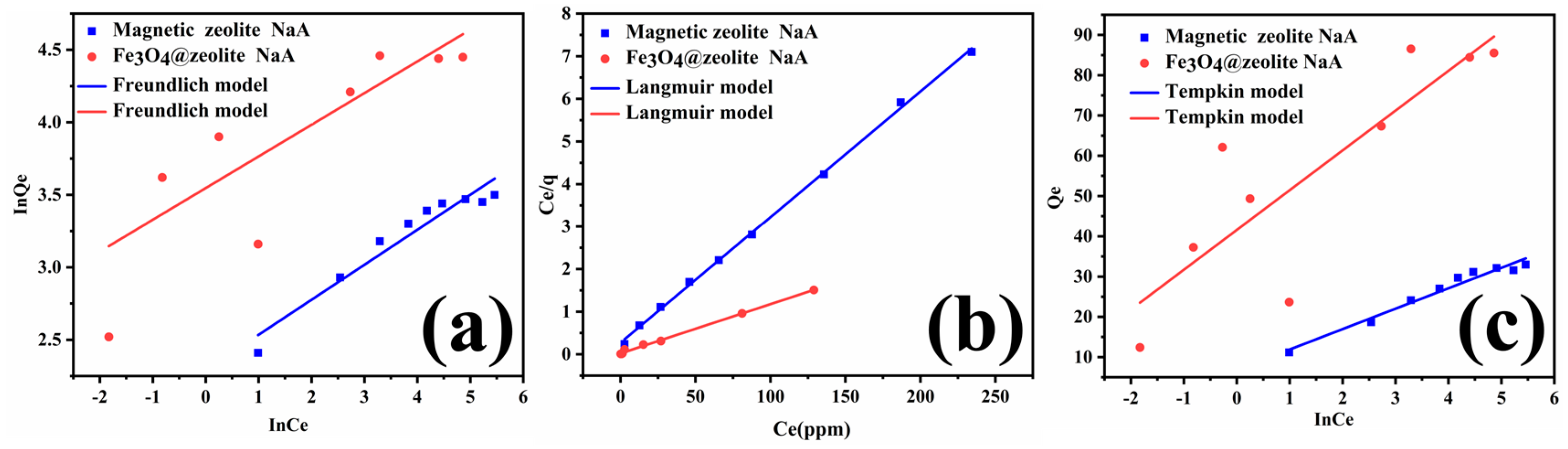
3.3. Adsorption Isotherm
3.4. Adsorption Kinetics
3.5. Adsorption Thermodynamics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lone, M.I.; He, Z.; Stoffella, P.J.; Yang, X. Phytoremediation of heavy metal polluted soils and water: Progresses and perspectives. J. Zhejiang Univ. Sci. B 2008, 9, 210–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onundi, Y.B.; Mamun, A.A.; Al Khatib, M.F.; Ahmed, Y.M. Adsorption of copper, nickel and lead ions from synthetic semiconductor industrial wastewater by palm shell activated carbon. Int. J. Environ. Sci. Tech. 2010, 7, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Meena, A.K. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. J. Hazard. Mater. 2005, 122, 161–170. [Google Scholar] [CrossRef]
- Li, N.; Bai, R. Copper adsorption on chitosan-cellulose hydrogel beads: Behaviors and mechanisms. Sep. Purif. Technol. 2005, 42, 237–247. [Google Scholar] [CrossRef]
- Larous, S.; Meniai, A.-H.; Lehocine, M.B. Experimental study of the removal of copper from aqueous solutions by adsorption using sawdust. Desalination 2005, 185, 483–490. [Google Scholar] [CrossRef]
- Mody, A.; Bellare, J. Zeolitic imidazolate framework-67/carboxylated graphene oxide nanosheets incorporated polyethersulfone hollow fiber membranes for removal of toxic heavy metals from contaminated water. Sep. Purif. Technol. 2020, 249, 117160. [Google Scholar] [CrossRef]
- Larsson, M.; Nosrati, A.; Kaur, S.; Wagner, J.; Baus, U.; Nydén, M. Copper removal from acid mine drainage-polluted water using glutaraldehyde-polyethyleneimine modified diatomaceous earth particles. Heliyon 2018, 4, e00520. [Google Scholar] [CrossRef] [Green Version]
- Thanh, D.N.; Novák, P.; Vejpravova, J.; Vu, H.N.; Lederer, J.; Munshi, T. Removal of copper and nickel from water using nanocomposite of magnetic hydroxyapatite nanorods. J. Magn. Magn. Mater. 2018, 456, 451–460. [Google Scholar] [CrossRef]
- Jang, J.; Lee, D.S. Magnetic Prussian blue nanocomposites for effective cesium removal from aqueous solution. Ind. Eng. Chem. Res. 2016, 55, 3852–3860. [Google Scholar] [CrossRef]
- Elkhatib, E.; Moharem, M.; Mahmoud, A. Low cost nanoparticles derived from nitrogen fertilizer industry waste for the remediation of copper contaminated soil and water. Environ. Eng. Res. 2020, 25, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Vecitis, C.D.; Schulze, A.; Cao, B.; Ismail, A.F.; Lu, X.; Chen, J.; Ramakrishna, S. Recent advances in nanomaterials for water protection and monitoring. Chem. Soc. Rev. 2017, 46, 6946–7020. [Google Scholar] [CrossRef] [PubMed]
