Preparation of CNF/PDMS Superhydrophobic Coatings with Good Abrasion Resistance Using a One-Step Spray Method
Abstract
:1. Introduction
2. Results and Discussion
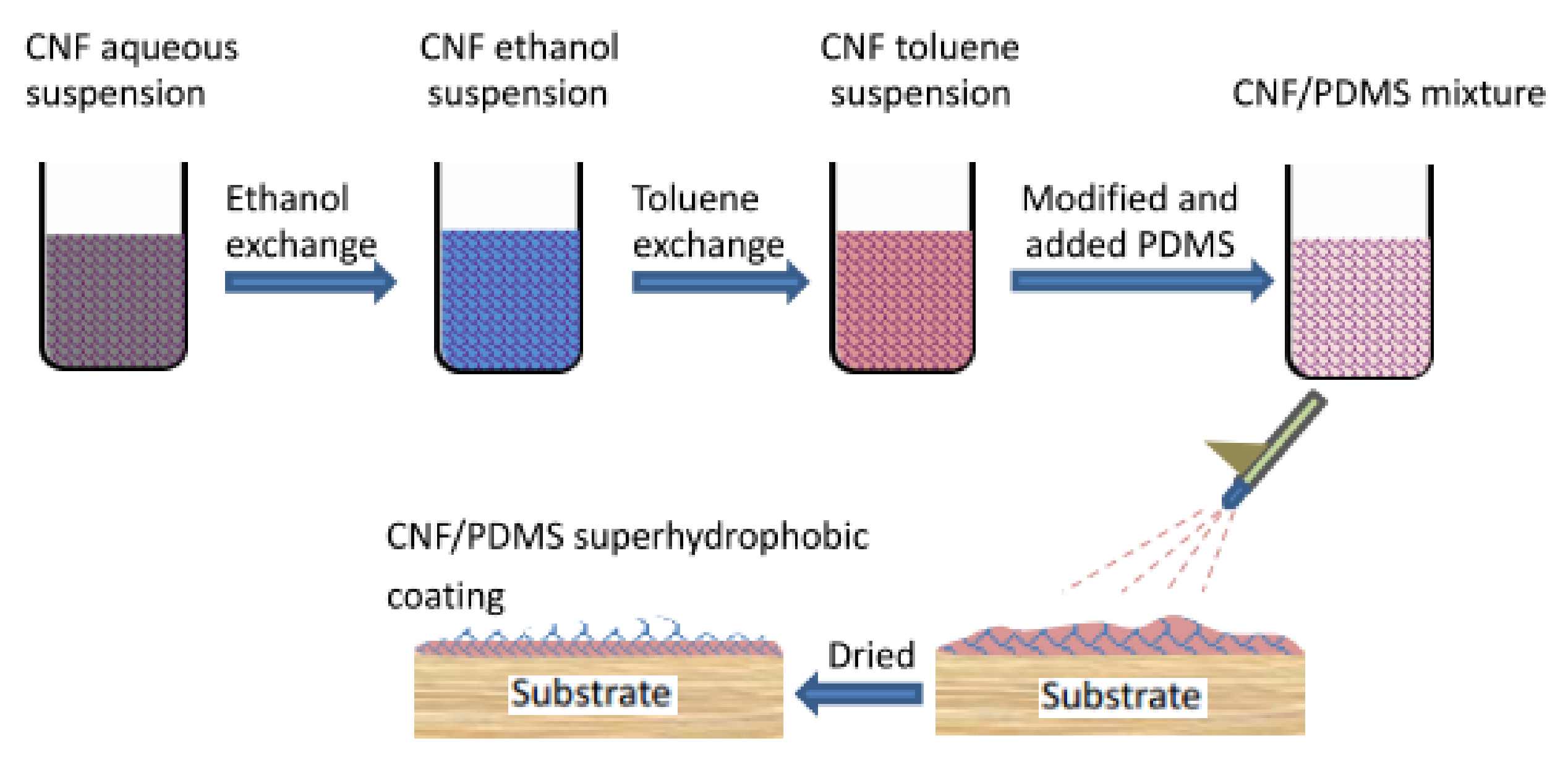
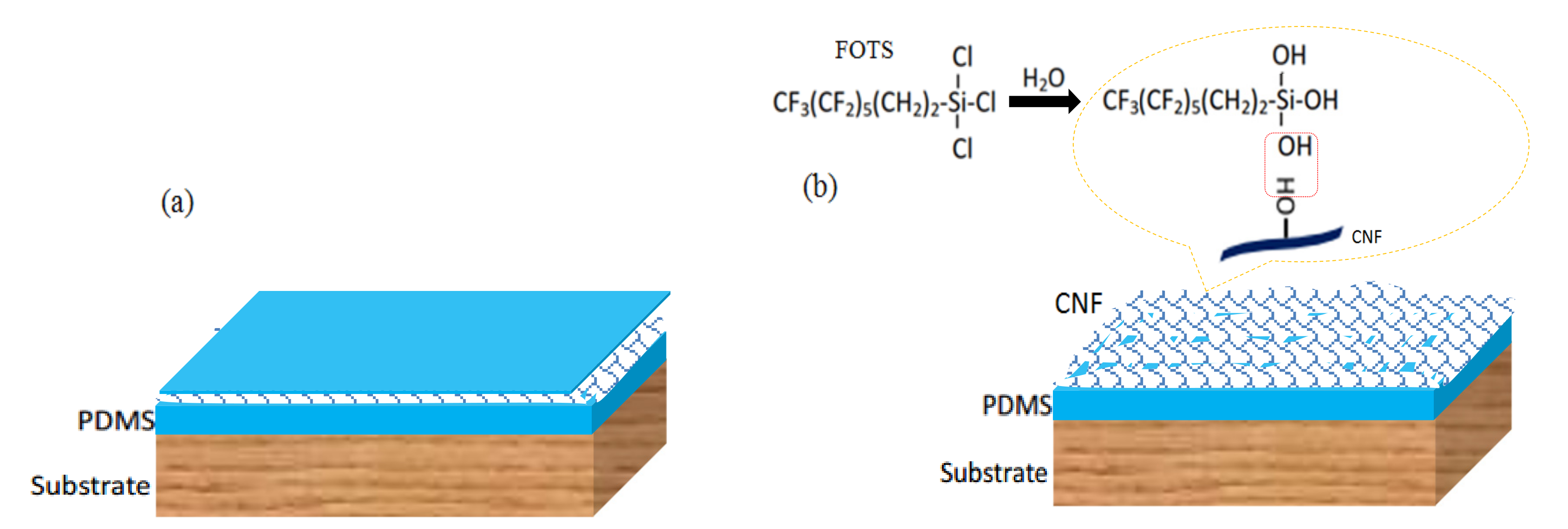
2.1. Formation Mechanism
2.2. Wettability
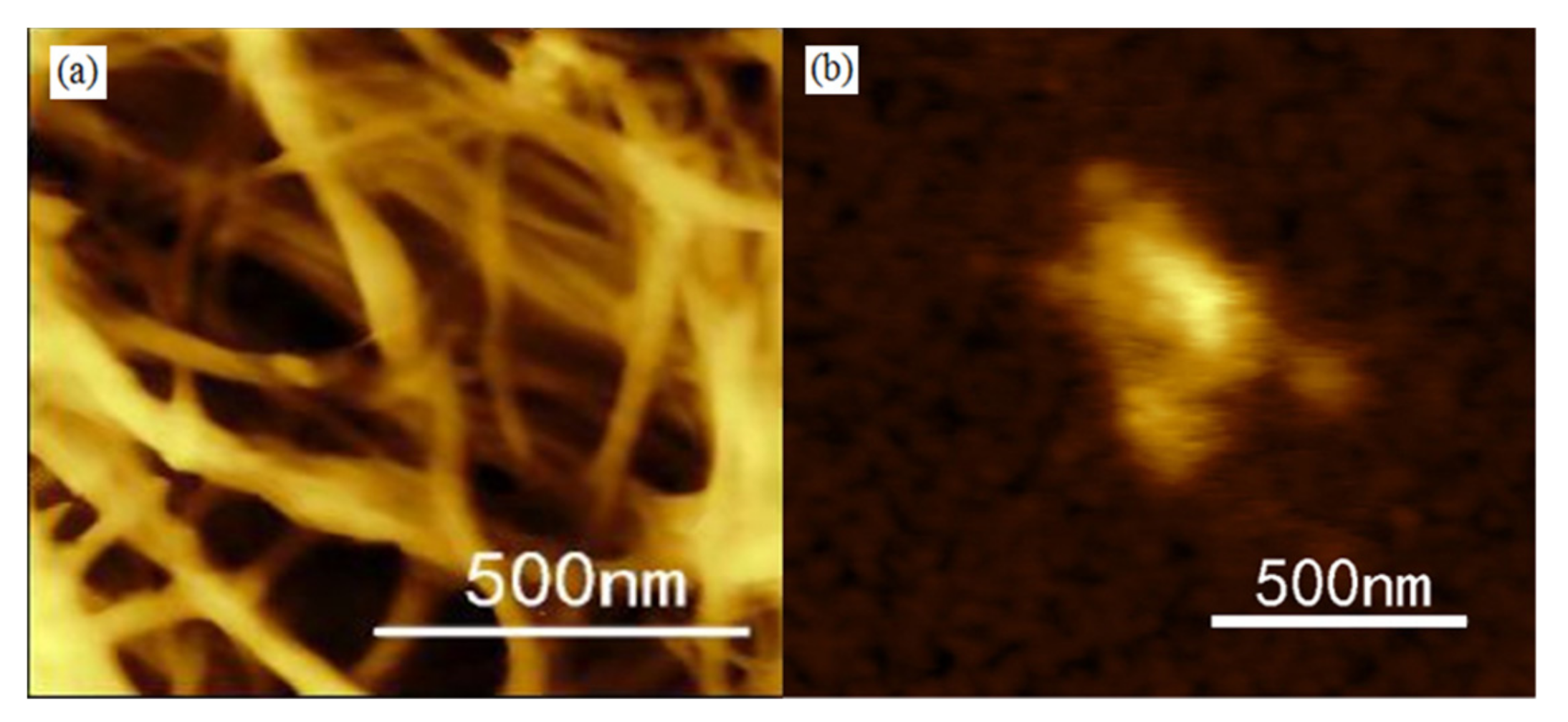
2.3. Surface Morphologies
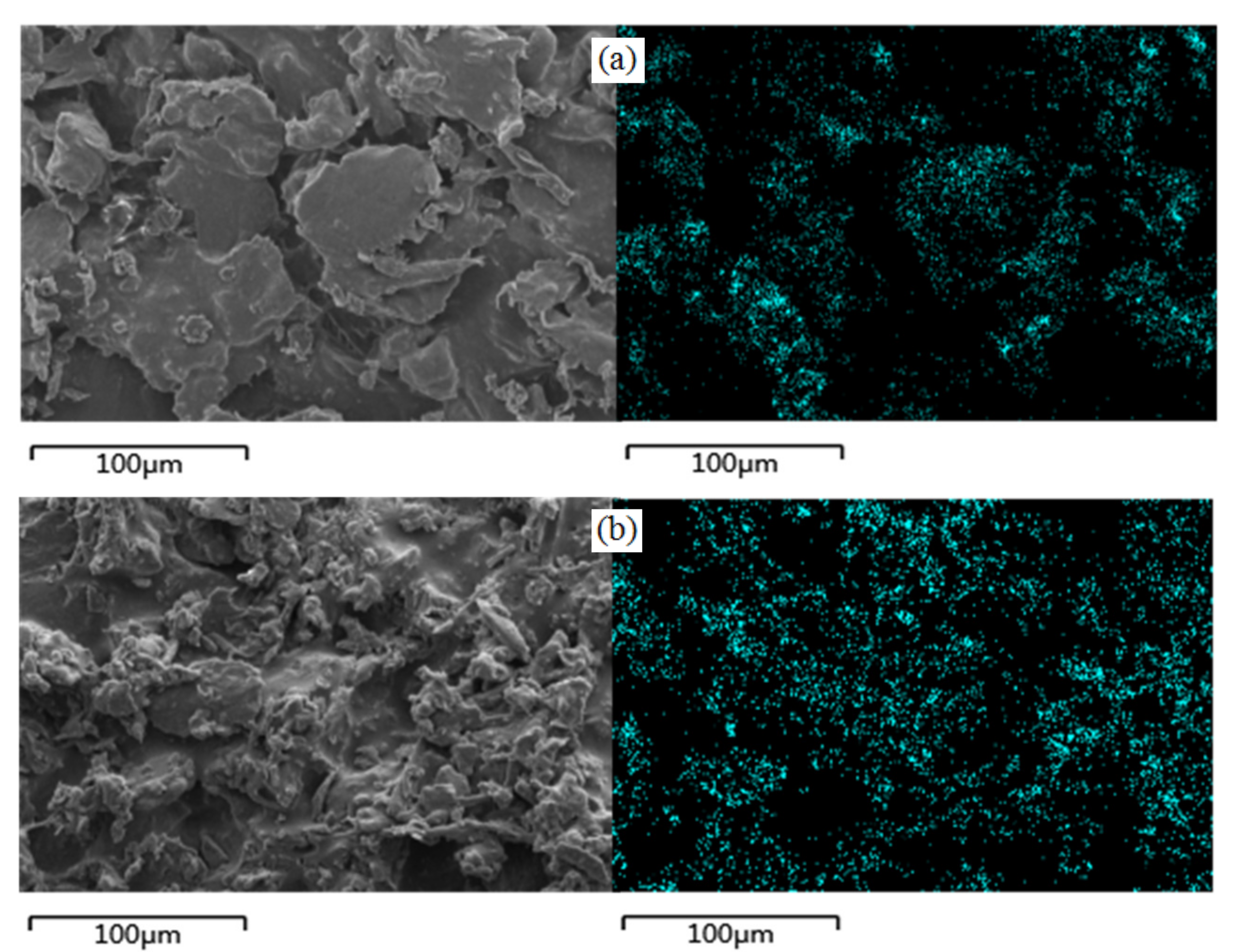
2.4. Chemical Composition Analysis
2.5. Sandpaper Abrasion Test
2.6. Knife-Scratch Tests
3. Materials and Methods
3.1. Materials
3.2. Preparation of CNF/PDMS Superhydrophobic Coating
3.2.1. Organic Solvent Exchange
3.2.2. Hydrophobic Toluene Suspended CNF
3.2.3. Spraying
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiaqiang, E.; Jin, Y.; Deng, Y.; Zuo, W.; Zhao, X.; Han, D.; Peng, Q.; Zhang, Z. Wetting Models and Working Mechanisms of Typical Surfaces Existing in Nature and Their Application on Superhydrophobic Surfaces: A Review. Adv. Mater. Interfaces 2018, 5, 1701052–1701091. [Google Scholar] [CrossRef]
- Zhu, J.; Wan, H.; Hu, X. A rapid one-step process for the construction of corrosion-resistant bionic superhydrophobic surfaces. Prog. Org. Coat. 2016, 100, 56–62. [Google Scholar] [CrossRef]
- Li, C.; Ma, R.; Du, A.; Fan, Y.; Zhao, X.; Cao, X. One-step fabrication of bionic superhydrophobic coating on galvanised steel with excellent corrosion resistance. J. Alloy. Compd. 2019, 786, 272–283. [Google Scholar] [CrossRef]
