Micro-Architectural Investigation of Teleost Fish Rib Inducing Pliant Mechanical Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
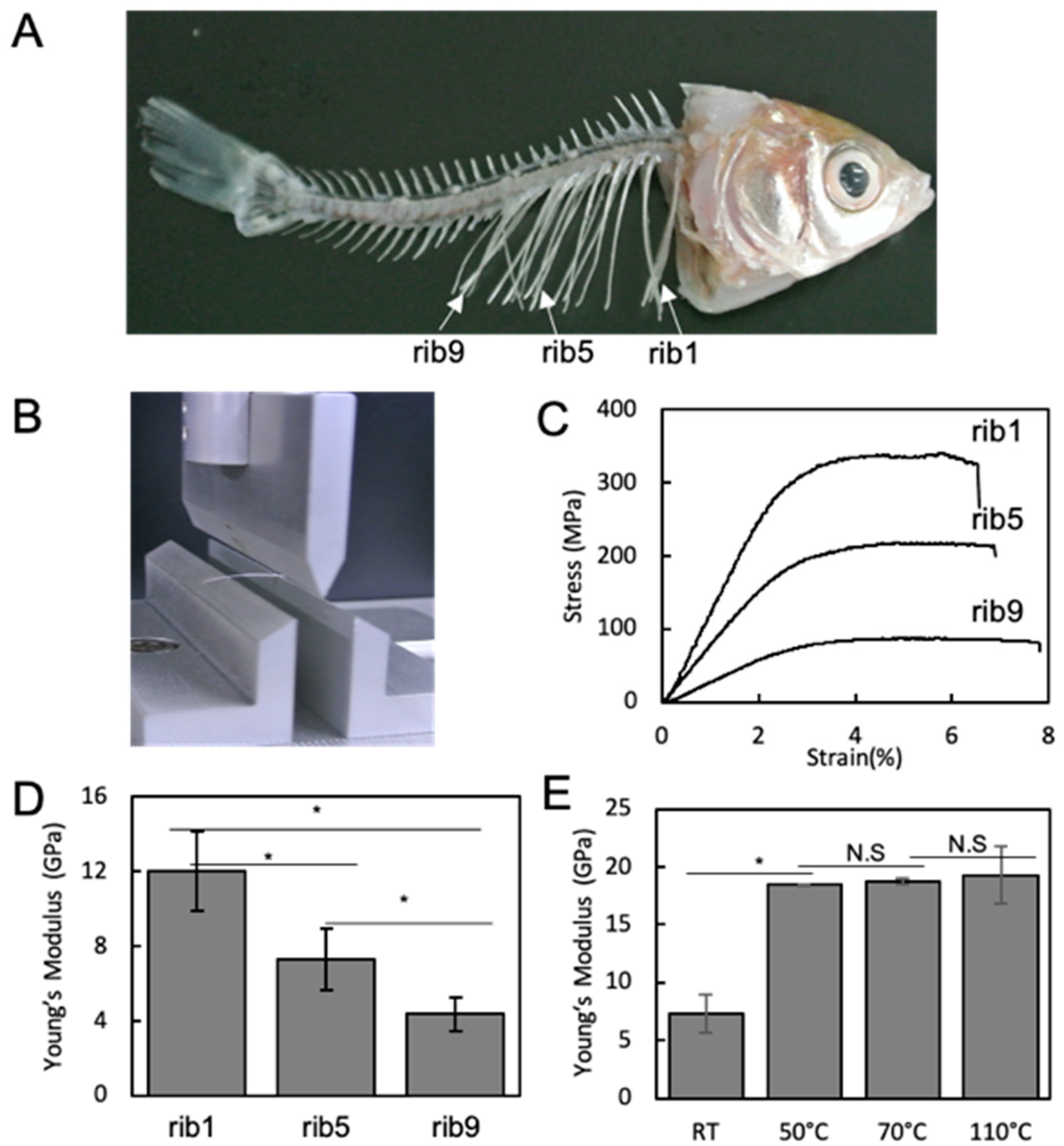
2.2. Mechanical Test
2.3. Characterization of Fish Rib Bone
2.4. Microstructure Observation
2.5. Statistical Analysis
3. Results
3.1. Mechanical Property of Rib Bone
3.2. Characterization of Fish Rib Bone
3.3. Microstructure Observation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Melo Pereira, D.; Habibovic, P. Biomineralization-inspired material design for bone regeneration. Adv. Healthc Mater. 2018, 7, e1800700. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.H.; Liu, S.W.; Xiong, L.; Qiu, P.; Ding, L.H.; Xiong, S.L.; Li, J.T.; Liao, X.G.; Tang, Z.M. Scaffolds for the repair of bone defects in clinical studies. J. Orthop. Surg. Res. 2018, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Casanova, S.; Martin-Saavedra, F.M.; Escudero-Duch, C.; Falguera Uceda, M.I.; Prieto, M.; Arruebo, M.; Acebo, P.; Fabiilli, M.L.; Franceschi, R.T.; Vilaboa, N. Local delivery of bone morphogenetic protein-2 from near infrared-responsive hydrogels for bone tissue regeneration. Biomaterials 2020, 241, 119909. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Witten, P.E.; Huysseune, A. A comparative view on mechanisms and functions of skeletal remodelling in teleost fish, with special emphasis on osteoclasts and their function. Biol. Rev. Camb. Philos. Soc. 2009, 84, 315–346. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Saini, M.; Dehiya, B.S.; Umar, A.; Sindhu, A.; Mohammed, H.; Al-Hadeethi, Y.; Guo, Z. Fabrication and in vitro biocompatibility of freeze-dried CTS-nHA and CTS-nBG scaffolds for bone regeneration applications. Int. J. Biol. Macromol. 2020, 149, 1–10. [Google Scholar] [CrossRef]
- Owen, R.; Sherborne, C.; Evans, R.; Reilly, G.C.; Claeyssens, F. Combined porogen leaching and emulsion templating to produce bone tissue engineering scaffolds. Int. J. Bioprint. 2020, 6, 265. [Google Scholar] [CrossRef]
- Ng, W.L.; Chua, C.K.; Shen, Y.-F. Print me an organ! why we are not there yet. Prog. Polym. Sci. 2019, 97, 10. [Google Scholar] [CrossRef]
- Huang, B.; Aslan, E.; Jiang, Z.; Daskalakis, E.; Jiao, M.; Aldalbahi, A.; Vyas, C.; Bártolo, P. Engineered dual-scale poly (ε-caprolactone) scaffolds using 3D printing and rotational electrospinning for bone tissue regeneration. Addit. Manuf. 2020, 36, 101452. [Google Scholar] [CrossRef]
- Schmidt, F.N.; Zimmermann, E.A.; Walsh, F.; Plumeyer, C.; Schaible, E.; Fiedler, I.A.K.; Milovanovic, P.; Rossle, M.; Amling, M.; Blanchet, C.; et al. On the origins of fracture toughness in advanced teleosts: How the swordfish sword’s bone structure and composition allow for slashing under water to kill or stun prey. Adv. Sci. 2019, 6, 1900287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhter, M.N.; Hara, E.S.; Kadoya, K.; Okada, M.; Matsumoto, T. Cellular fragments as biomaterial for rapid in vitro bone-like tissue synthesis. Int. J. Mol. Sci. 2020, 21, 5327. [Google Scholar] [CrossRef]