- Gougazeh, M.; Buhl, J.-C. Synthesis and characterization of zeolite A by hydrothermal transformation of natural Jordanian kaolin. J. Assoc. Arab Univ. Basic Appl. Sci. 2014, 15, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Broni, J.; Pal, A.; Suboti, B.; Itani, L.; Valtchev, V. Influence of alkalinity of the starting system on size and morphology of the zeolite A crystals. Mater. Chem. Phys. 2012, 132, 973–976. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Zhang, X.; Wang, J.; Liu, J.; Chen, R. Preparation of highly ordered cubic NaA zeolite from halloysite mineral for adsorption of ammonium ions. J. Hazard. Mater. 2010, 178, 658–664. [Google Scholar] [CrossRef]
- Jameson, C.J.; Jameson, A.K.; Lim, H.-M.; Baello, B.I. Grand canonical Monte Carlo simulations of the distribution and chemical shifts of xenon in the cages of zeolite NaA. II. Structure of the adsorbed fluid. J. Chem. Phys. 1994, 100, 5977–5987. [Google Scholar] [CrossRef]
- Sapawe, N.; Jalil, A.A.; Triwahyono, S.; Shah, M.I.A.; Jusoh, R.; Salleh, N.F.M.; Hameed, B.H.; Karim, A.H. Cost-effective microwave rapid synthesis of zeolite NaA for removal of methylene blue. Chem. Eng. J. 2013, 229, 388–398. [Google Scholar] [CrossRef]
- Liu, H.; Peng, S.; Shu, L.; Chen, T.; Bao, T.; Frost, R.L. Magnetic zeolite NaA: Synthesis, characterization based on metakaolin and its application for the removal of Cu2+, Pb2+. Chemosphere 2013, 91, 1539–1546. [Google Scholar] [CrossRef]
- Liu, H.; Peng, S.; Shu, L.; Chen, T.; Bao, T.; Frost, R.L. Effect of Fe3O4 addition on removal of ammonium by zeolite NaA. J. Colloid Interface Sci. 2013, 390, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.P.; Ou, S.; Tang, C.H. Core-shell soy protein-soy polysaccharide complex (nano)particles as carriers for improved stability and sustained release of curcumin. J. Agric. Food Chem. 2016, 64, 5053–5059. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Nasim, F.; Mishra, R.; Bharti, R.P.; Kundu, P.P. Polyurethane-incorporated chitosan/alginate core-shell nano-particles for controlled oral insulin delivery. J. Appl. Polym. Sci. 2018, 135, 46365. [Google Scholar] [CrossRef]
- Javid, M.; Zhou, Y.L.; Wang, D.X.; Liang, J.S. Strong microwave absorption of Fe@SiO2 nanocapsules fabricated by one-step high energy plasma. J. Phys. Chem. Solids 2019, 129, 242–251. [Google Scholar] [CrossRef]
- Liu, K.; Qin, Y.L.; Zhu, Y.; Tang, R. Effect of Fe3O4 content and microwave reaction time on the properties of Fe3O4/ZnO magnetic nanoparticles. J. Alloys Compd. 2019, 781, 790–799. [Google Scholar] [CrossRef]