- Huang, J.; Lyu, S.; Chen, Z.; Wang, S.; Fu, F. A facile method for fabricating robust cellulose nanocrystal/SiO2 superhydrophobic coatings. J. Colloid Interface Sci. 2019, 536, 349–362. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, H.; Tang, J.; Gong, H.; Liu, Y.; Wang, Z.; Shi, P.; Zhang, J.; Chen, X. A novel preparation of polystyrene film with a superhydrophobic surface using a template method. J. Phys. D: Appl. Phys. 2007, 40, 3485–3489. [Google Scholar] [CrossRef]
- Ebert, D.; Bhushan, B. Transparent, superhydrophobic, and wear-resistant surfaces using deep reactive ion etching on PDMS substrates. J. Colloid Interface Sci. 2016, 481, 82–90. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Lai, X.; Zhang, L.; Wang, J.; Liao, X.; Zeng, X. Vapor–Liquid Sol–Gel Approach to Fabricating Highly Durable and Robust Superhydrophobic Polydimethylsiloxane@Silica Surface on Polyester Textile for Oil–Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 28089–28099. [Google Scholar] [CrossRef]
- Tan, R.; Xie, H.; She, J.; Liang, J.; He, H.; Li, J.; Fan, Z.; Liu, B. A new approach to fabricate superhydrophobic and antibacterial low density isotropic pyrocarbon by using catalyst free chemical vapor deposition. Carbon 2019, 145, 359–366. [Google Scholar] [CrossRef]
- Yan, K.-K.; Jiao, L.; Lin, S.; Ji, X.; Lu, Y.; Zhang, L. Superhydrophobic electrospun nanofiber membrane coated by carbon nanotubes network for membrane distillation. Desalination 2018, 437, 26–33. [Google Scholar] [CrossRef]