- Kazi, G.A.S.; Rahman, K.A.; Farahat, M.; Matsumoto, T. Fabrication of single gel with different mechanical stiffness using three-dimensional mold. J Biomed. Mater. Res. A 2019, 107, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, E.S.; Okada, M.; Nagaoka, N.; Hattori, T.; Kuboki, T.; Nakano, T.; Matsumoto, T. Bioinspired mineralization using chondrocyte membrane nanofragments. ACS Biomater. Sci. Eng. 2018, 4, 617–625. [Google Scholar] [CrossRef]
- Blob, R.W.; Biewener, A.A. Mechanics of limb bone loading during terrestrial locomotion in the green iguana (Iguana iguana) and American alligator (Alligator mississippiensis). J. Exp. Biol. 2001, 204, 1099–1122. [Google Scholar]
- Cohen, L.; Dean, M.; Shipov, A.; Atkins, A.; Monsonego-Ornan, E.; Shahar, R. Comparison of structural, architectural and mechanical aspects of cellular and acellular bone in two teleost fish. J. Exp. Biol. 2012, 215, 1983–1993. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Kaibara, K.; Ishimoto, T.; Tabata, Y.; Umakoshi, Y. Biological apatite (BAp) crystallographic orientation and texture as a new index for assessing the microstructure and function of bone regenerated by tissue engineering. Bone 2012, 51, 741–747. [Google Scholar] [CrossRef]
- Atkins, A.; Dean, M.N.; Habegger, M.L.; Motta, P.J.; Ofer, L.; Repp, F.; Shipov, A.; Weiner, S.; Currey, J.D.; Shahar, R. Remodeling in bone without osteocytes: Billfish challenge bone structure-function paradigms. Proc. Natl. Acad. Sci. USA 2014, 111, 16047–16052. [Google Scholar] [CrossRef] [Green Version]
- Ozasa, R.; Nakatsu, M.; Moriguchi, A.; Sasaki, K.; Ishimoto, T.; Okada, M.; Matsumoto, T.; Nakano, T. Analysis of bone regeneration based on the relationship between the orientations of collagen and apatite in mouse femur. Mater. Trans. 2020, 61, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Kitamura, M.; Akamatsu, M.; Kawanami, M.; Furuichi, Y.; Fujii, T.; Mori, M.; Kunimatsu, K.; Shimauchi, H.; Ogata, Y.; Yamamoto, M.; et al. Randomized placebo-controlled and controlled non-inferiority phase III trials comparing trafermin, a recombinant human fibroblast growth factor 2, and enamel matrix derivative in periodontal regeneration in intrabony defects. J. Bone Miner. Res. 2016, 31, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Jeroen, A.; Steven, B.; Geert, L.; Jan, D. Interspecies differences in bone composition, density, and quality-potential implications for in vivo bone. J. Endocrinol. 1998, 139, 663–670. [Google Scholar] [CrossRef]