- Xu, J.J.; Liu, J.W.; Che, R.C.; Liang, C.Y. Polarization enhancement of microwave absorption by increasing aspect ratio of ellipsoidal nanorattles with Fe3O4 cores and hierarchical CuSiO3 shells. Nanoscale 2014, 7, 9734–9741. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, Y.; Xu, M.; Yang, H.; Wu, C.; Miyoshi, H. Core/shell Fe3O4 @SiO2 nanoparticles modified with PAH as a vector for EGFP plasmid DNA delivery into HeLa cells. Macromol. Biosci. 2011, 11, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Ma, H.; Chuan, X. Hydrothermal synthesis of zeolite A from K-feldspar and its crystallization mechanism. Adv. Powder Technol. 2015, 27, 139–144. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, X.-l.; Niu, H.-y.; Ma, Y.-r.; Li, W.-h.; Cai, Y.-q. In situ growth of gold nanoparticles onto polydopamine-encapsulated magnetic microspheres for catalytic reduction of nitrobenzene. Appl. Catal. B Environ. 2013, 134–135, 26–33. [Google Scholar] [CrossRef]
- Zheng, J.; Dong, Y.; Wang, W.; Ma, Y.; Hu, J.; Chen, X.; Chen, X. In situ loading of gold nanoparticles on Fe3O4@SiO2 magnetic nanocomposites and their high catalytic activity. Nanoscale 2013, 5, 4894–4901. [Google Scholar] [CrossRef]
- Xu, X.; Yang, W.; Liu, J.; Liu, L. Synthesis of a high-permeance NaA zeolite membrane by microwave heating. Adv Mater. 2010, 12, 195–198. [Google Scholar] [CrossRef]
- Barbosa, I.A.; de Sousa, P.C.F.; da Silva, D.L.; Zanardi, F.B.; Zanatta, L.D.; de Oliveira, A.J.A.; Iamamoto, Y. Metalloporphyrins immobilized in Fe3O4@SiO2 mesoporous submicrospheres: Reusable biomimetic catalysts for hydrocarbon oxidation. J. Colloid Interface Sci. 2016, 469, 296–309. [Google Scholar] [CrossRef]
- Wang, Z.H.; Fan, H.J.; Shi, B. The preparation and characterization of magnetic polymer. Leather Sci. Eng. 2014, 24, 23. [Google Scholar]
- Lai, L.; Xie, Q.; Chi, L.; Gu, W.; Wu, D. Adsorption of phosphate from water by easily separable Fe3O4@SiO2 core/shell magnetic nanoparticles functionalized with hydrous lanthanum oxide. J. Colloid Interface Sci. 2016, 465, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ping, T.; Maitlo, I.; Wang, B.; Akram, M.Y.; Nie, J.; Zhu, X. Regional selective construction of nano-Au on Fe3O4@SiO2@PEI nanoparticles by photoreduction. Nanotechnology 2016, 27, 215301. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.; Dondur, V.; Dimitrijevic, R. FTIR spectroscopy of framework aluminosilicate structures: Carnegieite and pure sodium nepheline. J. Mol. Struct. 2003, 654, 223–234. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, Y.; Xu, L.; Liu, G. Recovery and reduction of Au(III) from mixed metal solution by thiourea-resorcinol-formaldehyde microspheres. J. Hazard. Mater. 