- Huang, J.; Lyu, S.; Fu, F.; Chang, H.; Wang, S. Preparation of superhydrophobic coating with excellent abrasion resistance and durability using nanofibrillated cellulose. RSC Adv. 2016, 6, 106194–106200. [Google Scholar] [CrossRef]
- Wei, C.; Dai, F.; Lin, L.; An, Z.; He, Y.; Chen, X.; Chen, L.; Zhao, Y. Simplified and robust adhesive-free superhydrophobic SiO2-decorated PVDF membranes for efficient oil/water separation. J. Membr. Sci. 2018, 555, 220–228. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, S.; Guan, S.; Lv, Z.; Liu, E.; Zhao, Y. Fabrication and characterization of superhydrophobic TiO2 nanotube coating by a facile anodic oxidation approach. Surf. Coatings Technol. 2018, 354, 83–91. [Google Scholar] [CrossRef]
- Velayi, E.; Norouzbeigi, R. Robust superhydrophobic needle-like nanostructured ZnO surfaces prepared without post chemical-treatment. Appl. Surf. Sci. 2017, 426, 674–687. [Google Scholar] [CrossRef]
- Xu, C.-L.; Song, F.; Wang, X.-L.; Wang, Y.-Z. Surface modification with hierarchical CuO arrays toward a flexible, durable superhydrophobic and self-cleaning material. Chem. Eng. J. 2017, 313, 1328–1334. [Google Scholar] [CrossRef]
- Cao, C.; Cheng, J. Fabrication of superhydrophobic copper stearate@Fe3O4 coating on stainless steel meshes by dip-coating for oil/water separation. Surf. Coatings Technol. 2018, 349, 296–302. [Google Scholar] [CrossRef]