- Porter, M.E.; Beltran, J.L.; Koob, T.J.; Summers, A.P. Material properties and biochemical composition of mineralized vertebral cartilage in seven elasmobranch species (Chondrichthyes). J. Exp. Biol. 2006, 209, 2920–2928. [Google Scholar] [CrossRef] [Green Version]
- Mahamid, J.; Sharir, A.; Addadi, L.; Weiner, S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. USA 2008, 105, 12748–12753. [Google Scholar] [CrossRef] [Green Version]
- Liebschner, M.A.K. Biomechanical considerations of animal models used in tissue engineering of bone. Biomaterials 2004, 25, 1697–1714. [Google Scholar] [CrossRef]
- Burr, D.B.; Robling, A.G.; Turner, C.H. Effects of biomechanical stress on bones in animals. Bone 2002, 30, 781–786. [Google Scholar] [CrossRef]
- Song, H.D.; Sun, X.J.; Deng, M.; Zhang, G.W.; Zhou, Y.; Wu, X.Y.; Sheng, Y.; Chen, Y.; Ruan, Z.; Jiang, C.L.; et al. Hematopoietic gene expression profile in zebrafish kidney marrow. Proc. Natl. Acad. Sci. USA 2004, 101, 16240–16245. [Google Scholar] [CrossRef] [Green Version]
- Atkins, A.; Reznikov, N.; Ofer, L.; Masic, A.; Weiner, S.; Shahar, R. The three-dimensional structure of anosteocytic lamellated bone of fish. Acta Biomater. 2015, 13, 311–323. [Google Scholar] [CrossRef]
- Tetè, S.; Mastrangelo, F.; Bianchi, A.; Zizzari, V.; Scarano, A. Collagen fiber orientation around machined titanium and zirconia dental implant necks: An animal study. Int. J. Oral Maxillofac. Implant. 2009, 24, 52–58. [Google Scholar]
- Sekita, A.; Matsugaki, A.; Ishimoto, T.; Nakano, T. Synchronous disruption of anisotropic arrangement of the osteocyte network and collagen/apatite in melanoma bone metastasis. J. Struct. Biol. 2017, 197, 260–270. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.Y.; Okada, M.; Hara, E.S.; Xie, S.C.; Nagaoka, N.; Nakano, T.; Matsumoto, T. Micro-Architectural Investigation of Teleost Fish Rib Inducing Pliant Mechanical Property. Materials 2020, 13, 5099. https://doi.org/10.3390/ma13225099
Jiao YY, Okada M, Hara ES, Xie SC, Nagaoka N, Nakano T, Matsumoto T. Micro-Architectural Investigation of Teleost Fish Rib Inducing Pliant Mechanical Property. Materials. 2020; 13(22):5099. https://doi.org/10.3390/ma13225099
Chicago/Turabian StyleJiao, Yu Yang, Masahiro Okada, Emilio Satoshi Hara, Shi Chao Xie, Noriyuki Nagaoka, Takayoshi Nakano, and Takuya Matsumoto. 2020. "Micro-Architectural Investigation of Teleost Fish Rib Inducing Pliant Mechanical Property" Materials 13, no. 22: 5099. https://doi.org/10.3390/ma13225099
APA StyleJiao, Y. Y., Okada, M., Hara, E. S., Xie, S. C., Nagaoka, N., Nakano, T., & Matsumoto, T. (2020). Micro-Architectural Investigation of Teleost Fish Rib Inducing Pliant Mechanical Property. Materials, 13(22), 5099. https://doi.org/10.3390/ma13225099