2020, 397, 122812. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Belik, A.A.; Imura, M. Tailored design of multiple nanoarchitectures in metal-cyanide hybrid coordination polymers. J. Am. Chem. Soc. 2013, 135, 384–391. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, J.; Xiao, H.; Peng, H.; Bu, L.; Pan, Z.; He, Y.; Chen, F.; Wang, X.; Li, S. Adsorption behavior of hydrotalcite-like modified bentonite for Pb2+, Cu2+ and methyl orange removal from water. Appl. Surf. Sci. 2017, 420, 773–781. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; He, L.; Zhang, D.; Ye, M. Design of magnetic nanoparticles with high magnetic separation efficiencies and durability for Cu2+ adsorption. Nanotechnology 2019, 31, 085710. [Google Scholar] [CrossRef]
- Houming, C.; Zhu, Q.; Xing, Z. Adsorption of ammonia nitrogen in low temperature domestic wastewater by modification bentonite. J. Clean. Prod. 2019, 233, 720–730. [Google Scholar]
- Yang, X.; Zhang, X.; Ma, Y.; Huang, Y.; Wang, Y.; Chen, Y. Superparamagnetic graphene oxide–Fe3O4 nanoparticles hybrid for controlled targeted drug carriers. J. Mater. Chem. 2009, 19, 182710. [Google Scholar] [CrossRef]
- Neuberger, T.; Schpf, B.; Hofmann, H.; Hofmann, M.; Rechenberg, B.V. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Pham, T.D.; Nguyen, H.H.; Nguyen, N.V.; Vu, T.T.; Pham, T.N.M.; Doan, T.H.Y.; Ngo, T.M.V. Adsorptive removal of copper by using surfactant modified laterite soil. J. Chem. 2017, 2017, 1986071. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Gao, Y.; Yue, Q.; Kong, W.; Gao, B.; Wang, W.; Jiang, W. Degradation of chlortetracycline with simultaneous removal of copper (Ⅱ) from aqueous solution using wheat straw-supported nanoscale zero-valent iron. Chem. Eng. J. 2020, 379, 122384. [Google Scholar] [CrossRef]
- Jaiswal, A.; Banerjee, S.; Mani, R.; Chattopadhyaya, M.C. Synthesis, characterization and application of goethite mineral as an adsorbent. J. Environ. Chem. Eng. 2013, 1, 281–289. [Google Scholar] [CrossRef]
- Jiang, M.; Jin, X.; Lu, X.-Q.; Chen, Z. Adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) onto natural kaolinite clay. Desalination 2010, 252, 33–39. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Nguyen, X.T.; Nguyen, T.V.; Nguyen, T.T.; Vu, T.Q.; Nguyen, H.T.; Dinh, T.M.T. Treatment of Cd2+ and Cu2+ ions using modified apatite ore. J. Chem. 2020, 2020, 6527197. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liang, X.; Wang, Y.; Wang, X.; Liu, M.; Yin, D.; Xia, S.; Zhao, J.; Zhang, Y. Adsorption of Copper (II) onto activated carbons from sewage sludge by microwave-induced phosphoric acid and zinc chloride activation. Desalination 2011, 278, 231–237. [Google Scholar] [CrossRef]