- Xue, F.; Jia, D.; Li, Y.; Jing, X. Facile preparation of a mechanically robust superhydrophobic acrylic polyurethane coating. J. Mater. Chem. A 2015, 3, 13856–13863. [Google Scholar] [CrossRef]
- Huang, J.Y.; Li, S.H.; Ge, M.Z.; Wang, L.N.; Xing, T.L.; Chen, G.-Q.G.; Liu, X.F.; Aldeyab, S.S.; Zhang, K.-Q.; Chen, T.; et al. Robust superhydrophobic TiO2@fabrics for UV shielding, self-cleaning and oil–water separation. J. Mater. Chem. A 2015, 3, 2825–2832. [Google Scholar] [CrossRef]
- Siddaramanna, A.; Saleema, N.; Sarkar, D.K. A versatile cost-effective and one step process to engineer ZnO superhydrophobic surfaces on Al substrate. Appl. Surf. Sci. 2014, 311, 182–188. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose Nanofiber Supported 3D Interconnected BN Nanosheets for Epoxy Nanocomposites with Ultrahigh Thermal Management Capability. Adv. Funct. Mater. 2017, 27, 1604754. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, J.; Yao, Y.; Sun, R.; Xu, J.-B.; Wong, C.-P. A Combination of Boron Nitride Nanotubes and Cellulose Nanofibers for the Preparation of a Nanocomposite with High Thermal Conductivity. ACS Nano 2017, 11, 5167–5178. [Google Scholar] [CrossRef]