- Luo, B.; Chen, M.; Chen, F.; Xue, Z. L–cysteine/hydrotalcite hybrid for collaborative removal of Cu(II), Hg(II) and Pb(II) ions from aqueous solutions: Different metal ions require different mechanisms. Chem. Sel. 2020, 5, 4932–4942. [Google Scholar] [CrossRef]
- Wołowicz, A.; Hubicki, Z. Investigation of macroporous weakly basic anion exchangers applicability in palladium(II) removal from acidic solutions—Batch and column studies. Chem. Eng. J. 2011, 174, 510–521. [Google Scholar] [CrossRef]
- Basci, N.; Kocadagistan, E.; Kocadagistan, B. Biosorption of copper (II) from aqueous solutions by wheat shell. Desalination 2004, 164, 135–140. [Google Scholar] [CrossRef]
- Issa, M.A.; Abidin, Z.Z.; Pudza, M.Y.; Zentou, H. Efficient removal of Cu(ii) from aqueous systems using enhanced quantum yield nitrogen-doped carbon nanodots. RSC Adv. 2020, 10, 14979–14990. [Google Scholar] [CrossRef]
- Luo, X.; Lei, X.; Cai, N.; Xie, X.; Xue, Y.; Yu, F. Removal of heavy metal ions from water by magnetic cellulose-based beads with embedded chemically modified magnetite nanoparticles and activated carbon. ACS Sustain. Chem. Eng. 2016, 4, 3960–3969. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z.; Xiao, D.; Xiong, P.; Ye, N. Sulfonated multi-walled carbon nanotubes for the removal of copper (II) from aqueous solutions. J. Ind. Eng. Chem. 2014, 20, 1765–1771. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y. Eco-friendly floatable foam hydrogel for the adsorption of heavy metal ions and the generated waste for the catalytic reduction of organic dyes. Soft Matter 2020, 16, 6914–6923. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.K.; Mohamed, H.S.; Ahmed, S.A.; Sayed, F.H.; Elghandour, A.H.; Ahmed, S.A. Cd2+ and Cu2+ removal by the waste of the marine brown macroalga Hydroclathrus clathratus. Environ. Technol. Innov. 2019, 15, 100365. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, X.; Cao, Z.; Wang, S.; Zhong, H. Investigation on the selectivity of thioamide surfactants and adsorption mechanism of thio-p-toluamide for chalcopyrite. Appl. Surf. Sci. 2019, 484, 864–875. [Google Scholar] [CrossRef]
- Novak, I.; Klasinc, L.; McGlynn, S.P. Electronic structure and tautomerism of thioamides. J. Electron Spectrosc. Relat. Phenom. 2016, 209, 62–65. [Google Scholar] [CrossRef]
- Wang, X.; Hou, H.; Li, Y.; Wang, Y.; Hao, C.; Ge, C. A novel semi-IPN hydrogel: Preparation, swelling properties and adsorption studies of Co (II). J. Ind. Eng. Chem. 2016, 41, 82–90. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Mittal, H.; Waanders, F.; Ray, S.S. Thermodynamic properties and adsorption behaviour of hydrogel nanocomposites for cadmium removal from mine effluents. J. Ind. Eng. Chem. 2017, 48, 151–161. [Google Scholar] [CrossRef]