- Sethi, J.; Farooq, M.; Sain, S.; Sain, M.; Sirviö, J.A.; Illikainen, M.; Oksman, K. Water resistant nanopapers prepared by lactic acid modified cellulose nanofibers. Cellulose 2018, 25, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Cervin, N.T.; Aulin, C.; Larsson, P.T.; Wågberg, L. Ultra porous nanocellulose aerogels as separation medium for mixtures of oil/water liquids. Cellulose 2011, 19, 401–410. [Google Scholar] [CrossRef]
- Zheng, Q.; Cai, Z.; Gong, S. Green synthesis of polyvinyl alcohol (PVA)–cellulose nanofibril (CNF) hybrid aerogels and their use as superabsorbents. J. Mater. Chem. A 2014, 2, 3110–3118. [Google Scholar] [CrossRef]
- Benítez, A.J.; Walther, A. Cellulose nanofibril nanopapers and bioinspired nanocomposites: a review to understand the mechanical property space. J. Mater. Chem. A 2017, 5, 16003–16024. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Lyu, S.; Fu, F. Preparation of a robust cellulose nanocrystal superhydrophobic coating for self-cleaning and oil-water separation only by spraying. Ind. Crop. Prod. 2018, 122, 438–447. [Google Scholar] [CrossRef]
- Zhu, Q.; Chu, Y.; Wang, Z.; Chen, N.; Lin, L.; Liu, F.; Chu, Y. Robust superhydrophobic polyurethane sponge as a highly reusable oil-absorption material. J. Mater. Chem. A 2013, 1, 5386–5393. [Google Scholar] [CrossRef]
- Deng, X.; Mammen, L.; Zhao, Y.; Lellig, P.; Müllen, K.; Li, C.; Butt, H.-J.; Vollmer, D. Transparent, Thermally Stable and Mechanically Robust Superhydrophobic Surfaces Made from Porous Silica Capsules. Adv. Mater. 2011, 23, 2962–2965. [Google Scholar] [CrossRef]