- Cui, Y.; Ge, Q.; Liu, X.-Y.; Chung, T.-S. Novel forward osmosis process to effectively remove heavy metal ions. J. Membr. Sci. 2014, 467, 188–194. [Google Scholar] [CrossRef]



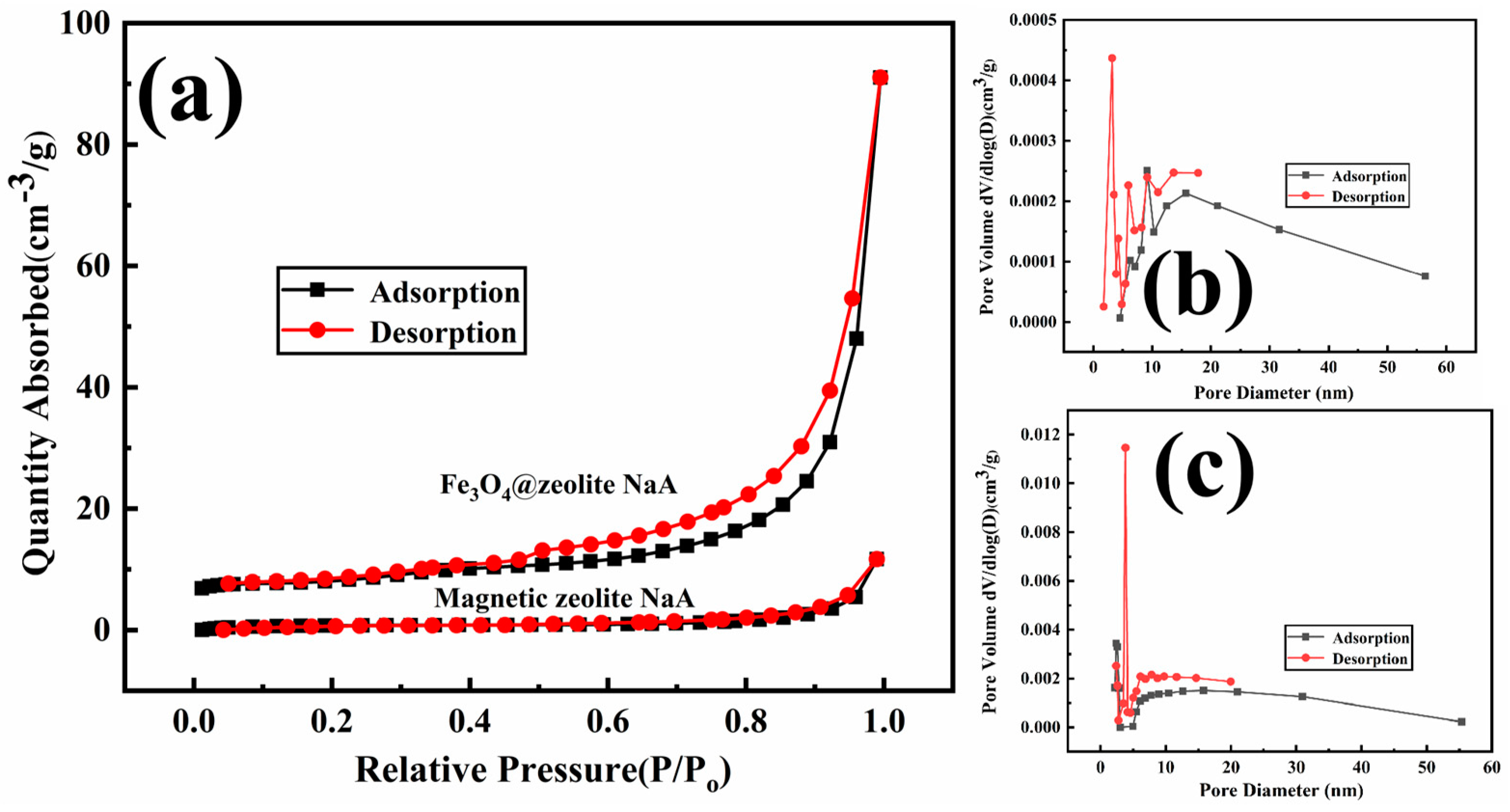



| Sample | BET Surfac Area (m2·g−1) | Pore Volume (cm3·g−1) | Average Pore Size (nm) |
|---|---|---|---|
| Magnetic zeolite NaA | 2.731 | 0.017872 | 31.7899 |
| Fe3O4@zeolite NaA | 26.846 | 0.136697 | 20.5846 |
| Models | Parameter | Magnetic zeolite NaA | Fe3O4@zeolite NaA |
|---|---|---|---|
| R2 | 0.93447 | 0.61082 | |
| Freundich | n | 4.1406 | 4.5773 |
| KF (mg/g) | 9.9094 | 34.6927 | |
| R2 | 0.99890 | 0.99734 | |
| Langmuir | Qmax (mg/g) | 33.89 | 86.58 |
| KL (L/mg) | 0.10835 | 0.53177 | |
| R2 | 0.96782 | 0.73113 | |
| Tempkin | B1 | 5.07216 | 9.8778 |
| Kt (L/mg) | 3.85743 | 67.35653 |
| Adsorbent | Qm (mg/g) | Ref. |
|---|---|---|
| Sewage sludge AC | 7.73 | [47] |
| Wheat shell | 10.84 | [50] |
| Lignocellulosic waste N-CDs | 26.95 | [51] |
| Mg2Al-LS-LD | 71.4 | [52] |
| Sulfonated MWCNT | 36.8 | [53] |
| Magnetic zeolite NaA | 32.12 | This study |
| Fe3O4@zeolite NaA | 86.54 | This study |
| Models | Parameter | Magnetic Zeolite NaA | Fe3O4@zeolite NaA |
|---|---|---|---|
| K1 (min−1) | 0.099 | 0.07775 | |
| Pseudo-first-order | Qe (mg/g) | 13.898 | 41.698 |
| R2 | 0.89008 | 0.84336 | |
| K2 (min−1) | 0.00969 | 0.00224 | |
| Pseudo-second-order | Qe (mg/g) | 34.282 | 95.147 |
| R2 | 0.99702 | 0.99305 |
| Sample | ΔHo | ΔSo | ΔGo(KJ·mol−1) | |||
|---|---|---|---|---|---|---|
| (KJ·mol−1) | [J·(K·mol)−1] | 288.15 K | 298.15 K | 308.15 K | 318.15 K | |
| Magnetic zeolite NaA | 8.05 | 14.86 | −4.27 | −4.42 | −4.57 | −4.72 |
| Fe3O4@zeolite NaA | 9.64 | 42.38 | −12.19 | −12.63 | −13.05 | −13.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Wang, P.; Shen, J.; Sun, Q. Core-shell Fe3O4@zeolite NaA as an Adsorbent for Cu2+. Materials 2020, 13, 5047. https://doi.org/10.3390/ma13215047
Cao J, Wang P, Shen J, Sun Q. Core-shell Fe3O4@zeolite NaA as an Adsorbent for Cu2+. Materials. 2020; 13(21):5047. https://doi.org/10.3390/ma13215047
Chicago/Turabian StyleCao, Jun, Peng Wang, Jie Shen, and Qi Sun. 2020. "Core-shell Fe3O4@zeolite NaA as an Adsorbent for Cu2+" Materials 13, no. 21: 5047. https://doi.org/10.3390/ma13215047
APA StyleCao, J., Wang, P., Shen, J., & Sun, Q. (2020). Core-shell Fe3O4@zeolite NaA as an Adsorbent for Cu2+. Materials, 13(21), 5047. https://doi.org/10.3390/ma13215047