- Chang, H.; Tu, K.; Wang, X.; Liu, J. Facile Preparation of Stable Superhydrophobic Coatings on Wood Surfaces using Silica-Polymer Nanocomposites. Bioresources 2015, 10, 2585–2596. [Google Scholar] [CrossRef] [Green Version]
- Tu, K.; Wang, X.; Kong, L.; Chang, H.; Liu, J. Fabrication of robust, damage-tolerant superhydrophobic coatings on naturally micro-grooved wood surfaces. RSC Adv. 2016, 6, 701–707. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Lyu, S. Facile Preparation of a Robust and Durable Superhydrophobic Coating Using Biodegradable Lignin-Coated Cellulose Nanocrystal Particles. Materials 2017, 10, 1080. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Cai, P.; Li, M.; Wu, Q.; Li, Q.; Wang, S. Preparation of CNF/PDMS Superhydrophobic Coatings with Good Abrasion Resistance Using a One-Step Spray Method. Materials 2020, 13, 5380. https://doi.org/10.3390/ma13235380
Huang J, Cai P, Li M, Wu Q, Li Q, Wang S. Preparation of CNF/PDMS Superhydrophobic Coatings with Good Abrasion Resistance Using a One-Step Spray Method. Materials. 2020; 13(23):5380. https://doi.org/10.3390/ma13235380
Chicago/Turabian StyleHuang, Jingda, Peihao Cai, Mengmeng Li, Qiang Wu, Qian Li, and Siqun Wang. 2020. "Preparation of CNF/PDMS Superhydrophobic Coatings with Good Abrasion Resistance Using a One-Step Spray Method" Materials 13, no. 23: 5380. https://doi.org/10.3390/ma13235380





